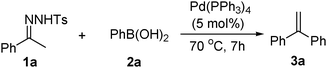
Pd-catalyzed oxidative cross-coupling of N-tosylhydrazones with arylboronic acids†
Xia
Zhao
a,
Jing
Jing
a,
Kui
Lu
a,
Yan
Zhang
a and
Jianbo
Wang
*ab
aBeijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China. E-mail: wangjb@pku.edu.cn
bThe State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China
First published on 3rd February 2010
Abstract
The Pd-catalyzed reaction of N-tosylhydrazones and arylboronic acids provides olefin derivatives. This oxidative cross-coupling is suggested to proceed through a migratory insertion process of a Pd carbene intermediate.
Pd-catalyzed cross-coupling reactions of diazo compounds have recently emerged as a new type of cross-coupling reaction.1–4 We have reported a Pd-catalyzed cross-coupling reaction of α-diazocarbonyl compounds with boronic acids, which affords α,β-unsaturated carbonyl compounds in high yields.1e The reaction presumably proceeds through a migratory insertion process of the Pd carbene intermediate.5 To further extend the scope of this cross-coupling reaction, we conceived to employ diazo compounds that do not bear electron withdrawing substituents as the substrates in this reaction. Diazo compounds without electron withdrawing groups are usually unstable and thus difficult to handle. The recently developed methods of in situ generation of diazo compounds have largely circumvented this problem. Treatment of N-tosylhydrazones with base is the most widely practised method for this purpose.6 The reaction conditions are compatible with the Pd-catalyzed cross-coupling reaction, as demonstrated by Barluenga and co-workers’ reports on a Pd-catalyzed reaction of N-tosylhydrazones with aryl bromides in the presence of lithium tert-butoxide,1b,c and by our own recent report on the Pd-catalyzed reaction of N-tosylhydrazones with benzyl halides.2c As a continuation, we report in this communication the Pd-catalyzed oxidative cross-coupling of N-tosylhydrazone with aromaticboronic acids.
Initially, the cross-coupling reaction between acetophenone N-tosylhydrazone 1a and phenyl boronic acid2a was carried out under various conditions. From the mechanistic rational (vide infra), the cross-coupling reaction starts from the transmetallation of boronic acid to the Pd(II) catalyst.7 At the end of the reaction, a Pd(0) species is released, which needs to be oxidized in order to regenerate the Pd(II) catalyst. Initially, Ag2CO3 was employed as the oxidant. Thus, with Pd(PPh3)4 as catalyst and LiOtBu as base, the reaction of N-tosylhydrazone 1a and boronic acid2a in dioxane at 70 °C affords 1,1-diphenylethylene3a in 16% yield (Table 1, entry 1). Further optimization of the reaction was focused on the search for a suitable oxidant. A number of oxidants such as Ag2CO3, tBuOOtBu, KBrO3, CuCl2 and Cu(OAc)2 were screened and none of them was efficient for the reaction (entries 2–6). To our delight, we observed that the combination of CuCl and oxygen under balloon pressure was effective and promoted the reaction in moderate to high yield (entry 7).
| Entry | Oxidant (% mol) | Base (equiv.) | Solvent | Yield (%)b |
|---|---|---|---|---|
a Reaction conditions: 1a (0.25 mmol), 2a (0.75 mmol), Pd(PPh3)4 (5 mol%), solvent (2 mL).
b Isolated yield.
c In an atmosphere of O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1 N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4.
|
||||
| 1 | Ag2CO3 (200) | LiOtBu (3) | Dioxane | 16 |
| 2 | t BuOOtBu (200) | LiOtBu (3) | Dioxane | 10 |
| 3 | t BuOOtBu (300) | LiOtBu (3) | Dioxane | 13 |
| 4 | Cu(OAc)2 (200) | LiOtBu (4) | Dioxane | Trace |
| 5 | CuCl2 (200) | LiOtBu (4) | Dioxane | Trace |
| 6 | KBrO3 (150) | LiOtBu (5) | Dioxane | 8 |
| 7 | O2, CuCl (20) | LiOtBu (4) | Dioxane | 47 |
| 8 | O2, CuCl (20) | LiOtBu (4) | Toluene | 34 |
| 9 | O2, CuCl (20) | LiOtBu (4) | MeCN | 22 |
| 10 | O2, CuCl (10) | LiOtBu (5) | DMSO | 7 |
| 11 | O2, CuCl (20) | LiOtBu (5) | Dioxane | 68 |
| 12 | O2, CuCl (10) | LiOtBu (5) | Dioxane | 71 |
| 13 | O2, CuBr (20) | LiOtBu (5) | Dioxane | 60 |
| 14 | O2, CuCl2 (20) | LiOtBu (5) | Dioxane | 64c |
| 15 | O2, CuI (20) | LiOtBu (5) | Dioxane | 13 |
| 16 | O2, CuOTf (10) | LiOtBu (5) | Dioxane | 26 |
Next, we observed that solvent significantly affected this coupling reaction. Toluene, MeCN and DMSO were tested, but they were all less effective as compared with dioxane (entries 8–10). To further optimize the reaction, some copper salts were examined.† We found that both Cu(II) and Cu(I) could work for the reaction (entries 13–16). For CuCl, the molar percentage could be reduced to 10%, affording the highest yield (entry 12). Since the base used in the reaction also plays an important role, several bases such as KOtBu, NaOtBu, Cs2CO3, were also examined. However, they are all less efficient compared with LiOtBu. Finally, we studied different palladium catalysts.† Although either Pd(0) or Pd(II) catalysts could catalyze the cross-coupling reaction, none of them exceeded the simple Pd(PPh3)4. Thus, the optimized reaction conditions were as follows: 1a (0.25 mmol), 2a (0.75 mmol), Pd(PPh3)4 (5 mol%), CuCl (10 mol%), LiOtBu (1.25 mmol), in dioxane at 70 °C.
A series of substituted acetophenone N-tosylhydrazones 1a–i and arylboronic acids 2a–e were examined under the optimal reaction conditions. As shown in Table 2, the reaction gave moderate to good yields in most cases. The results do not show a significant electronic effect in this reaction. Since the homocoupling of arylboronic acids is inevitable as a side reaction,8 in all cases 3 equiv. of arylboronic acids were needed in order to completely transform the N-tosylhydrazones to the products.
| Entry | 1, Ar1 | 2, Ar2 | t/h | Yield (3, %)b |
|---|---|---|---|---|
| a Reaction conditions: 1a–i (0.25 mmol), 2a–e (0.75 mmol), Pd(PPh3)4 (5 mol%), CuCl (10 mol%), LiOtBu (1.25 mmol), dioxane (2 mL), 70 °C. b Yield of isolated product after chromatography. c The product and by-product biphenyl were inseparable on a column. The yield was determined by 1H NMR. d The product and by-product 4,4′-dimethylbiphenyl were inseparable on a column. The yield was determined by 1H NMR. | ||||
| 1 | 1a, C6H5 | 2a, C6H5 | 7 | 3a, 71c |
| 2 | 1a, C6H5 | 2b, p-MeOC6H4 | 7 | 3b, 70 |
| 3 | 1a, C6H5 | 2c, m-ClC6H4 | 7 | 3c, 47 |
| 4 | 1b, p-MeC6H4 | 2a, C6H5 | 6.5 | 3d, 70c |
| 5 | 1b, p-MeC6H4 | 2b, p-MeOC6H4 | 10 | 3e, 71 |
| 6 | 1b, p-MeC6H4 | 2d, p-MeC6H4 | 10 | 3f, 67d |
| 7 | 1c, p-ClC6H4 | 2a, C6H5 | 9 | 3g, 52 |
| 8 | 1c, p-ClC6H4 | 2b, p-MeOC6H4 | 8 | 3h, 67 |
| 9 | 1d, m-MeOC6H4 | 2a, C6H5 | 8.5 | 3i, 57 |
| 10 | 1d, m-MeOC6H4 | 2d, p-MeC6H4 | 7 | 3j, 68 |
| 11 | 1e, p-MeOC6H4 | 2a, C6H5 | 7 | 3b, 67 |
| 12 | 1e, p-MeOC6H4 | 2b, p-MeOC6H4 | 7 | 3k, 76 |
| 13 | 1e, p-MeOC6H4 | 2c, m-ClC6H4 | 7 | 3l, 40 |
| 14 | 1e, p-MeOC6H4 | 2d, p-MeC6H4 | 7 | 3e, 82 |
| 15 | 1e, p-MeOC6H4 | 2e, m-MeC6H4 | 7 | 3m, 71 |
| 16 | 1f, 3,4-Me2C6H3 | 2a, C6H5 | 9 | 3n, 57c |
| 17 | 1g, 3,5-Me2C6H3 | 2a, C6H5 | 7 | 3o, 63c |
| 18 | 1g, 3,5-Me2C6H3 | 2b, p-MeOC6H4 | 9 | 3p, 30 |
| 19 | 1h, p-CF3C6H4 | 2b, p-MeOC6H4 | 6 | 3q, 64 |
| 20 | 1i, p-NCC6H4 | 2a, C6H5 | 5 | 3r, 51 |
The reaction could be extended to hydrazone substrates bearing substituents other than a methyl group. Thus, the Pd-catalyzed reaction of 4a–g and 2a,b under identical reaction conditions afforded 5a–h in good yields in most cases (Scheme 1).
 | ||
| Scheme 1 Pd-catalyzed reaction of 4a–g with 2a,b. | ||
A plausible mechanism for this Pd-catalyzed oxidative coupling is proposed in Scheme 2. The reaction is initiated by the oxidation of the CuCl to Cu(II) species by oxygen, which then oxidize Pd(0) to Pd(II) species. Transmetallation of the Pd(II) species with the arylboronic acid affords arylpalladium species A, which reacts with the in situ generated diazo substrate to give Pd carbene complex B. Migratory insertion of the aryl group to the carbenic carbon of the Pd carbene species affords the intermediate C.1–5 Finally, β-hydride elimination of C affords the product and regenerates the Pd(0) species in the presence of base.
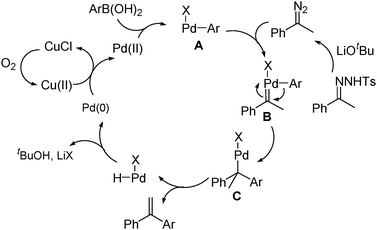 | ||
| Scheme 2 Possible reaction pathways. | ||
However, the possibility exists that the Pd carbene is generated directly from Pd(II) and the diazo substrate. The formation of the olefin product may be due to the 1,2-H shift of the Pd carbene intermediate and subsequent Heck–Mizoroki-type reaction.9,10 To confirm this possible pathway, styrene6 and phenyl boronic acid2a were reacted under the identical conditions (eqn (1)). Only a trace amount of 1,2-diphenylethylene7 was observed and no 1,1-diphenylethylene could be detected in the 1H NMR spectrum of the crude product. Consequently, this pathway can be excluded.
 | (1) |
To gain further insight into the reaction mechanism, we examined the kinetic isotope effect (KIE) of the reaction (Scheme 3). At first, intermolecular competition was carried out with d3-deuterated N-tosylhydrazone 8 and 1a. The competition reaction gave 3b and 9 in equal amount (kH/kD = 1.0). On the other hand, the intramolecular competition experiment with d1-deuterated N-tosylhydrazone 10 gave a KIE of 2.54. The significant KIE in the β-hydride elimination step and lack of KIE for the overall reaction (intermolecular competition) clearly suggest that β-hydride elimination is not in the rate determining step. It is noted that the KIE value observed in the intramolecular competition is comparable to those reported for β-hydride elimination in Pd-catalyzed reactions.11 Thus, the KIE results are in accordance with the proposed reaction mechanism shown in Scheme 2.
 | ||
| Scheme 3 Kinetic isotopic effect experiment. | ||
In conclusion, we have reported the first oxidative cross-coupling reaction between N-tosylhydrazone and arylboronic acids. This study shows that the coupling of N-tosylhydrazone and arylboronic acids under oxidative conditions can compete with the oxidative homocoupling of arylboronic acids, which indicates that the interaction of arylpalladium species with diazo substrates and the subsequent processes are both highly efficient. This study further demonstrates the generality of the transformations based on Pd carbene processes.
The project was supported by the NSFC (Grant No. 20832002, 20772003, 20821062) and 973 Program (No. 2009CB825300).
Notes and references
- (a) K. L. Greenman, D. S. Carter and D. L. Van Vranken, Tetrahedron, 2001, 57, 5219 CrossRef CAS; (b) J. Barluenga, P. Moriel, C. Valdés and F. Aznar, Angew. Chem., Int. Ed., 2007, 46, 5587 CrossRef CAS; (c) J. Barluenga, M. Tomás-Gamasa, P. Moriel, F. Aznar and C. Valdés, Chem.–Eur. J., 2008, 14, 4792 CrossRef CAS; (d) R. Kudirka and D. L. Van Vranken, J. Org. Chem., 2008, 73, 3585 CrossRef CAS; (e) C. Peng, Y. Wang and J. Wang, J. Am. Chem. Soc., 2008, 130, 1566 CrossRef CAS.
- (a) K. L. Greenman and D. L. Van Vranken, Tetrahedron, 2005, 61, 6438 CrossRef CAS; (b) W.-Y. Yu, Y.-T. Tsoi, Z. Zhou and A. S. C. Chan, Org. Lett., 2009, 11, 469 CrossRef CAS; (c) Q. Xiao, J. Ma, Y. Yang, Y. Zhang and J. Wang, Org. Lett., 2009, 11, 4732 CrossRef CAS.
- (a) S. K. J. Devine and D. L. Van Vranken, Org. Lett., 2007, 9, 2047 CrossRef CAS; (b) S. K. J. Devine and D. L. Van Vranken, Org. Lett., 2008, 10, 1909 CrossRef CAS; (c) R. Kudirka, S. K. J. Devine, C. S. Adams and D. L. Van Vranken, Angew. Chem., Int. Ed., 2009, 48, 3677 CrossRef CAS.
- S. Chen and J. Wang, Chem. Commun., 2008, 4198 RSC.
- For reports on the migratory insertion process of stable Pd carbene species, see: (a) A. C. Albéniz, P. Espinet, R. Manrique and A. Pérez-Mateo, Angew. Chem., Int. Ed., 2002, 41, 2363 CrossRef CAS; (b) M. Bröring, C. D. Brandt and S. Stellwag, Chem. Commun., 2003, 2344 RSC; (c) D. Solé, L. Vallverdú, X. Solans, M. Font-Bardia and J. Bonjoch, Organometallics, 2004, 23, 1438 CrossRef CAS; (d) A. C. Albéniz, P. Espinet, R. Manrique and A. Pérez-Mateo, Chem.–Eur. J., 2005, 11, 1565 CrossRef CAS; (e) A. C. Albéniz, P. Espinet, A. Pérez-Mateo, A. Nova and G. Ujaque, Organometallics, 2006, 25, 1293 CrossRef CAS.
- J. R. Fulton, V. K. Aggarwal and J. de Vicente, Eur. J. Org. Chem., 2005, 1479 CrossRef CAS.
- For selected reviews on Pd-catalyzed coupling reactions with arylboronic compounds, see: (a) A. Suzuki, Acc. Chem. Res., 1982, 15, 178 CrossRef CAS; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (c) N. Miyaura, J. Organomet. Chem., 2002, 653, 54 CrossRef CAS; (d) N. Miyaura, in Advances in Metal Organic Chemistry, ed. L. S. Liebeskind, J AI, London, 1998, vol. 6, p. 187 Search PubMed; (e) A. Suzuki, in Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, New York, 1998, ch. 2 Search PubMed; (f) N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CAS.
- For examples of Pd-catalyzed homocoupling of arylboronic compounds under oxidative conditions, see: (a) H. Yoshida, Y. Yamaryo, J. Ohshita and A. Kunai, Tetrahedron Lett., 2003, 44, 1541 CrossRef CAS; (b) S. Punna, D. D. Diaz and M. G. Finn, Synlett, 2004, 2351 CAS; (c) C. Zhou and R. C. Larock, J. Org. Chem., 2006, 71, 3184 CrossRef CAS; (d) A. Christian, C. Chama and J. Anny, Eur. J. Org. Chem., 2008, 4567.
- For a recent report on the 1,2-H shift of Rh(II) carbenes, see: F. Xiao and J. Wang, J. Org. Chem., 2006, 71, 5789 Search PubMed.
- For examples, see: (a) X. Du, M. Suguro, K. Hirabayashi and A. Mori, Org. Lett., 2001, 3, 3313 CrossRef CAS; (b) P.-A. Enquist, J. Lindh, P. Nilsson and M. Larhed, Green Chem., 2006, 8, 338 RSC; (c) K. S. Yoo, C. H. Yoon and K. W. Jung, J. Am. Chem. Soc., 2006, 128, 16384 CrossRef CAS.
- (a) E. Keinan, S. Kumar, V. Dangur and J. Vaya, J. Am. Chem. Soc., 1994, 116, 11151 CrossRef CAS; (b) M. R. Netherton and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 3910 CrossRef CAS; (c) D. R. Chrisope, P. Beak and W. H. Saunders, Jr., J. Am. Chem. Soc., 1988, 110, 230 CrossRef CAS; (d) J. M. Takacs, E. C. Lawson and F. Clement, J. Am. Chem. Soc., 1997, 119, 5956 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/b925590g |
| This journal is © The Royal Society of Chemistry 2010 |