Light controlled protein release from a supramolecular hydrogel†
Ke
Peng
,
Itsuro
Tomatsu
and
Alexander
Kros
*
Leiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands. E-mail: a.kros@chem.leidenuniv.nl; Fax: +31 71 527 4397
First published on 13th May 2010
Abstract
Using the inclusion complex of trans azobenzene and cyclodextrin as a photo-switchable crosslinker, a dextran based photo-responsive hydrogel system has been constructed and employed for a light controlled protein release system.
In the past few years, a number of therapeutic proteins have been developed against a broad range of diseases such as cancers, autoimmune diseases and metabolic disorders.1 However, most of these effective therapeutic proteins are prevented from clinical use by fundamental technical hurdles particularly with regard to delivery.2
Hydrogels are an ideal candidate for protein delivery, as they contain large amounts of water in the polymer network in a way similar to body tissues. Thus it allows to retain the proteins in the protective 3-D network in their active forms and prevents them from denaturation during administrations.3 Recently, sustained delivery of proteins using hydrogel systems composed of a highly biocompatible biopolymer dextran4 or other polymers5 has been reported, in which the loaded proteins are released from the hydrogel matrix in a time dependent manner regulated by the change of mesh size due to the erosion of the hydrogel network.6
On another front, stimuli-responsive hydrogels have been developed using supramolecular crosslinkers, whose network can be eroded responding to temperature,7 pH,8 light,9 and other stimuli.10 These stimuli-responsive hydrogels are potentially beneficial for an efficient drug delivery system because it can be employed to transport the entrapped drug to the target site and to trigger the release by a stimulus at the desired point and time.11 Among these stimuli, light is a particularly interesting option as it is a remote stimulus that can be controlled spatially and temporally with great ease and convenience.12 More importantly, the light irradiation does not have a harmful effect on the activity of most proteins.13
Previously, we have functionalized dextran with maleimide moiety, which can react rapidly with thiols via the thiol–maleimide “click” reaction.14 We have shown that it can be used for in situ hydrogel formation15 and the resulting nanogel particles can be used as a drug carrier for hydrophobic drugs in zebrafish embryos exhibiting an excellent biocompatibility.16
Taking the advantage of the efficient thiol–maleimide reaction, in the present work, we functionalized dextrans with either azobenzene (AB) or β-cyclodextrin (CD) moieties. These polymers can be used as building blocks of a supramolecularly crosslinked hydrogel for a light controlled release of proteins. Upon mixing these two polymers, an inclusion of trans AB in CD induces the gel formation when the ABs are in the trans configuration. The inclusion complexes dissociate upon trans–cis isomerization of the ABs after irradiation with UV light (365 nm) resulting in a dissolution of the hydrogel.17,18 Furthermore, proteins can be physically entrapped in this supramolecular gel matrix simply by dissolving it into the polymer solutions before the gel preparation. Using green fluorescent protein (GFP) as a model protein the light controlled in vitro release was demonstrated. These characteristics of the current system will be beneficial for the future drug screening technology using zebrafish embryos as these are transparent for the 365 nm UV light used in this study.19
Azobenzene carrying dextran (AB–Dex) was prepared by the thiol–maleimide reaction of 3-[4-(phenylazo)-phenoxy]-propane-1-thiol (1) and maleimide functionalized dextran (Mal–Dex) in DMSO. The reaction was allowed to proceed for 12 hours at room temperature, and the resulting AB modified dextran was isolated by ultrafiltration and lyophilization (Fig. 1a).
 | ||
| Fig. 1 (a) Preparation of azobenzene modified dextran (AB–Dex) and cyclodextrin modified dextran (CD–Dex) through the thiol–maleimide reaction. (b) Schematic representation of photoresponsive protein release from the gel composed of trans AB–Dex and CD–Dex. Upon the UV light irradiation azobenzene moieties isomerise from trans to cis configurations, resulting in the dissociation of crosslinking points, and allow the entrapped protein to migrate into the media. | ||
The conjugation of AB to dextran was confirmed by 1H NMR. On the spectra, besides signals attributed to dextran, peaks at δ = 7.2, 7.6 and 7.9 due to the AB were observed. The reaction was further confirmed by the disappearance of the peak of maleimide at δ = 6.9. The degree of substitution (DS: defined as the number of substituents per 100 anhydroglucosidic rings) was determined to be 6.
β-Cyclodextrin modified dextran (CD–Dex) was also obtained by the thiol–maleimide reaction of mono-6-thio-β-cyclodextrin (2) and Mal–Dex in water. The reaction mixture was stirred for 4 hours at room temperature. The resulting CD–Dex was obtained after ultrafiltration and lyophilization. The conjugation of CD was confirmed by the 1H NMR with a peak at δ = 5.1 attributed to the anomeric protons of CD and disappearance of the maleimide peak at δ = 6.9. The DS of CD–Dex was determined to be 5 in the same way as AB–Dex.
The photoisomerization of AB–Dex was confirmed by 1H NMR (see ESI†). Before irradiation with UV light, peaks ascribable to the aromatic protons of trans-AB were observed with a trace amount of cis-AB indicated by the small peaks at δ = 6.8 and 7.3. After 4 hour irradiation with UV light, the peaks due to trans-AB moiety were disappeared and the peaks ascribable to the aromatic protons of cis-AB were observed. From the ratios of the integrals in these spectra, fractions of trans configuration were determined to be ∼95 and ∼0% before and after UV irradiation, respectively.
We wondered if the newly synthesised AB functionalized polymer AB–Dex can also form inclusion complex strongly with CD only when AB is in trans configuration as seen in other reported systems.17,18 To address this issue, NOESY spectrum of the mixture of trans and cis AB–Dex was measured in the presence of CD. As shown in Fig. 2, correlation peaks between the protons attributed to trans AB and inner protons of CD were observed which shows the inclusion complex formation of trans AB and CD. There was no correlation peak between the protons of cis AB and CD. These data indicate that the inclusion complex formation of AB–Dex with CD occurs predominantly with the trans configuration and not with the cis configuration, indicating it can act indeed as a photoresponsive switch.
 | ||
| Fig. 2 NOESY spectrum of a mixture of the isomers of AB–Dex in D2O in the presence of CD. NOE signals were observed between the protons attributed to trans AB and the inner (C-3, C-6, C-5) protons of CD. | ||
A hydrogel was obtained from a mixture of solutions of trans AB–Dex and CD–Dex. Since trans AB binds inside the CD cavity as seen in Fig. 2, supramolecular crosslinking points were formed among the polymers. After UV irradiation, the gel mixture turned into a solution. This is because trans-AB moieties were isomerized into the cis configuration, which did not show an interaction with CD–Dex. It is notable that the transition from gel to sol is reversible by changing the wavelength of the light17 (Fig. 3).
 | ||
| Fig. 3 Photographs of a mixture of AB–Dex (67 mg mL−1) and CD–Dex (100 mg mL−1) in PBS; taken after mixing (a) and after irradiation with UV light (b). | ||
To investigate the potential of the hydrogel as a light-controlled protein release system, green fluorescent protein (GFP) was used as a model protein and encapsulated inside the hydrogel. The GFP carrying gel (18 mg) was placed into a cuvette with 2 mL of fresh PBS and the fluorescence intensities of the solution part were measured as a function of time (Fig. 4). Prior to the UV light irradiation, the emission intensities of GFP were practically constant, indicating that the GFP remains entrapped inside the hydrogel network. After 10 minutes of UV light irradiation, the fluorescence intensity started to increase which was pronounced after 20 minutes. The release of GFP can be continued; 40% of the loaded proteins was released in 60 min under UV irradiation and reached ca. 65% in 2 hours. In contrast, a significant increase in the release of GFP was not observed without UV light irradiation even after 60 minutes (ca. 10%). Therefore we conclude that the release of GFP was in a UV light responsive manner (Fig. 1b).
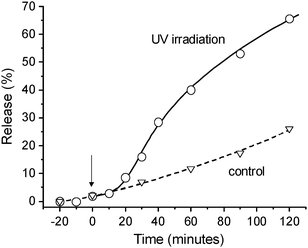 | ||
| Fig. 4 Release of green fluorescent protein (GFP) from the supramolecular hydrogel. From the time 0, the sample was placed under UV light. With a delay of 10 min, GFP was released from the hydrogel matrix. | ||
The stability of the current gel can be enhanced by using a dextran polymer with a significant higher molecular weight and by increasing the number of cyclodextrins and azobenzenes per dextran chain. These two changes combined would result in a lowered release of protein before UV-irradiation.
In conclusion, a light responsive hydrogel system composed of azobenzene functionalized dextran (AB–Dex) and β-cyclodextrin functionalized dextran (CD–Dex) has been prepared for the light controlled release of proteins. AB–Dex and CD–Dex were prepared efficiently from maleimide modified dextran via thiol–maleimide “click” reaction. Using NMR techniques we confirmed that AB–Dex can be isomerized from trans to cis upon UV light irradiation and trans isomers can form inclusion complex with cyclodextrins more firmly than do cis isomers. Using this photoresponsive supramolecular interaction as a molecular switch, we constructed a photoresponsive hydrogel system. Upon UV light irradiation, trans-AB moieties were isomerized to cis configurations resulting in the dissociation of the network formed with CD–Dex, converting the hydrogel into a sol. The light responsive gel-to-sol transition was successfully employed for the controlled release of an entrapped model protein, green fluorescent protein (GFP). This hydrogel system equipped with both biocompatibility and stimuli-responsivity will contribute to the future protein administrations where UV irradiation is applicable such as a light controlled transdermal delivery system. Currently we are planning to apply this system for the light controlled protein delivery in transparent zebrafish embryos, which are relevant for the fast screening of new potential drugs.19
The authors are grateful to Prof. Dr H. P. Spaink for the supply of green fluorescent protein. We thank Mr Wim Jesse for his technical assistance and we also thank Mr Kees Erkelens and Mr Fons Lefeber for 2D NOESY data. The authors (A. K. and I. T.) acknowledge the support of the Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.
Notes and references
- C. Krejsa, M. Rogge and W. Sadee, Nat. Rev. Drug Discovery, 2006, 5, 507 CrossRef CAS.
- G. Walsh, Nat. Biotechnol., 2006, 24, 769 CrossRef CAS.
- S. Kiyonaka, K. Sada, I. Yoshimura, S. Shinkai, N. Kato and I. Hamachi, Nat. Mater., 2004, 3, 58 CrossRef CAS; R. Singh, S. Singh and J. W. Lillard, J. Pharm. Sci., 2008, 97, 2497 CrossRef CAS.
- J. A. Cadée, C. J. de Groot, W. Jiskoot, W. den Otter and W. E. Hennink, J. Controlled Release, 2002, 78, 1 CrossRef CAS; M. Maire, F. Chaubet, P. Mary, C. Blanchat, A. Meunier and D. Logeart-Avramoglou, Biomaterials, 2005, 26, 5085 CrossRef CAS; C. Hiemstra, Z. Y. Zhong, M. J. van Steenbergen, W. E. Hennink and J. Feijen, J. Controlled Release, 2007, 122, 71 CrossRef CAS; S. R. van Tomme, A. Mens, C. F. van Nostrurn and W. E. Hennink, Biomacromolecules, 2008, 9, 158 CrossRef CAS.
- F. van de Manakker, K. Braeckmans, N. el Morabit, S. C. De Smedt, C. F. van Nostrum and W. E. Hennink, Adv. Funct. Mater., 2009, 19, 2992 CrossRef CAS; F. Khan, R. S. Tare, R. O. C. Oreffo and M. Bradley, Angew. Chem., Int. Ed., 2009, 48, 978 CrossRef CAS.
- N. A. Peppas, Y. Huang, M. Torres-Lugo, J. H. Ward and J. Zhang, Annu. Rev. Biomed. Eng., 2000, 2, 9 CrossRef CAS.
- T. Aoki, M. Kawashima, H. Katono, K. Sanui, N. Ogata, T. Okano and Y. Sakurai, Macromolecules, 1994, 27, 947 CrossRef CAS; G. H. Chen and A. S. Hoffman, Nature, 1995, 373, 49 CrossRef CAS.
- M. Annaka and T. Tanaka, Nature, 1992, 355, 430 CrossRef CAS; T. Sakiyama, T. Tsutsui, E. Masuda, K. Imamura and K. Nakanishi, Macromolecules, 2003, 36, 5039 CrossRef CAS; Z. S. Ge, J. M. Hu, F. H. Huang and S. Y. Liu, Angew. Chem., Int. Ed., 2009, 48, 1798 CrossRef CAS.
- C. T. Lee, K. A. Smith and T. A. Hatton, Macromolecules, 2004, 37, 5397 CrossRef; I. Tomatsu, A. Hashidzume and A. Harada, Macromolecules, 2005, 38, 5223 CrossRef CAS; T. Suzuki, S. Shinkai and K. Sada, Adv. Mater., 2006, 18, 1043 CrossRef CAS; F. Peng, G. Z. Li, X. X. Liu, S. Z. Wu and Z. Tong, J. Am. Chem. Soc., 2008, 130, 16166 CrossRef CAS.
- W. G. Weng, J. B. Beck, A. M. Jamieson and S. J. Rowan, J. Am. Chem. Soc., 2006, 128, 11663 CrossRef CAS; T. Ogoshi, Y. Takashima, H. Yamaguchi and A. Harada, J. Am. Chem. Soc., 2007, 129, 4878 CrossRef CAS; I. Tomatsu, A. Hashidzume and A. Harada, Macromol. Rapid Commun., 2006, 27, 238 CrossRef CAS.
- L. E. Bromberg and E. S. Ron, Adv. Drug Delivery Rev., 1998, 31, 197 CrossRef CAS; D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655 CrossRef CAS.
- S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520 RSC.
- S. Matsumoto, S. Yamaguchi, A. Wada, T. Matsui, M. Ikeda and I. Hamachi, Chem. Commun., 2008, 1545 RSC; A. A. Aimetti, A. J. Machen and K. S. Anseth, Biomaterials, 2009, 30, 6048 CrossRef CAS.
- R. J. Pounder, M. J. Stanford, P. Brooks, S. P. Richards and A. P. Dove, Chem. Commun., 2008, 5158 RSC.
- K. Peng, I. Tomatsu, A. V. Korobko and A. Kros, Soft Matter, 2010, 6, 85 RSC.
- K. Peng, C. Cui, I. Tomatsu, A. H. Meijer, H. P. Spaink and A. Kros, Soft Matter, in press Search PubMed.
- I. Tomatsu, A. Hashidzume and A. Harada, J. Am. Chem. Soc., 2006, 128, 2226 CrossRef CAS; I. Tomatsu, A. Hashidzume and A. Harada, Angew. Chem., Int. Ed., 2006, 45, 4605 CrossRef CAS; G. Pouliquen, C. Amiel and C. Tribet, J. Phys. Chem. B, 2007, 111, 5587 CrossRef CAS; C. H. Luo, F. Zuo, Z. H. Zheng, X. B. Ding and Y. X. Peng, J. Macromol. Sci., Part A: Pure Appl. Chem., 2008, 45, 364 Search PubMed; Y. L. Zhao and J. F. Stoddart, Langmuir, 2009, 25, 8442 CrossRef CAS.
- Y. P. Wang, M. Zhang, C. Moers, S. L. Chen, H. P. Xu, Z. Q. Wang, X. Zhang and Z. B. Li, Polymer, 2009, 50, 4821 CrossRef CAS.
- S. A. Brittijn, S. J. Duivesteijn, M. Belmamoune, L. F. M. Bertens, W. Bitter, J. D. De Bruijn, D. L. Champagne, E. Cuppen, G. Flik, C. M. Vandenbroucke-Grauls, R. A. J. Janssen, I. M. L. De Jong, E. R. De Kloet, A. Kros, A. H. Meijer, J. R. Metz, A. M. Van der Sar, M. J. M. Schaaf, S. Schulte-Merker, H. P. Spaink, P. P. Tak, F. J. Verbeek, M. J. Vervoordeldonk, F. J. Vonk, F. Witte, H. P. Yuan and M. K. Richardson, Int. J. Dev. Biol., 2009, 53, 835 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional NMR data of photo-isomerization. See DOI: 10.1039/c002565h |
| This journal is © The Royal Society of Chemistry 2010 |
