Chemoselective reaction system using a two inlet micro-flow reactor: application to carbonyl allylation†
Fumihiro
Amemiya
,
Keishi
Fuse
,
Toshio
Fuchigami
and
Mahito
Atobe
*
Department of Electronic Chemistry, Tokyo Institute of Technology, Yokohama, 226-8502, Japan. E-mail: atobe@echem.titech.ac.jp; Fax: +81-45-924-5407; Tel: +81-45-924-5407
First published on 5th March 2010
Abstract
A new strategy for chemoselective reaction using a two inlet micro-flow reactor is described. In this system, the combined use of suitable flow mode and corresponding cathode material enables chemoselective cathodic reduction to control the product regioselectivity in carbonyl allylation.
Chemoselective control is crucial to the synthesis of complex molecules of natural products or other materials.1 Methodologies for chemoselective control in many reactions have been well established recently, however, existing methods often involve practical difficulties. Although the use of catalysts such as enzymes and metal complexes is the most common approach to the control of chemoselectivity, these methods require special techniques to use or synthesize such catalysts.2 In this study, a new strategy for chemoselective reaction with simple operation is proposed.
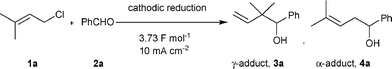
Carbonyl allylation is one of the most important and most utilized reactions in organic synthesis.3 This reaction usually provides two regioisomers, γ- and α-adducts (Scheme 1), and both products are very useful and valuable as the framework for the synthesis of natural compounds and pharmaceuticals. Therefore, the development of a facile regioselective synthesis for either γ- or α-adducts is important.
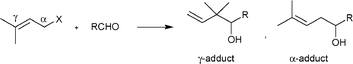 | ||
| Scheme 1 Carbonyl allylation between allylic halides and aldehydes. | ||
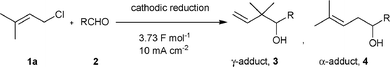
Among the several methods employed for this cross-coupling reaction, the electrochemical method serves as a straightforward and powerful method, and cathodic carbonyl allylation has been reported by many researchers since 1972.4 Although the regioselectivity of this reaction is influenced by electrode materials, the concentration of supporting electrolytes and current density, Tokuda et al. clarified that the regioselectivity was mainly related to the difference in the reduction potentials between the two starting substrates.5 Electrochemical allylation with aldehydes, which have higher reduction potential than allylic halides, occurs predominantly at the γ-position. On the other hand, when aldehydes are more readily reduced than allylic halides, the reduction yields α-adducts preferentially (Scheme 2). Therefore, control of the regioselectivity requires that either the allylic halides or aldehydes should be reduced chemoselectively, regardless of their reduction potentials.
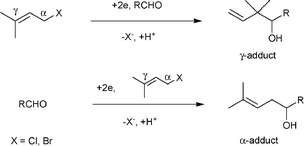 | ||
| Scheme 2 The reaction pathway of cathodic carbonyl allylation between an allylic halide and an aldehyde. | ||
To perform chemoselective cathodic reduction, we conceived the use of parallel laminar flow in a micro-flow reactor.6 The channel of the micro-flow reactor is sufficiently small to ensure stable and laminar flow of solutions. As shown in Fig. 1, when two solutions (allylic halide solution and aldehyde solution) are introduced through respective inlets (inlet 1 and inlet 2) of the two inlet micro-flow reactor, a stable liquid–liquid interface can be formed, and mass transfer between the input streams occurs only by means of diffusion. Therefore, the substrate introduced through inlet 1 could be predominantly reduced, whereas the reduction of the inlet 2 substrate could be avoided. Consequently, chemoselective cathodic reduction would proceed and an intentional cross-coupling product would be obtained regioselectively.
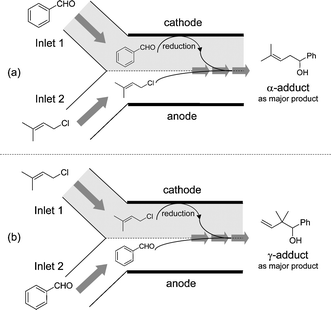 | ||
| Fig. 1 Chemoselective cathodic reduction using parallel laminar flow in the two inlet micro-flow reactor. (a) Flow mode for the selective reduction of benzaldehyde (2a). (b) Flow mode for the selective reduction of 1-chloro-3-methyl-2-butene (1a). | ||
In our previous work, we successfully demonstrated that by using such a parallel laminar flow in a micro-flow reactor selective oxidation of substrates could be achieved without affecting oxidation of the nucleophile in anodic substitution reactions.7
First, cyclic voltammograms (CVs) were measured for the reduction of benzaldehyde (2a) in the micro-flow reactor. A reduction peak of 2a, as shown in Fig. 2(b), was clearly observed at −1.89 V vs. Ag wire when an electrolytic stream containing 2a entered only through inlet 1. On the other hand, the peak current was significantly decreased when an electrolytic solution with 2a was introduced through inlet 2, and a solution without 2a was introduced through inlet 1 (Fig. 2(c)). This result indicated that the use of parallel laminar flow did prevent 2a from reaching the cathode when it was introduced through inlet 2.
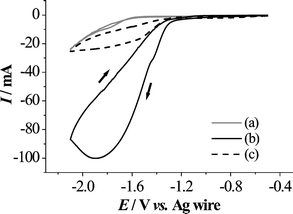 | ||
| Fig. 2 Cyclic voltammograms for the reduction of benzaldehyde (2a) in 200 mM n-Bu4NClO4–hexamethylphosphoric triamide (HMPA) using a two inlet micro-flow reactor with a Pt cathode. Ag wire as a reference electrode was placed externally, downstream near the outlet of the micro-flow reactor. The scan rate was 100 mV s−1. (a) HMPA solution without 2a was introduced through inlet 1 at a flow rate of 0.02 mL min−1 (background). (b) HMPA solution with 2a (1 M) was introduced through inlet 1 at a flow rate of 0.02 mL min−1. (c) HMPA solution with 2a (2 M) was introduced through inlet 2, and HMPA solution without 2a was introduced through inlet 1, both at a flow rate of 0.01 mL min−1. | ||
To verify this point, the diffusion coefficient for 2a in HMPA was estimated from Stokes–Einstein theory (see ESI†). If the effective radius of the diffusing species modeled as a sphere was set as 0.28 nm, which was obtained from a comparable size organic compound reported in the literature,8 the diffusion coefficient (D) was determined as 2.4 × 10−6 cm2 s−1. A residence time (t) in the reactor was 18 s corresponding to a total flow rate of 0.02 mL min−1. Therefore, the diffusion length (x) of 2a was estimated as 66 μm according to equation x = (Dt)1/2. As the channel depth is 20 μm, this suggests that the first 2a molecule reaches the cathode surface in approximately one thirds of the channel length. Therefore, the concentration of 2a on the cathode surface would be diluted compared to that under the condition (b) of Fig. 2.
Next, preparative scale experiments of the cathodic cross-coupling reaction were carried out using a 1-chloro-3-methyl-2-butene (1a) and benzaldehyde (2a) substrate combination. The reduction potential of 2a is much lower than that of 1a at the Pt electrode (see ESI†). Hence, the α-adduct was obtained as a major product when the reaction was carried out using a conventional batch type cell with the Pt electrode (Table 1, entry 1). The selectivity of α-adduct was improved by using the two inlet micro-flow reactor with the flow mode (a) of Fig. 1 (entry 2). 90% of substrate 1a was converted under these conditions, however, hydrobenzoin, benzyl alcohol and homo-coupling products of 1a were also detected as by-products. (The oxidation of HMPA occurred simultaneously at the anode because HMPA has a lower oxidation potential.) Since these homo-coupling side-reactions would occur simultaneously in entry 2, the yield was improved by decreasing the concentration of 2a as shown in entry 3. (The current efficiency for 4a was 27.1% and the productivity was 26.8 mg h−1.) On the other hand, the use of the flow mode (b) of Fig. 1 significantly increased the γ-adduct selectivity. These results indicated that our concept for chemoselective cathodic reduction by using a parallel laminar flow in the two inlet micro-flow reactor works. In addition, since the Ag electrode has electrocatalytic activity for the reduction of organic halides,9 the γ-adduct was obtained selectively by a combination of the use of the Ag cathode and the flow mode (b) of Fig. 1. (In entry 5, the current efficiency for 3a was 33.4% and the productivity was 32.9 mg h−1.) It should be noted that this was opposite selectivity compared to that obtained in entry 3.
| Entry | Cathode material | Flow mode | Total yield (%)b3a + 4a | Selectivityb3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a 4a |
|---|---|---|---|---|
| a Experimental conditions: The concentration of 1a in HPMA was 500 mM, and that for 2a was 2 M. n-Bu4NClO4 was used as a supporting electrolyte in all solutions (200 mM). Flow rates of each solution were 0.01 mL min−1 and the total flow rate was 0.02 mL min−1. b Determined by GC and 1H NMR analysis. c Reported in the literature.5 d The concentration of 2a was 1 M. e Isolated yield in parenthesis. | ||||
| 1c | Pt | Batch type cell | 69 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
| 2 | Pt | Mode (a) of Fig. 1 | 44 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 91 |
| 3d | Pt | Mode (a) of Fig. 1 | 55 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 92 |
| 4 | Pt | Mode (b) of Fig. 1 | 58 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
| 5 | Ag | Mode (b) of Fig. 1 | 75 (67)e | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
On the basis of this chemoselective micro-flow reactor system, cathodic cross-coupling reactions between 1a and various aldehydes (2b–e) were investigated. The use of the Pt cathode with flow mode (a) of Fig. 1 provided the corresponding α-adducts as major products in reasonable yields (Table 2, entries 1, 3, 5, and 7). In contrast, the use of the Ag cathode with flow mode (b) of Fig. 1 afforded the corresponding γ-adducts as major products in good to moderate yields (entries 2, 4, 6 and 8). From these general experiments, it can be concluded that this micro-flow reactor system enables chemoselective reduction and intentional regioselective control of the cathodic cross-coupling reaction between allylic chloride and a wide range of aldehydes.
| Entry | R (Ep,red)b | Cathode material | Flow modec | Total yield (%)d3 + 4 | Selectivityd3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|---|---|---|---|---|---|
| a Experimental conditions are described in footnote a of Table 1. b Reduction peak potentials recorded at the Pt cathode. c (a) is the flow mode for the selective reduction of aldehyde and (b) is the flow mode for the selective reduction of 1a, as shown in Fig. 1. d Determined by 1H NMR analysis. | |||||
| 1 | Ph-p-CF3 (2b, −1.76 V) | Pt | (a) | 63 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85 |
| 2 | Ag | (b) | 65 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
|
| 3 | 2-Naphthyl (2c, −2.05 V) | Pt | (a) | 59 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88 |
| 4 | Ag | (b) | 54 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
|
| 5 | Ph-p-Me (2d, −2.28 V) | Pt | (a) | 67 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
| 6 | Ag | (b) | 68 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
|
| 7 | 3-Furanyl (2e, −2.51 V) | Pt | (a) | 52 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
| 8 | Ag | (b) | 49 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
In summary, intentional chemoselective reduction in a cathodic cross-coupling reaction was successfully demonstrated using the two inlet micro-flow reactor. The combined use of suitable flow mode and corresponding cathode material enables control of the regioselectivity in this reaction.
This work was financially supported in part by the Global COE program (Tokyo Institute of Technology) and a Grant-in-Aid for Scientific Research (20350046 and 21656205) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Notes and references
- For selected reviews, see: M. Nahmany and A. Melman, Org. Biomol. Chem., 2004, 2, 1563 Search PubMed; C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Li, Chem. Soc. Rev., 2008, 37, 527 RSC; S. Reymond, L. Ferrie, A. Guerinot, P. Capdevielle and J. Cossy, Pure Appl. Chem., 2008, 80, 1683 RSC; C. P. R. Hackenberger and D. Schwarzer, Angew. Chem., Int. Ed., 2008, 47, 10030 CrossRef CAS; R. A. Shenvi, D. P. O’Malley and P. S. Baran, Acc. Chem. Res., 2009, 42, 530 CrossRef CAS; I. S. Young and P. S. Baran, Nat. Chem., 2009, 1, 193 CrossRef CAS.
- For selected examples, see: V. Gotor, J. Mol. Catal. B: Enzym., 2002, 19–20, 21 Search PubMed; P. Mäki-Arvela, J. Hajek, T. Salmi and D. Y. Murzin, Appl. Catal., A, 2005, 292, 1 CrossRef CAS; R. Winkler, M. E. A. Richter, U. Knüpfer, D. Merten and C. Hertweck, Angew. Chem., Int. Ed., 2006, 45, 8016 CrossRef; T. Ohshima, T. Iwasaki, Y. Maegawa, A. Yoshiyama and K. Mashima, J. Am. Chem. Soc., 2008, 130, 2944 CrossRef CAS; S. Riva, Organic Synthesis with Enzymes in Non-Aqueous Media, ed. G. Carrea and S. Riva, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008, p. 145 CrossRef CAS.
- For selected reviews and examples of carbonyl allylation, see: Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 Search PubMed; Y. Tamaru, Perspectives in Organopalladium Chemistry for the XXI Century, ed. J. Tsuji, Elsevier, Amsterdam, 1999, p. 215 CrossRef CAS; S. E. Denmark and J. Fu, Chem. Rev., 2003, 103, 2763 CrossRef CAS; J. W. J. Kennedy and D. G. Hall, Angew. Chem., Int. Ed., 2003, 42, 4732 Search PubMed; M. Kimura, M. Shimizu, K. Shibata, M. Tazoe and Y. Tamaru, Angew. Chem., Int. Ed., 2003, 42, 3392 CrossRef CAS; G. Zanoni, S. Gladiali, A. Marchetti, P. Piccinini, I. Tredici and G. Vidari, Angew. Chem., Int. Ed., 2004, 43, 846 CrossRef CAS; I. Marek and G. Sklute, Chem. Commun., 2007, 1683 CrossRef CAS; L. F. Tietze, T. Kinzel and C. C. Brazel, Acc. Chem. Res., 2009, 42, 367 CrossRef CAS; J. E. Bower, I. S. Kim, R. L. Patman and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 34 RSC; Y. Lu, I. S. Kim, A. Hassan, V. Del, J. David and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 5018 CrossRef CAS.
- M. M. Baizer and J. L. Chruma, J. Org. Chem., 1972, 37, 1951 CrossRef CAS.
- S. Satoh, H. Suginome and M. Tokuda, Bull. Chem. Soc. Jpn., 1981, 54, 3456 CrossRef CAS; M. Tokuda, S. Satoh and H. Suginome, J. Org. Chem., 1989, 54, 5608 CrossRef CAS.
- For selected reviews on microreactor technology, see: Microreaction Technology, ed. W. Ehrfeld, Springer, Berlin, 1998 Search PubMed; S. J. Haswell, P. D. I. Fletcher, G. M. Greenway, V. Skelton, P. Styring, D. O. Morgan, S. Y. F. Wong and B. H. Warrington, Automated Synthetic Methods for Speciality Chemicals, ed. W. Hoyle, Royal Society of Chemistry, Cambridge, 1999, p. 26 Search PubMed; Microsystem Technology in Chemistry and Life Sciences, ed. A. Manz and H. Becker, Springer, Berlin, 1999 Search PubMed; Microreactors, ed. W. Ehrfeld, V. Hessel and H. Löwe, Wiley-VCH, Weinheim, 2000 Search PubMed; K. F. Jensen, Chem. Eng. Sci., 2001, 56, 293 Search PubMed; B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 Search PubMed; J. Yoshida and S. Suga, Electrochemistry, 2007, 75, 58 Search PubMed; J. Yoshida, A. Nagaki and T. Yamada, Chem.–Eur. J., 2008, 14, 7450 Search PubMed.
- D. Horii, T. Fuchigami and M. Atobe, J. Am. Chem. Soc., 2007, 129, 11692 CrossRef CAS; D. Horii, F. Amemiya, T. Fuchigami and M. Atobe, Chem.–Eur. J., 2008, 14, 10382 CrossRef CAS.
- W. R. Fawcett and J. S. Jaworski, J. Phys. Chem., 1983, 87, 2972 CrossRef CAS; M. Hoon and W. R. Fawcett, J. Phys. Chem. A, 1997, 101, 3726 CrossRef CAS.
- S. Rondinini, P. R. Mussini, P. Muttini and G. Sello, Electrochim. Acta, 2001, 46, 3245 CrossRef CAS; J. Simonet, J. Electroanal. Chem., 2005, 583, 34 CrossRef CAS; C. Bellomunno, D. Bonanomi, L. Falciola, M. Longhi, P. R. Mussini, L. M. Doubova and G. Di Silvestro, Electrochim. Acta, 2005, 50, 2331 CrossRef CAS; A. A. Isse, S. Gottardello, C. Maccato and A. Gennaro, Electrochem. Commun., 2006, 8, 1707 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals and experimental details. See DOI: 10.1039/b926943f |
| This journal is © The Royal Society of Chemistry 2010 |