Simultaneous determination of paclobutrazol and myclobutanil enantiomers in water and soil using enantioselective reversed-phase liquid chromatography
Qin
Tian
*a,
Zhiqiang
Zhou
b,
Chunguang
Lv
b,
Yi
Huang
a and
Liping
Ren
c
aNational Research Center for Geoanalysis, Beijing, 100037, China. E-mail: tqname81@163.com; Tel: +86-10-68999599
bDepartment of Applied Chemistry, College of Science, China Agricultural University, Beijing, 100094, China
cCollege of Animal Science and Technology, China Agricultural University, Beijing, 100094, China
First published on 17th May 2010
Abstract
A sensitive reversed phase-liquid chromatography (RP-LC) method for the simultaneous separation and determination of paclobutrazol and myclobutanil enantiomers in water and soil was developed. The method involved a solid phase extraction of paclobutrazol and myclobutanil enantiomers from water and soil by using C18 Bond-Elut column. The LC system consisted of a reversed phase cellulose tris-3,5-dimethylphenyl carbamate (CDMPC) chiral stationary phase (CSP) with a mobile phase of methanol–water with UV detection. The calibration curve for each enantiomer was linear from 0.5–20 μg mL−1. Under suitable conditions, recoveries for all enantiomers were above 75% at two concentration levels in water and soil. Within-day and day-to-day assay precisions (RSDs, %) were below 9% for each enantiomer at concentrations of 0.5, 1.0, 5.0 μg mL−1. The method precisions (RSDs, %) were in the range of 3.83–11.5%. The limit of quantification (LOQ) of each enantiomer was 0.0025 μg mL−1 using 200.0 ml of water and 0.020 μg g−1 using 25.0 g of soil. The limit of detection (LOD) for each enantiomer in water was 0.0015 μg mL−1 and in soil 0.012 μg g−1 (S/N = 3). In addition, circular dichroism (CD) and UV spectra were obtained by stopped flow scanning.
Instruction
Paclobutrazol and myclobutanil (Fig. 1) are systemic pesticides belonging to the triazole family of chemicals and are merchandised under different trade names. They have different physicochemical properties and are widely used in various countries. Myclobutanil is a high-efficiency, low toxicity and wide spectrum fungicide. Its chemical name is 2-p-chlorophenyl-2-(1H-1,2,4-triazole-1-ylmethyl)hexanenitrile. Paclobutrazol (PAC) [(2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol] is used extensively in agriculture and horticulture as both plant growth regulator and fungicide. In ornamental crops, PAC is used for reducing the size of plants, improving compactness, and increasing other functional aspects, such as the ability to resist both abiotic and biotic stresses. Triazoles have both fungitoxic and plant-growth regulatory effects. With the exception a few cases such as diniconazole and uniconazole, most triazole-type fungicides are presently marketed in their racemic forms, making the single isomers of these fungicides practically unavailable to common researchers. It is well known that optical isomers show different pharmacological and toxicological activities and one of the optical isomers may be inactive or toxic.1 For example, the (2R,3R)(+)-enantiomer of paclobutrazol shows stronger fungicidal activity than the (2S,3S)(−)-enantiomer, whereas the latter has higher plant growth regulating activity.2,3 However, achiral analysis of chiral compounds often gives only partial information, and chiral analysis is required for a full understanding of the biological behavior of such compounds. The development of analytical methods that can identify, quantify, and control the enantiomers play a very important role in the development process of chiral agrochemical compounds. Therefore, it is essential and urgent to provide enantiomeric analysis methods of chiral pesticides to help evaluate the risks posed to environment and public health. | ||
| Fig. 1 The structures of the chiral pesticides in this study. | ||
According to previous work, the achiral analysis or residue determinations of paclobutrazol and myclobutanil and their metabolites in water, soil, wine, strawberries, farming foodstuff, soya grain, fruits and vegetables by traditional methods have been reported using gas chromatography-mass spectrometry(GC-MS),4–7 liquid chromatography-tandem mass spectrometry (LC-MS/MS),8–10 thin layer chromatography (TLC),11 ultra-high-performance liquid chromatography coupled with time-of-flight mass spectrometry(UPLC-TOF MS).12 The enantiomeric quantitative determination of paclobutrazol and myclobutanil in soil and water was reported, respectively.13 As much as we know, no previous work has been done on simultaneous enantioselective analysis of paclobutrazol and myclobutanil.
In this study, a valid method for the simultaneous separation and determination of paclobutrazol and myclobutanil enantiomers in water and soil was developed by LC using cellulose tris-3,5-dimethylphenyl carbamate chiral stationary phase (CDMPC-CSP) under reversed phase (RP) conditions. The linearity, accuracy, precision, recovery and limit of detection (LOD) of each enantiomer in water and soil were also determined. Circular dichroism (CD) and UV spectra were obtained by stopped flow scanning.
Experimental
Reagents and chemicals
Paclobutrazol (95.0%) and myclobutanil (96.5%) were supplied by the Laboratory of Pesticide Analysis and Environmental Toxicology, China Agricultural University (Beijing, China). Macro spherical silica was prepared in our laboratory with the following properties: average pore diameter, 6.7 nm; particle size, 5–7 μm; specific surface area, 110 m2 g−1. 3-Aminopropyltriethoxy-silane (KH-550) was purchased from Liaoning GaiXian Chemicals Plant. 3,5-Dimethylphenylisocyanate (99%) was purchased from Sigma-Aldrich Inc (USA). All other reagents were of analytical or LC grade. Double distilled water was used for the preparation of mobile phase. Stock solutions of 1000 μg mL−1 were prepared by weighting and dissolving each pesticide in methanol and storing in glass-stopper bottles at −18 °C.Water sample: Tap water sample was collected from the lab of the China Agriculture University and filtered through 0.45 μm pore-size filters.
Soil sample: 5 kg soils were collected from the China Agriculture University, air-dried and homogenized with particle diameters less than 1 mm before further handling.
Apparatus
Chromatography was carried out on an Agilent 1100 with quaternary-pump system, autosampler, mobile phase vacuum degassers, and an ultraviolet detector. The output signal was acquired and processed on a HP1100 workstation. Solid phase extraction cartridges (AccuBond II, ODS-C18 Cartridges, 500 mg/6 ml) were purchased from Agilent Company (U.S). Solid phase extraction equipment (Lichrolut TM) was purchased from Merck Company (Germany). Water-Circulation Multifunction Vacuum Pump (SHB-III) was purchased from Zhengzhou Great Wall Scientific Industry and Trade Co. Ltd Henan province, China.Preparation of the CSP
The CSP was prepared and packed following the guidance of the literature.14,15Macrocrystalline cellulose (1.0 g) reacted with 3,5-dimethylphenylisocyanate (3.5 g) in pyridine at 110 °C for 24 h to synthesize CDMPC. After cooling to room temperature, the product was precipitated by methanol (30 mL), filtered, washed with methanol twice, and dried for 24 h under vacuum. Aminopropylsilica (APS) was prepared by treating 3-aminopropyltriethoxysilane with spherical silica at 110 °C for 24 h in toluene. The CSP was prepared by coating CDMPC to APS. The slurry of the CSP in n-hexane-IPA (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) solution was packed into a stainless steel column (250 mm × 4.6 mm id) under 3.7 × 107 Pa in the laboratory.
10 v/v) solution was packed into a stainless steel column (250 mm × 4.6 mm id) under 3.7 × 107 Pa in the laboratory.
Chromatographic conditions
Separation was accomplished on a reversed phase cellulose-based chiral column at ambient temperature. The mobile phase compositions after optimization were methanol–water (65/35, v/v). The flow rate was set to 1.0 ml min−1 with UV at 225 nm. The samples were injected with an amount of 20 μl. The CD and UV spectra were obtained by stopped flow scanning by JASCO 2000 HPLC system (JASCO Corporation, Japan) equipped with PU-2089 plus pump and CD-2095 plus circular dichroism detector. The mobile phase compositions were methanol–water (60/40, v/v). The flow rate was set to 0.8 ml min−1 with CD at 230 nm.Sample preparation procedure
Water sample: AccuBond SPE ODS-C18 cartridges were used for extraction and clean up of the water sample. The SPE cartridges were conditioned with 5 ml × 2 of methanol, then, 5 ml × 2 distilled water. The passage of sample (volume 200 ml) through the cartridges was carried out at a flow-rate of 4 ml min−1 by means of a vacuum pump. Once the retention step had been completed, the cartridges were dried for 30 min under a vacuum of 15 mmHg (1 mmHg = 133.32 Pa). The components retained were eluted with 5 ml methanol. The eluate was collected and concentrated to near dryness under a stream of nitrogen. The residue was finally reconstituted in 0.5 ml of methanol. After being filtered through a filter (0.45 μm pore-size), an aliquot (20 μL) was injected into the RP-LC.Soil sample: The soil sample (25 g) was extraction with acetone (50 ml) in a 250 mL conical flask. After shaking for 30 min (machine) and sonicating for 3 min, the soil was allowed to settle for 15 min and then filtered through a Buchner funnel with glass fiber filter paper. Use acetone (20 ml) to rinse the homogenizer jar and the Buhner funnel to further extract the pesticides. Most of the acetone was removed by rotary evaporation and 100 ml water added, then cleaned-up through SPE procedures.
Method validation
The method validation was carried out by assessing lineatity, accuracy and precision. Linearity of calibration curves was studied in the concentration range between 0.5 and 20 μg mL−1 using six calibration standards at 0.5, 1.0, 2.5, 5.0, 10, 20 μg mL−1 for each enantiomer of paclobutrazol and myclobutanil by appropriate dilution of aliquots of the stock solutions in methanol. The accuracy of the method was calculated in terms of recoveries. The recovery rate of each enantiomer of two pesticides at two different fortification levels was evaluated in order to assess the extraction efficiency of the proposed method. The precision of the method was assessed in terms of repeatability of recoveries. The run-to-run precisions of three concentrations (0.5, 1.0 and 5.0 μg mL−1) were determined by injections in six replicates and the day-to-day precisions were also tested over 6 days by six successive injections each day.Results and discussion
Calibration curve, limit of detection (LOD) and limit of quantification (LOQ)
Under the working conditions, calibration curves were generated by plotting peak area of each enantiomer versus the concentration of the standard enantiomers. Quantitation was calculated on the bases of linear regression analysis. The limit of detection (LOD) for each enantiomer was considered to be the concentration that produced a signal-to-noise (S/N) ratio of 3. The limit of quantification (LOQ) was defined as the lowest pesticide concentration on the calibration curve, which can be determined with an accuracy and precision <20%.16Table 1 showed linear ranges, linear equations, correlation coefficients (R2) and limits of detection. For each enantiomer of the two pesticides, good linearity was obtained with the R2 value more than 0.99 in the tested range. LOD for each enantiomer was 0.0015 μg mL−1 in water and 0.012 μg g−1 in soil. LOQ of each enantiomer was 0.0025 μg−1 mL using 200 ml of water and 0.020 μg g−1 using 25 g of soil.| Compound | Enantiomer | Linear range/μg mL−1 | Linear equation (R2) | LOD | LOQ | ||
|---|---|---|---|---|---|---|---|
| Water/μg mL−1 | Soil/μg g−1 | Water/μg mL−1 | Soil/μg g−1 | ||||
| Paclobutrazol | E 1 | 0.5–20 | A 1 = 58.188ρ1 − 16.573 (0.9986) | 0.0015 | 0.012 | 0.0025 | 0.020 |
| E 2 | 0.5–20 | A 2 = 56.994ρ2 − 14.205 (0.9987) | 0.0015 | 0.012 | 0.0025 | 0.020 | |
| Myclobutanil | E 3 | 0.5–20 | A 3 = 61.092ρ3 − 11.409 (0.9989) | 0.0015 | 0.012 | 0.0025 | 0.020 |
| E 4 | 0.5–20 | A 4 = 60.523ρ4 − 10.573 (0.9986) | 0.0015 | 0.012 | 0.0025 | 0.020 | |
Precision and accuracy
Under the working conditions, each enantiomer of paclobutrazol and myclobutanil was baseline separated. Complete separation of the each enantiomer made their quantitative analysis possible. The repeatabilities of retention times and peak areas were also good with relative standard deviations 0.19–0.76% and 3.22–8.64% for each enantiomer of paclobutrazol and myclobutanil (Table 2).| Standard solution/μg mL−1 | RSD (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Paclobutrazol (E1) | Paclobutrazol (E2) | Myclobutanil (E3) | Myclobutanil (E4) | |||||
| Retention time (t1) | Area1 | Retention time (t2) | Area2 | Retention time (t3) | Area3 | Retention time (t4) | Area4 | |
| a Number of samples. b Number of days. Area1 and Area2 represent the areas of the first and the second eluted enantiomer of paclobutrazol. Area3 and Area4 represent the areas of the first and the second eluted enantiomer of myclobutanil. | ||||||||
| Within-day (n = 6)a | ||||||||
| 0.5 | 0.27 | 5.42 | 0.41 | 4.87 | 0.47 | 6.45 | 0.54 | 6.14 |
| 1.0 | 0.34 | 4.19 | 0.34 | 3.64 | 0.29 | 4.18 | 0.43 | 5.01 |
| 5.0 | 0.31 | 4.26 | 0.19 | 3.28 | 0.36 | 3.76 | 0.39 | 3.22 |
| Day to day (n = 6)b | ||||||||
| 0.5 | 0.32 | 7.11 | 0.38 | 7.55 | 0.51 | 8.64 | 0.76 | 8.34 |
| 1.0 | 0.28 | 7.19 | 0.26 | 5.24 | 0.24 | 6.06 | 0.54 | 5.67 |
| 5.0 | 0.21 | 5.29 | 0.29 | 4.85 | 0.20 | 4.46 | 0.28 | 6.32 |
Acetone was selected as the solvent for extraction of the enantiomers in soil because of its effectiveness and solubility in water. C18 SPE was a powerful method for enrichment and cleanup and environmently friendly.13 As shown in Table 3 and Table 4, good recoveries and precisions were obtained. Recoveries of paclobutrazol enantiomers and myclobutanil enantiomers were established by analyzing two concentrations of solutions. Typical chromatograms of paclobutrazol and myclobutanil of blank sample and fortified sample with the racemic standard solution were shown in Fig. 2 for water and Fig. 3 for soil samples. There were no interference peaks eluted at the same retention times of each enantiomer. Precisions of the proposed method were calculated by analyzing replicate (n = 6) water and soil samples spiked with two different concentration levels (0.0025, 0.025 μg mL−1 or 0.02, 0.20 μg g−1) of paclobutrazol enantiomers and myclobutanil enantiomers (Table 3 and Table 4). As can be seen from Table 3 and Table 4, the precisions in water and soil were in the range of 3.83–11.5%. The method was then applied to determine tap water and environmental soil sample, no target compounds were detected.
| Concentration added | Paclobutanil (E1) | Paclobutanil (E2) | ||||
|---|---|---|---|---|---|---|
| Conc. found | Recovery (%) | RSD (%) | Conc. found | Recovery (%) | RSD (%) | |
| a Recovery values represent the mean ± standard deviations. | ||||||
| Water/μg mL−1 | ||||||
| 0.0025 | 0.0023 | 90.96 ± 9.06 | 9.96 | 0.0023 | 90.81 ± 8.88 | 9.78 |
| 0.025 | 0.0243 | 97.37 ± 4.01 | 4.12 | 0.0245 | 98.06 ± 4.14 | 4.22 |
| Soil/μg g−1 | ||||||
| 0.02 | 0.017 | 85.42 ± 8.76 | 10.3 | 0.017 | 86.09 ± 6.85 | 7.96 |
| 0.20 | 0.189 | 94.63 ± 4.80 | 5.07 | 0.190 | 95.22 ± 3.65 | 3.83 |
| Concentration added | Myclobutranil (E3) | Myclobutranil (E4) | ||||
|---|---|---|---|---|---|---|
| Conc. found | Recovery (%) | RSD (%) | Conc. found | Recovery(%) | RSD (%) | |
| a Recovery values represent the mean ± standard deviations. | ||||||
| Water/μg mL−1 | ||||||
| 0.0025 | 0.0021 | 83.13 ± 9.47 | 11.4 | 0.0021 | 85.06 ± 7.78 | 9.15 |
| 0.025 | 0.0229 | 91.75 ± 5.50 | 5.99 | 0.0235 | 93.84 ± 5.60 | 5.97 |
| Soil/μg g−1 | ||||||
| 0.02 | 0.016 | 82.40 ± 8.72 | 10.6 | 0.016 | 82.98 ± 9.58 | 11.5 |
| 0.20 | 0.180 | 89.89 ± 4.06 | 4.52 | 0.181 | 90.69 ± 4.95 | 5.46 |
 | ||
| Fig. 2 Chromatograms of (a) paclobutrazol and myclobutanil of blank water sample, (b) water sample fortified with racemic paclobutrazol and myclobutanil at 0.0025 μg mL−1 for each enantiomer. (peak 1, peak 2—one pair of enantiomers of paclobutrazol; peak 3, peak 4—one pair of enantiomers of myclobutanil). | ||
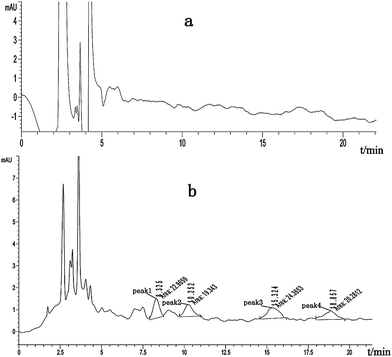 | ||
| Fig. 3 Chromatograms of (a) paclobutrazol and myclobutanil of blank soil sample, (b) soil sample fortified with racemic paclobutrazol and myclobutanil at 0.020 μg g−1 for each enantiomer. (peak 1, peak 2—one pair of enantiomers of paclobutrazol; peak 3, peak 4—one pair of enantiomers of myclobutanil). | ||
UV and circular dichroism (CD) spectrum
The enantiomers of a chiral compound are usually identified with their absolute configuration or optical rotation. LC coupled with a CD detector has recently become a powerful tool for determining the optical property of the resolved enantiomers.17 The CD spectra of paclobutrazol and myclobutanil enantiomers with wavelengths of 220–400 nm were obtained using the on-line CD detector by stopped-flow scanning at chromatographic peak tops in CD detection. The UV and CD spectra of paclobutrazol and myclobutanil enantiomers were shown in Fig. 4: as expected the UV absorbance spectra were identical, but the CD spectra of the two enantiomers exhibited completely opposite features. In this study, the CD spectra of paclobutrazol and myclobutanil enantiomers were not yet known and it may be useful to assign the absolute configuration of the resolved enantiomers by comparing the CD curve of the molecule under study with that of a similar compound having known absolute configuration.18–20 However, the separated enantiomers were distinguished based upon their CD signs 230 nm. Theoretically, when taking the axis of CD = 0 as a mirror, the CD spectrum of one enantiomer is the mirror image of its antipode. The absorbance intensity changed with the wavelength and there was a maximum value. For paclobutrazol and myclobutanil, there were no or weak CD absorbance above 240 nm, and only the wavelength 220–240 nm was appropriate for identifying the enantiomers. This is very important to denote the wavelength for determination of elution orders using CD signals. Meanwhile, the results shown in Fig. 4 confirmed that the resolved peaks in Fig. 5 corresponded to one pair of enantiomers.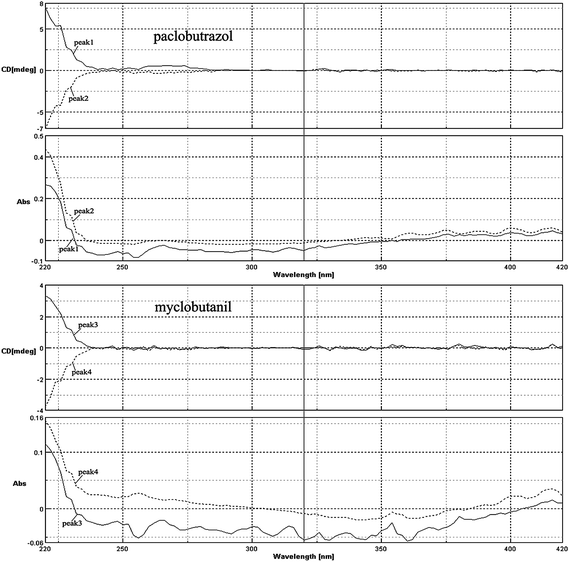 | ||
| Fig. 4 The CD, UV spectra of paclobutrazol and myclobutanil on CDMPC-CSP. | ||
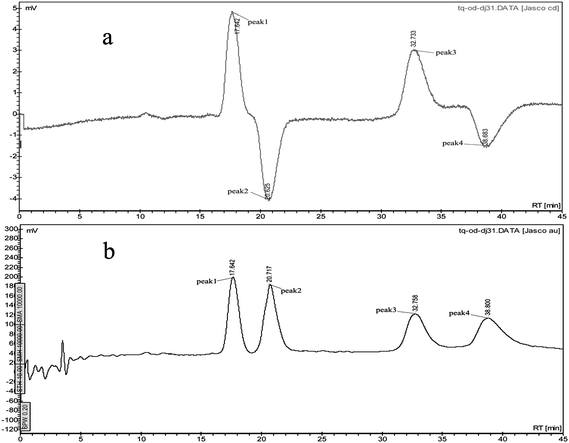 | ||
| Fig. 5 The CD (a), UV (b) chromatograms of paclobutrazol and myclobutanil on CDMPC-CSP (peak 1, peak 2—one pair of enantiomers of paclobutrazol; peak 3, peak 4—one pair of enantiomers of myclobutanil). | ||
Conclusion
A reversed phase LC method using the CDMPC-CSP and methanol–water mobile phase was applied for the simultaneous enantiomeric separation of the two chiral pesticides. There was excellent simultaneous separation of the enantiomers of the two pesticides. The structures of paclobutrazol and myclobutanil (Fig. 1) have an electronegative atom (nitrogen or oxygen) or phenyl ring directly linking to the chiral center. Therefore, the separation of the enantiomers of two pesticides occurred through the different hydrogen bond, π–π, and dipole–dipole induced interactions between the chiral stationary phase and the analyte enantiomers.In this article, a simple, rapid, economic and sensitive method was developed for the simultaneous separation and determination of paclobutrazol and myclobutanil enantiomers in water and soil using CDMPC-CSP under RP. RP conditions have good solubility of polar compounds, easier sample preparation from water or soil, use of less costly solvents, and especially useful pharmacokinetics study in biological matrices. Meanwhile the method was validated, and good linearity, LOD, LOQ, recovery and precision obtained mean it can be applied to help further studies in tracing different bioactivities, metabolism and environmental behaviors of each enantiomer, and finally helping minimize the risks posed by fungicides to the environment and public health. In addition, optical characters of individual enantiomers prepared were studied by CD spectroscopy.
References
- H. Y. Aboul-Enein and L. I. Abou-Basha, Chirality and drug hazards. In: H. Y. Aboul-Enein and I. W. Wainer, ed. The Impact of Stereochemistry On Drug Development and Use, 1997, Wiley, New York. ch.1. pp.1 Search PubMed.
- R. S. Burden, G. A. Carter, T. Clark, D. T. Cooke, S. J. Croker, A. H. B. Deas, P. Hedden and J. R. Lenton, Pestic. Sci., 1987, 21, 253–267 CrossRef CAS.
- B. Sugavanam, Pestic. Sci., 1984, 15, 296–302 CrossRef CAS.
- M. Natangelo, S. Tavazzi, R. Fanelli and E. Benfenati, J. Chromatogr., A, 1999, 859, 193–201 CrossRef CAS.
- C. G. Zambonin, A. Cilenti and F. Palmisano, J. Chromatogr., A, 2002, 967, 255–260 CrossRef CAS.
- D. Sharma and M. D. Awasthi, Chemosphere, 2005, 60, 164–169 CrossRef CAS.
- C. Lesueur, P. Knittl, M. Gartner, A. Mentler and M. Fuerhacker, Food Control, 2008, 19, 906–914 CrossRef CAS.
- A. Sannino, L. Bolzoni and M. Bandini, J. Chromatogr., A, 2004, 1036, 161–169 CrossRef CAS.
- I. R. Pizzutti, André de Kok, R. Zanella, M. B. Adaime, M. Hiemstra, C. Wickert and O. D. Prestes, J. Chromatogr., A, 2007, 1142, 123–136 CrossRef CAS.
- I. R. Pizzutti, André de Kok, M. Hiemstra, C. Wickert and O. D. Prestes, J. Chromatogr., A, 2009, 1216, 4539–4552 CrossRef CAS.
- A. Balinova, Anal. Chim. Acta, 1995, 311, 423–427 CrossRef CAS.
- O. Lacina, J. Urbanova, J. Poustka and J. Hajslova, J. Chromatogr., A, 2010, 1217, 648–659 CrossRef CAS.
- P. Wang, D. Liu, X. Gu, S. Jiang and Z. Zhou, J. AOAC Int., 2008, 91, 1007–1012 CAS.
- Y. Okamoto, M. Kawashima and K. Hatada, J. Chromatogr., A, 1986, 363, 173–186 CrossRef CAS.
- Z. Q. Zhou, P. Wang, S. R. Jiang, M. Wang and L. Yang, J. Liq. Chromatogr. Relat. Technol., 2003, 26, 2873–2880 CrossRef CAS.
- J. Qiu, Q. Wang, W. Zhu, G. Jia, X. Wang and Z. Zhou, Chirality, 2007, 19, 51–55 CrossRef CAS.
- D. R. Bobbitt and S. W. Linder, Trends Anal. Chem., 2001, 20, 111–123 CrossRef CAS.
- G. Blaschke, Angew. Chem., 1980, 92, 14–25 CrossRef CAS.
- P. Salvadori, C. Bertucci and C. Rosini, Chirality, 1991, 3, 376–385 CAS.
- G. Cannazza, D. Braghiroli, M. Baraldi and C. Parenti, J. Pharm. Biomed. Anal., 2000, 23, 117–125 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
