Tetragonal faceted-nanorods of anatase TiO2 single crystals with a large percentage of active {100} facets†
Jianming
Li
and
Dongsheng
Xu
*
Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: dsxu@pku.edu.cn; Fax: (+86)10-62760360; Tel: (+86)10-62753580
First published on 23rd January 2010
Abstract
Tetragonal faceted-nanorods of single-crystalline anatase TiO2 with a large percentage of higher-energy {100} facets have been synthesized by hydrothermal transformation of alkali titanate nanotubes in basic solution.
In recent years, intensive studies have been reported to prepare TiO2 because it has been widely used in photocatalysts, photosplitting water, dye-sensitized solar cells, photochromic devices, and gas sensing.1–4 In particular, anatase had been undoubtedly proved to show the most excellent applications among all the TiO2 crystallographic phases.5,6 It has been demonstrated that the intrinsic properties of anatase TiO2 depended on shapes, sizes and especially the exposed facets.7–9 According to the Wulff construction,10,11 the equilibrium crystal shape of a basic anatase crystal closely resembles the shape of the naturally occurring anatase mineral, which is a truncated bipyramidal structure enclosed by eight {101} facets and two {001} facets, and the exposed {101} facets amounted to more than 94% (Scheme 1(a)). Recently, micron-sized anatase crystals which exposed a large percentage of {001} facets have been synthesized by using fluorine to stabilize the higher surface energy facets of {001} (Scheme 1(b)).12 Since then, tremendous efforts have been conducted on the degree of truncation,13,14 the particle sizes15 and the synthesis methods16,17 for truncated octahedral anatase (Scheme 1, path 2). However, anatase TiO2 crystals with four well-defined lateral active {100} facets (Scheme 1(c)) have never been synthesized. Theoretical studies demonstrated that anatase {100} facets are more active and accordingly exhibit higher catalytic activity than {001} and {101} facets.17–19 Barnard et al.20,21 have theoretically demonstrated that hydroxyl can lower the surface free energy of {100} facets. However, this process has never been verified in experiments, because most available titanium precursors are very reactive under basic conditions. Synthesis of anatase TiO2 single crystals which predominantly expose higher-energy {100} facets is still a big challenge.
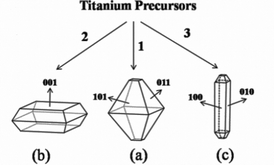 | ||
| Scheme 1 Schematic drawings of anatase shapes: (a) equilibrium crystal shape with large percentage of {101} facets; (b) truncated octahedral shape with large percentage of {001} facets; (c) tetragonal faceted-nanorod with four well lateral {100} facets. | ||
Herein, we first report a facile hydrothermal route for the synthesis of tetragonal faceted-nanorods (TFNRs) of anatase TiO2 which predominantly exposed higher-energy {100} facets. This process involves the formation of Na-titanate nanotubes by hydrothermal reaction of P25 in NaOH solution22 first and hydrothermal transformation of the precursors into anatase phase of TiO2 in basic solution. Traditionally, transformation of Na-titanate to pure anatase TiO2 needed exchanging alkali ions with protons to form the H-titanates in advance.23–25 This is also the first report on one-step transformation of Na-titanate precursor into anatase TiO2.
Typically, the Na-titanate nanotubes are 5–20 nm in diameter and several micrometres in length. Energy dispersive X-ray spectroscopy reveals that the atomic ratio of sodium to titanium was ca. 18% (Fig. S1 in ESI† ). Fig. 1 shows the scanning electron microscopy (SEM) image of the products obtained by hydrothermal reaction of Na-titanate nanotubes, indicating a tetragonal nanorod shape. All the particles had well-defined lateral facets with sharp edges and the adjacent facets were perpendicular as well as with the same width (inset of Fig. 1(b)). Fig. 2 shows X-ray diffraction patterns of the Na-titanate precursors and the TFNR particles. The diffraction peaks in Fig. 2(b) match well with the crystal structure of the anatase TiO2 phase (tetragonal, I41/amd, JCPDS 21-1272), indicating all of the Na-titanate precursors have been transferred into an anatase TiO2 phase.
 | ||
| Fig. 1 (a) Lower and (b) higher SEM images of tetragonal faceted-nanorods. The inset of (b) is the top view of a single TFNR. | ||
 | ||
| Fig. 2 X-Ray diffraction patterns of: (a) Na-titanate precursors and (b) the TFNR products. | ||
More detailed structural information of the TFNRs was revealed by transmission electron microscopy (TEM) and selected-area electron diffraction (SAED). By measuring the SAED patterns in Fig. 3(a), the axis of the rod was parallel to the 〈001〉 direction, indicating that the rod grows along the 〈001〉 direction. The angle between 〈100〉 and 〈001〉 directions is 90°, in good agreement with the model of TFRN anatase TiO2 enclosed by {100} facets projected along the [010] direction (Fig. 3(b)). Fig. 3(c) shows the high-resolution TEM image taken from Fig. 3(a) indicated by the rectangle. Three sets of lattice fringes with spacings of 0.35, 0.35 and 0.48 nm can be attributed corresponding to (101), (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and (002) of the anatase phase, respectively. The exposed facet (under electron-beam irradiation) was flat and the thickness of the rod was found to be constant by measuring the titanium content across the plane (Fig. S2(a) and 2(b) in ESI† ). To further confirm the exposed facets of the TFNR, the same rod was rotated to the [1
) and (002) of the anatase phase, respectively. The exposed facet (under electron-beam irradiation) was flat and the thickness of the rod was found to be constant by measuring the titanium content across the plane (Fig. S2(a) and 2(b) in ESI† ). To further confirm the exposed facets of the TFNR, the same rod was rotated to the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] zone axis from the [010] zone axis. As shown in Fig. 3(d), the diameter of the rod was 1.4 times larger than that in Fig. 3(a). The rod exhibited a sharp edge and a symmetric sharp peak of the titanium content was observed (Fig. S2(c) and 2(d) in ESI† ). Both the SEAD pattern (inset of Fig. 3(d)) and HRTEM image (Fig. 3(f)) also confirm that the TFNR has a single-crystalline structure of anatase phase with a growth direction along 〈001〉, which matches well with the TFNR model enclosed by {100} facets, projected along [1
0] zone axis from the [010] zone axis. As shown in Fig. 3(d), the diameter of the rod was 1.4 times larger than that in Fig. 3(a). The rod exhibited a sharp edge and a symmetric sharp peak of the titanium content was observed (Fig. S2(c) and 2(d) in ESI† ). Both the SEAD pattern (inset of Fig. 3(d)) and HRTEM image (Fig. 3(f)) also confirm that the TFNR has a single-crystalline structure of anatase phase with a growth direction along 〈001〉, which matches well with the TFNR model enclosed by {100} facets, projected along [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction (Fig. 3(e)). On the basis of the above observations and structural analysis, we conclude that the exposed lateral facets of the as-prepared TFNRs are mainly the {100} facets.
0] direction (Fig. 3(e)). On the basis of the above observations and structural analysis, we conclude that the exposed lateral facets of the as-prepared TFNRs are mainly the {100} facets.
![(a) Typical TEM image of an individual TFNR viewed along the [010] direction; inset: the corresponding SEAD pattern. (b) Schematic model of an ideal TFNR with four lateral {100} facets, projected along the [010] direction. (c) HRTEM image taken from (a) indicated by the rectangle. (d) TEM image of the same TFNR viewed along the [11̄0] direction; inset: the corresponding SAED pattern. (e) Schematic model of an ideal TFNR with four lateral {100} facets, projected along the [11̄0] direction. (f) HRTEM image taken from (c) indicated by the rectangle.](/image/article/2010/CC/b923755k/b923755k-f3.gif) | ||
Fig. 3 (a) Typical TEM image of an individual TFNR viewed along the [010] direction; inset: the corresponding SEAD pattern. (b) Schematic model of an ideal TFNR with four lateral {100} facets, projected along the [010] direction. (c) HRTEM image taken from (a) indicated by the rectangle. (d) TEM image of the same TFNR viewed along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction; inset: the corresponding SAED pattern. (e) Schematic model of an ideal TFNR with four lateral {100} facets, projected along the [1 0] direction; inset: the corresponding SAED pattern. (e) Schematic model of an ideal TFNR with four lateral {100} facets, projected along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction. (f) HRTEM image taken from (c) indicated by the rectangle. 0] direction. (f) HRTEM image taken from (c) indicated by the rectangle. | ||
To investigate the transformation process from Na-titanate nanotubes to the TFNRs of anatase TiO2, a detailed time-dependent morphology evolution study was conducted at 200 °C (Fig. S3 in ESI† ). It was observed that more particles appeared and the particles gradually grew up while the diameter and length of Na-titanate nanotubes decreased. When the hydrothermal reaction time was longer than 6 h, the Na-titanate completely transformed to TFNRs of anatase TiO2. Furthermore, we measured the pH value of the reaction solution. Before hydrothermal reaction, the solution pH was 10.1. When reaction was stopped at 1 h, the solution pH was 10.9 and the obtained products contained both Na-titanate and anatase phases (Fig. S4(a) in ESI† ). It was found that the pH would gradually increase with the reaction time and the final pH reached 11.8. In addition, no anatase was found if the Na-titanate precursors were treated in an ethanol bath at 200 °C for 24 h (Fig. S4(b) in ESI† ).
Upon hydrothermal treatment of the Na-titanate nanotubes, the transformation would proceed with dissolution and nucleation. At the beginning of the transformation, the surface of Na-titanate nanotubes was gradually decomposed and produced Ti(OH)4 fragments, hydroxyl and sodium ions under hydrothermal condition, according to eqn (1).
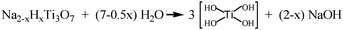 | (1) |
 | (2) |
In general, the catalytic activity of inorganicnanocrystals depends greatly on the exposed crystal facets.28,29 These TFNR anatase TiO2 single crystals are expected to have higher reactivity due to the large percentage of {100} facets compared with crystals having normal majority {101} facets. Here, we have investigated the photocatalytic activity of the TFNRs by measuring the formation of active hydroxyl radicals (˙OH), the most important oxidative species in photocatalysis reactions.30Terephthalic acid (TA) was used as a fluorescence probe. Fig. 4 displays the fluorescence spectra of the UV light irradiated tetragonal nanorod anatase TiO2 in 3 mM terephthalic acid and 0.01 M NaOH solution at different irradiation times. For comparison, the capability of forming ˙OH of commercially available anatase powder (Sinopharm Chemical Reagent Co., Ltd, purity >99%) was also studied due to their similar BET surface area. The unique fluorescence peak at 426 nm is originated from 2-hydroxyterephthalic (TAOH) acid produced by the reaction of TA with ˙OH in basic solution.31 The linear relationships between fluorescence intensity and irradiation time are found for both the TFNRs and commercial anatase powders, as shown in the inset of Fig. 4. We found that the TFNRs exhibit much higher activities (2.2 times) than that of the commercial anatase powders (inset of Fig. 4). This result suggests that the reactive {100} facets may play an important role in the photocatalytic reaction.
 | ||
| Fig. 4 Fluorescence spectra of UV light (maximum emission was 254 nm) irradiation of the TFNRs in 3 mM terephthalic acid and 0.01 M NaOH solution at different irradiation times. Inset is the time dependences of fluorescence intensity at 426 nm, where squares and circles correspond to the TFNR sample and the commercial TiO2 powder, respectively. | ||
In summary, we have reported a novel and facile route for the preparation of tetragonal faceted-nanorods of single-crystalline anatase TiO2 with predominately exposed higher-energy {100} facets by hydrothermal transformation of Na-titanate in alkaline solution. This is the first report that alkali titanate (instead of the usual H-titanate) can be completely transferred to pure anatase TiO2 in basic conditions viahydrothermal reaction. A “dissolution–nucleation” process is proposed to explain the transformation from alkali titanate to TFNR anatase TiO2. Furthermore, an enhanced photocatalytic activity for the TFNR sample is observed.
This work was supported by the NSFC (Grant Nos. 20525309, 20673008, 50821061) and MSTC (MSBRDP, Grant Nos. 2006CB806102, 2007CB936201).
Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CAS.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. A. Fox and M. T. Dulay, Chem. Rev., 1993, 93, 341 CrossRef CAS.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- R. Hengerer, L. Kavan, P. Krtil and M. Gratzel, J. Electrochem. Soc., 2000, 147, 1467 CrossRef CAS.
- L. Kavan, M. Gratzel, S. E. Gilbert, C. Klemenz and H. J. Scheel, J. Am. Chem. Soc., 1996, 118, 6716 CrossRef CAS.
- H. H. Deng, H. Zhang and Z. H. Lu, Chem. Phys. Lett., 2002, 363, 509 CrossRef CAS.
- M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 155409 CrossRef.
- U. Diebold, N. Ruzycki, G. Herman and A. Selloni, Catal. Today, 2003, 85, 93 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152 CrossRef CAS.
- Y. Dai, C. M. Cobley, J. Zeng, Y. Sun and Y. Xia, Nano Lett., 2009, 9, 2455 CrossRef CAS.
- D. Zhang, G. Li, X. Yang and J. C. Yu, Chem. Commun., 2009, 4381 RSC.
- B. Wu, C. Guo, N. Zheng, Z. Xie and G. D. Stucky, J. Am. Chem. Soc., 2008, 130, 17563 CrossRef CAS.
- A. Beltrán, J. R. Sambrano, M. Calatayud, F. R. Sensato and J. Andrés, Surf. Sci., 2001, 490, 116 CrossRef CAS.
- M. Calatayud and C. Minot, Surf. Sci., 2004, 552, 169 CrossRef CAS.
- A. S. Barnard, P. Zapol and L. A. Curtiss, Surf. Sci., 2005, 582, 173 CrossRef CAS.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261 CrossRef CAS.
- T. Kasuga, M. Hiramatsu, A. Hoson, T. Sckino and K. Niihara, Langmuir, 1998, 14, 3160 CrossRef CAS.
- Y. Yu and D. Xu, Appl. Catal., B, 2007, 73, 166 CrossRef CAS.
- Y. Mao and S. S. Wong, J. Am. Chem. Soc., 2006, 128, 8217 CrossRef CAS.
- H. Zhu, Y. Lan, X. Gao, S. Ringer, Z. Zheng, D. Song and J. Zhao, J. Am. Chem. Soc., 2005, 127, 6730 CrossRef CAS.
- A. Chemseddine and T. Mortiz, Eur. J. Inorg. Chem., 1999, 235 CrossRef CAS.
- Y. Gao and S. A. Elder, Mater. Lett., 2000, 44, 228 CrossRef CAS.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- L. Hu, Q. Peng and Y. Li, J. Am. Chem. Soc., 2008, 130, 16136 CrossRef CAS.
- T. Hirakawa and Y. Nosaka, Langmuir, 2002, 18, 3247 CrossRef CAS.
- J. C. Yu, J. Yu, W. Ho, Z. Jiang and L. Zhang, Chem. Mater., 2002, 14, 3808 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and figures. See DOI: 10.1039/b923755k |
| This journal is © The Royal Society of Chemistry 2010 |
