Development of a modified copper-cadmium reduction method for rapid assay of total nitric oxide
Kranti
Sorte
and
Anjan
Basak
*
Department of Biochemistry, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (D. Univ.), Wardha-442004, Maharashtra, India. E-mail: drabasak1@yahoo.com
First published on 18th May 2010
Abstract
The biomolecule, nitric oxide (NO) is short lived and is converted to two stable products—nitrite (NO2) and nitrate (NO3). NO3 can be measured by colorimetric procedure involving enzymatic conversion to NO2 and by HPLC. But these methods, though sensitive and specific, require expensive reagents or equipment. Reduction of NO3 to NO2 by copper-coated cadmium granules is a popular method devised by Cortas and Wakid (Clin. Chem., 1990, 36, 1440), but it takes 120 min. Our aim of this study is to modify the Cortas and Wakid method for rapid reduction of NO3 to NO2 for total NO estimation in serum. After reduction, NO2 is estimated by Griess reaction. As per our results, complete reduction of NO3 could be achieved by both the methods, but it took only 5 min by our method. Standard graphs were linear by both of the methods. Factors were found to be 143.4 and 196.7 by the Cortas and Wakid method and by our method, respectively. Recovery of NO2 from NO3 was found to be 105% and 106%, respectively. We measured total NO2 in sixteen sera by both the methods and our method's coefficient of correlation was found to be 0.9924. Our method is comparable to that of Cortas and Wakid for complete reduction of NO3 with the greatest advantage of decrease in the time span from 120 to 5 min. This is due to our development of an increased copper coating method on cadmium granules. Our rapid method saves much time for total NO estimation.
Introduction
Nitric Oxide (NO) is a short lived product of a five-electron oxidation of amino acid L-arginine catalysed by nitric oxide synthase. The transient and volatile nature of NO makes it unsuitable for estimation by convenient methods; however, two stable oxidation products, nitrate (NO3) and nitrite (NO2), are formed. In biological fluids, nitrate can be measured by colorimetric procedure involving enzymatic conversion to NO2 by nitrate reductase and by ion-exchange chromatography.1,2 Though the methods are more sensitive and specific, but these require costly reagents or equipments. Reduction of nitrate to nitrite by a metal and subsequent estimation of NO2 by diazotization (Griess reaction) has become a common procedure as it is sensitive, specific and inexpensive.3,4 But this method is not free of drawbacks. Some metals reduce nitrite further to NO and some biological constituents (e.g. ascorbic acid and phosphate) interfere.5Reduction of nitrate to nitrite by copper-coated cadmium granules is a popular method.5 The method has been refined by use of standard addition and by precipitation with zinc hydroxide. But the drawback of this method is that a long time (120 min) is required for reduction of NO3.5
Aims and objectives
Development of a modified Copper-Cadmium Reduction method for rapid assay of total Nitric Oxide in serumMaterials and methods
Materials
All the chemicals used were of analytical grade.B) 200 mmol L−1 in glycine buffer for our Rapid Cadmium-copper reduction method.
Methods
Activation of cadmium granules
 | ||
| Fig. 1 Changes in colour of cadmium granule from native to activated and then after prolong air exposure. | ||
Reduction procedures
Results
We compared the effectivity of our method with that of the Cortas and Wakid Cd reduction method for reduction of nitrate to nitrite. The efficiency of our method to coat cadmium granules by copper was much greater (Fig. 2). | ||
| Fig. 2 Colours of cadmium granules. | ||
Reduction of standard solutions of NO3 to NO2 (recovery test) were found to be 105% by the Cortas and Wakid method and 106% by our method (Tables 1 and 2). Standard graphs were also linear by both of the methods (Fig. 3a and b). We have checked linearity from 10–100 μmol L−1. The factor (concentration of standard divided by absorbance of standard at 545 nm) was found to be 143.4 by the Cortas and Wakid method and 196.7 by our method. The blank absorbances were 0.036 and 0.024, respectively. Total NO2 was measured in 16 serum samples by both of the methods (Table 2). Mean values were found to be 35.53 ± 33.82 μmol L−1 by the Cortas and Wakid method and 39.03 ± 35.86 μmol L−1 by our method, but the difference was insignificant (P > 0.40). Linear regression analysis gave a co-efficient of correlation (R2) 0.9924 with an X-axis intercept of 1.4884 (Fig. 4). Intra-assay coefficient of variation (C.V.) of samples (n = 8) were 5.2% by both the methods.
| μmol/L | NO2 | NO3 → NO2 |
|---|---|---|
| 10 | 0.068 | 0.070 |
| 25 | 0.160 | 0.179 |
| 50 | 0.332 | 0.343 |
| 75 | 0.492 | 0.522 |
| 100 | 0.696 | 0.709 |
| μmol/L | NO2 | NO3 → NO2 |
|---|---|---|
| 10 | 0.048 | 0.051 |
| 25 | 0.122 | 0.127 |
| 50 | 0.238 | 0.265 |
| 75 | 0.360 | 0.392 |
| 100 | 0.489 | 0.487 |
 | ||
| Fig. 3 (a) Standard graph of total NO assay by the Cortas and Wakid Cd–Cu reduction method. (b) Standard graph of total NO assay by our Rapid Cd-Cu reduction method. | ||
| Sr. No. | Cortas and Wakid Cd reduction method | Our rapid Cd reduction method |
|---|---|---|
| 1 | 7.74 | 9.83 |
| 2 | 23.51 | 28.71 |
| 3 | 19.19 | 13.96 |
| 4 | 38.00 | 35.2 |
| 5 | 13.62 | 16.32 |
| 6 | 30.10 | 34.80 |
| 7 | 124.16 | 132.94 |
| 8 | 18.92 | 23.40 |
| 9 | 28.10 | 34.02 |
| 10 | 23.08 | 28.12 |
| 11 | 27.96 | 34.61 |
| 12 | 83.30 | 88.10 |
| 13 | 92.32 | 100.29 |
| 14 | 13.48 | 14.16 |
| 15 | 11.32 | 14.16 |
| 16 | 13.76 | 15.93 |
| Mean ± S.D. | 35.535 ± 33.82 | 39.034 ± 35.86 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
t = 0.284, P > 0.40 | |
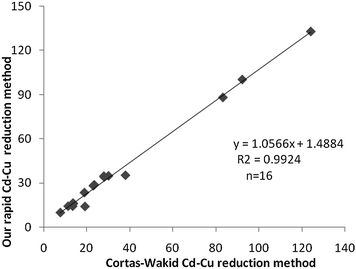 | ||
| Fig. 4 Correlation of serum total NO assay (μmol L−1). | ||
Reduction of NO3 to NO2 with time had been studied. We found complete reduction by 4 min and increasing the time up to 8 min did not change the reduction process (Table 3).
| Time/min | A 545 |
|---|---|
| 0 | 0.001 |
| 1 | 0.112 |
| 2 | 0.251 |
| 3 | 0.364 |
| 4 | 0.470 |
| 5 | 0.470 |
| 6 | 0.473 |
| 7 | 0.471 |
| 8 | 0.473 |
Discussion
Estimation of the total NO is of importance for understanding of many biological processes. The enzymatic procedure using nitrate reductase enzyme is accurate, but it takes 15 min of time and is also expensive. The NO assay by Cortas and Wakid is also accurate, but very time consuming. Copper-cadmium alloy used by Sastry et al. for their NO assay also takes 60 min of time for reduction.7 So, we were compelled to do an experiment to shorten the time. The greatest advantage of our method is the time reduction from 120 min to only 5 min for reduction of NO3 to NO2. This was possible due to the ability of our method for increased copper coating on cadmium granules (Fig. 2). This increased copper coating increased the efficiency of conversion of NO3 to NO2. We checked the linearity of standard graph up to 100 μmol L−1 of NO2. However, upper limit of linearity was 250 μmol L−1 as found by Cortas and Wakid.5 ¦The sensitivity of assay by our method is less as compared to the Cortas and Wakid method (0.197 vs. 0.143 μmol L−1).Values of serum NO2 estimation by both the methods (Table 2) showed very good correlation (Fig. 4). Intra-assay C.V. were found to be same by both the methods (5.2%).
We did not add glycine buffer during the reduction of NO3 as we found that the increased copper coating on Cd granules used to get dissolved in the buffer. In our experimental conditions, the time required for complete reduction of NO3 was 4 min (Table 3). After 4 min there were no changes in absorbance up to 8 min which indicates that NO2 was not further reduced to NO. Interference by other metal ions were not studied as it is a small modification of the widely used Cortas and Wakid method5 with the exceptions that
i) increased copper coating on cadmium where chemicals were the same and
ii) during reduction procedure we omitted addition of the glycine buffer.
Sastry et al. used copper-cadmium alloy in acidic media,7 Cortas and Wakid used alkaline media,5 whereas we used neutral media for reduction of nitrate to nitrite to measure NO in biological samples.
Our copper coated cadmium granules effectivity is not stable. The granules begin loosing reduction capacity after about 20 min and turns to a black colour on prolonged standing (Fig. 1). Thus we have developed a rapid method that anyone can adopt for total NO assay. It is a fast and reliable method and will provide the choice of a cheaper alternative.
Conclusion
Our rapid method saves much time for total NO assay.References
- J. Schild and J. H. Kleimne, Z. Naturforch., 1985, 40, 134–137 Search PubMed.
- D. P. Jong and M. Burgraff, Clin. Chim. Acta, 1983, 132, 63–71 CrossRef.
- E. Hegesh and J. Shiloah, Clin. Chim. Acta, 1982, 125, 107–115 CrossRef CAS.
- C. D. Usher and T. M. Telling, J. Sci. Food Agric., 1975, 26, 138–144.
- N. K. Cortas and N. W. Wakid, Clin. Chem., 1990, 36, 1440–1443 CAS.
- M. Somogyi, J. Biol. Chem., 1930, 86, 655–663 CAS.
- K. V. H. Sastry, R. P. Moudgal, J. Mohan, J. S. Tyagi and G. S. Rao, Anal. Biochem., 2002, 306, 79–82 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
