Evaluation of analytical instrumentation
Part XXIV. Instrumentation for quadrupole inductively coupled plasma mass spectrometry†
First published on 9th August 2010
Abstract
The Analytical Methods Committee has received and approved the following report from the Instrumental Criteria Sub-Committee.
Introduction
The following report was compiled by the above Sub-Committee of the AMC, which consisted of Prof. S. Greenfield (Chairman), Dr C. Burgess, Prof. S. J. Hill, Prof. K. E. Jarvis, Dr G. Lord, Dr M. Sargent, Prof. P. J. Potts, Dr J. Price and Dr M. West with Dr E. J. Newman as Secretary. The initial input of the features for consideration was undertaken by a working party comprising Prof. K. E. Jarvis and Dr M. Sargent to whom the committee expresses its thanks.The purchase of analytical instrumentation is an important function of many laboratory managers, who may be called upon to choose between wide ranges of competing systems that are not always easily comparable. The objectives of the Instrumental Criteria Sub-Committee are to tabulate a number of features of analytical instruments that should be considered when making a comparison between various systems. As is explained below, it is then possible to score these features in a rational manner, which allows a scientific comparison to be made between instruments as an aid to selection.
The over-all object is to assist purchasers in obtaining the best instrument for their analytical requirements. It is hoped that this evaluation will, to some extent, also help manufacturers to supply the instrument best suited to their customers' needs. It is perhaps pertinent to note that a number of teachers have found the reports to be of use as teaching aids.
No attempt has been made to lay down a specification. In fact, the Committee considers that it would be invidious to do so. It has tried to encourage the purchasers to make up their own minds as to the importance of the features that are on offer by the manufacturers.
The XXIVth report of the Sub-Committee deals with instrumentation for quadrupole inductively coupled plasma mass spectrometry.
Notes on the use of this document
The guidelines are intended to be used as a checklist of features to be considered, mostly of the instrument itself, but also of service requirements and any existing relationship between the user and manufacturer. The relative importance of these features will depend on a number of factors which in some circumstances could be subjective. However, if all the points have been considered, the choice should be informed.| Column 1. | The features of interest. |
| Column 2. | What the feature is and how it can be evaluated. |
| Column 3. | The Sub-Committee has indicated the relative importance of each feature to enable users to decide on a weighting factor according to their own application. |
| Column 4. | Here the Sub-Committee has given reasons for its opinion as to the importance of each feature. |
It is suggested that scores are given for each feature of each instrument and that these scores are modified by a weighting factor and sub-totals obtained. The grand total will give the final score that can contribute to the selection of the instrument that best suits the user's requirements. It is further suggested, that the scoring of features will be by proportion, e.g., Worst/0 to Best/100. The weighting factor (WF) will depend on individual requirements. All features mentioned in the tables have some importance. If, in the Sub-Committee's opinion, some features are considered to be of greater importance they are marked I. Those features of greatest importance are marked as VI (very important). A scale should be chosen for the weighting factor that allows the user to discriminate according to needs, e.g., ×1 to ×3 or ×1 to ×10. Multiplying proportional scoring (PS) by WF obtains the sub-total.
In some circumstances, where there is a fundamental incompatibility between a feature of the instrument and the intended application, it may be necessary to exclude an instrument completely from further consideration. It is also recognised that some instrumental aspects can not be scored and this is stated in the reports where appropriate. It is further recognised that some of the tests cannot be undertaken for several reasons such as an inability to change features under computer control. However, it is thought that the theory behind the production of the tests has educational value
With these requirements in mind, the user should then evaluate the instruments available on the market taking into account the following guidelines and any financial limitations. In many instances it will quickly become clear that a number of different instruments could be satisfactory and non-instrumental criteria may then become important. However, in some specialized cases only one or two instruments will have the ability or necessary features to be used in the intended application.
Finally, as many laboratories are now working to established quality standards, some consideration should be given to third party certification of the manufacturer to standards such as the ISO Guide 9000 series. Such certification should extend to the service organisation.
Other reports
The Analytical Methods Committee has published the following reports in the series:Part I. Atomic absorption spectrophotometers, primarily for use with flames, Anal. Proc., 1984, 21, 45. Revised in Analyst, 1998, 123. 1407.
Part II. Atomic absorption spectrophotometers, primarily for use with electrothermal atomizers, Anal. Proc., 1985, 22, 128. Revised in Analyst, 1998, 123, 1415.
Part III. Polychromators for use in emission spectrometry with ICP sources, Anal. Proc., 1986, 23, 109.
Part IV. Monochromators for use in emission spectrometry with ICP sources, Anal. Proc., 1987, 24, 3.
Part V. Inductively coupled plasma sources for use in emission spectrometry, Anal. Proc., 1987, 24, 266.
Part VI. Wavelength dispersive X-ray spectrometers, Anal. Proc., 1990, 27, 324.
Part VII. Simultaneous wavelength dispersive X-ray spectrometers, Anal. Proc., 1991, 28, 312.
Part VIII. Instrumentation for gas–liquid chromatography, Anal. Proc., 1993, 30, 296.
Part IX. Instrumentation for high-performance liquid chromatography, Analyst, 1997, 122, 387.
Part X. Instrumentation for inductively coupled plasma mass spectrometry, Analyst, 1997, 122, 393.
Part XI. Instrumentation for molecular fluorescence spectrometry, Analyst, 1998, 123, 1649.
Part XII. Instrumentation for capillary electrophoresis, Analyst, 2000, 125, 361.
Part XIII. Instrumentation for UV-VIS-NIR spectrometry, Analyst, 2000, 125, 367.
Part XIV. Instrumentation for Fourier transform infrared spectrometry, Analyst, 2000, 125, 375.
Part XV. Instrumentation for Gas chromatography-ion-trap mass spectrometry, Analyst, 2001, 126, 953.
Part XVI. Evaluation of general user NMR spectrometers, Accred. Qual. Assur., 2006, 11, 130.
Part XVII. Instrumentation for inductively coupled plasma atomic emission spectrometers, Accred. Qual. Assur., 2005, 10, 155.
Part XVIII. Differential scanning calorimetry, Accred. Qual. Assur., 2005, 10, 160.
Part XIX. CHNS elemental analysers, Accred. Qual. Assur., 2006, 11, 569.
Part XX. Instrumentation for energy dispersive X-ray fluorescence spectrometry, Accred. Qual. Assur., 2006, 11, 610.
Part XXI. NIR instrumentation for process control, Accred. Qual. Assur., 2006, 11, 236.
Part XXII. Instrumentation for liquid chromatography/mass spectrometry, Accred. Qual. Assur., 2007, 12, 3.
Part XXIII. Portable XRF instrumentation, Accred. Qual. Assur., 2008, 13, 453.
An overview of inductively coupled plasma mass spectrometry (ICP-MS)
Introduction
Inductively coupled plasma mass spectrometry (ICP-MS) brings together the advantages of the ICP source and mass spectrometry instrumentation for routine elemental analysis. Since its commercial introduction it has been applied to the determination of trace, minor and major elements in almost every area of application. A wide range of instrumentation is now available, from small bench-top ‘quadrupole’ instruments to large floor-standing ‘multi-collectors’, but all adopt the same basic principle. A solid, liquid or gaseous analytical sample is introduced into a high temperature argon annular plasma (the ICP) and the matrix is atomised and ionised. An aliquot of the ionised sample is extracted from the plasma and passed into a high vacuum mass spectrometer where the ions are separated according to their mass and charge, allowing the analyte ions of interest to be collected and measured. Quadrupole ICP-MS instrumentation is used most widely for routine applications and the tabular part of this report is concerned only with that type of instrument. A brief description of the other main instrument types is given in this overview as background information for the reader.Key features
The introduction of ICP-MS followed many years of routine commercial application of optical emission and atomic absorption spectrometry. The unique combination of features offered by the new technique at the time of its introduction was a multi-element and multi-isotope capability combined with high selectivity and sensitivity. These capabilities facilitate measurement of almost every common element over a very wide concentration range with relative freedom from interferences. This combination remains the key to its success but current instrumentation also offers rapid automated analysis, even at ultra-trace levels (see table below), with relatively modest effort on method development and validation. Numerous accessories are readily available for introduction of almost any sample type, including the ability to directly couple both gas and liquid chromatography systems to the ICP. The latter have opened up a whole new field of application, namely speciation of organo-metallic molecules. Finally, ICP-MS greatly facilitates routine acquisition of isotopic information for applications as diverse as isotope dilution mass spectrometry (IDMS) and ‘fingerprinting’ of forensic samples.Basic principles
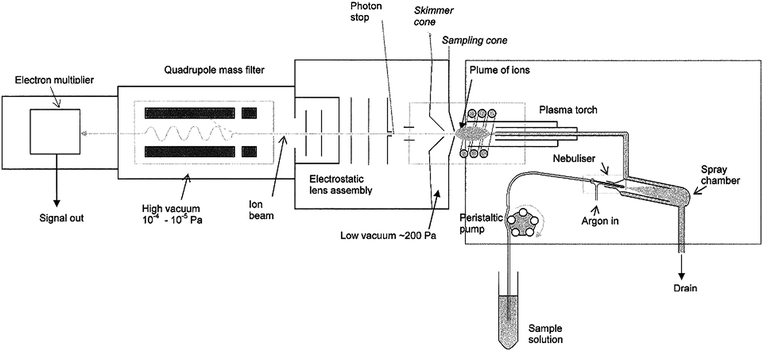
In a typical implementation, the sample is a solution which is passed through a nebuliser and introduced into the ICP as an argon aerosol. Larger droplets are removed in a spray chamber and the remainder swept into the plasma which is generated in a stream of argon contained in a quartz tube or "torch". The torch is surrounded by a copper coil carrying a high frequency (HF) current produced by an HF generator which may operate at powers up to 1.5 kW or more. The high frequency currents flowing in the induction coil generate oscillating magnetic fields whose lines of force flow axially through the quartz tube and follow an elliptical closed path outside the coil. The induced magnetic fields, in turn, cause the electrons and ions (which have previously been "seeded" in the argon gas by means of a Tesla coil) to flow in closed annular paths inside the quartz tube. Since the induced magnetic fields are time varying in their direction and strength, the electrons are accelerated on each half cycle. The accelerated electrons and ions meet resistance to their flow and Joule heating follows. Argon is used as the plasma gas because it is relatively cheap and has a higher first ionization potential than most other elements. The high ionization energy ensures efficient production of ions from the sample.A typical instrument comprises four main stages within the vacuum system: the interface, an ion focussing device, the mass “analyser”, and an ion detection system. The interface was a critical step in the development of ICP-MS and comprises metal plates with central orifices (the “cones”) which allow ions produced in the hot atmospheric pressure plasma to be extracted into the cool high vacuum system of the mass spectrometer. The ions are focussed into a narrow beam by electrostatic lenses which also separate them from the photons and extraneous material extracted from the plasma. By far the most common type of mass analyser used with ICP-MS is the quadrupole, followed by magnetic sector and a few time-of-flight analysers. Quadrupole instruments offer only unit mass resolution but are less expensive than magnetic sector instruments, have less stringent vacuum requirements and do not require “baking out” after changing a detector. They offer a faster scan rate than current magnetic sector instruments and are, therefore, more suitable for the detection of transient signals (e.g. when using electrothermal vaporisation, laser ablation or a coupled chromatographic system).
The quadrupole analyser uses electrical fields to separate ions based on their mass to charge ratio (m/z). For a given voltage setting, only one m/z has stable trajectory through the analyser and the quadrupole scans rapidly across the mass range, passing each required mass sequentially to the ion detector. In quadrupole instruments this is almost always an electron multiplier detector which detects the ions as they arrive and has appropriate electronics to count and store the total signal for each mass (m/z). The output is a mass spectrum which provides a simple representation of the sample as a series of peaks, one for each m/z (or mass, since in most cases z = 1). The magnitude of each peak is directly proportional to the concentration of the corresponding element or isotope in the sample so that quantitative measurements can be obtained using calibrants. A specialised and expensive type of magnetic sector instrument uses multiple detectors (collectors) to achieve very high precision isotope ratio measurements for applications such as geological aging.
Sample preparation
A typical ICP-MS application involves preparation of a solution for introduction to the nebuliser although, as mentioned above, many other types of application have been described including use of gaseous samples, slurries, laser ablation of solids, and use of ICP-MS as a chromatographic detector. In the simplest case, preparation of the solution involves no more than dilution or dissolution of the sample. Many samples, however, require a ‘sample digestion’ process to obtain the required solution. Key aspects of this process are removal of high solid (salt) content or organic matter from the sample. High solid content produces rapid deterioration of the cones whilst, for the latter, the plasma is not tolerant of high carbon content at typical operating powers. It is customary to use an internal standard, particularly for quantitative applications and where the samples and calibration standards have significantly different compositions. The internal standard is an element with similar behaviour to the analyte(s) which is added to all solutions prior to nebulisation into the plasma. It is used as a reference to facilitate electronic offset of changes arising from sample transport effects, nebulisation efficiency and long-term drift in the sensitivity or baseline of the spectrum. One or more internal standards may be required to cover the mass range of interest, typical elements being Ga, Ge, In, Rh and Tl.Control of interferences
ICP-MS produces spectra which are relatively simple compared with optical emission spectrometry because of the isotopes of the common elements have a small number of peaks compared with the enormous number of spectral lines in an emission spectrum. Hence only a few ‘isobaric’ interferences arise from direct overlap between the isotopes of two elements (e.g.114Cd overlaps 114Sn). However, the plasma also produces polyatomic ions, which are derived from the argon, the sample matrix or the sample solvent, and some doubly charged ions. A number of common isobaric interferences arise from overlap of the analytical isotope of interest with ions produced by both these mechanisms (e.g.56Fe with 40Ca16O and 68Zn+ with 136Ba2+). In many cases, such interferences can be overcome by selection of an alternative isotope of the analyte, although this is likely to be one with lower abundance and result in reduced sensitivity. It is also possible to remove or avoid a number of common polyatomic species by careful selection of the plasma operating conditions or by appropriate pre-treatment of the sample. The latter approach includes chemical separation of the sample matrix or interfering species and techniques such as ion chromatography or flow injection analysis. A facility is provided with the software of most spectrometers to automate interference corrections, particularly for rapid, semi-quantitative multi-element analysis. The correction software uses empirical correction factors and an iterative procedure to estimate an apparent concentration for each analyte of interest arising from known interfering species. The corrections are based on an estimate of the concentration of each interfering species.There are also two common instrumental approaches to control isobaric interferences. The magnetic sector mass spectrometer has the potential for significantly higher mass resolution than the quadrupole, offering the potential to resolve the interfering species. However, this adds considerably to instrumental cost and frequently involves a trade-off between resolution and sensitivity. A more recent approach to the removal of polyatomic interferences is the collision or reaction cell which is available as an option with most current quadrupole instruments. The ion beam is passed through the cell prior to entering the quadrupole and encounters gas molecules which selectively remove a single interfering species. The gas may be reactive (e.g. hydrogen or oxygen), inert (e.g. helium or xenon) or a mixture of the two. The type and amount of each gas is critical and conditions must be carefully established for each application, particularly for multi-element analysis (for which a helium-hydrogen mixture is frequently recommended).
Range of applications
Typical ICP-MS instrumentation allows determination of elements with atomic mass in the range 7 to 250 which includes all elements from Li to U. This can be achieved over a concentration range which may extend across seven or eight orders of magnitude, i.e. a range from nanograms to tens or hundreds of milligrams per litre. Coupled with the multi-element capability, this has encouraged adoption of the technique in virtually every application area including medical, forensic, toxicology, environmental, food, agriculture and industry. In some applications a semi-quantitative ‘fingerprint’ including many elements and/or individual isotopes may serve the required purpose. At the other extreme, the technique may be used with IDMS calibration to achieve a highly accurate analysis of a trace constituent of a complex sample matrix.Recent developments in the biological field have seen rapid expansion of ICP-MS coupled with gas or liquid chromatography for speciation of organo-metallic compounds, both simple molecules and large species such as proteins. The rapid growth in biological applications has also stimulated interest in less easily ionised elements (e.g. P, S, Se). Greatly improved sensitivity can be achieved for such elements by adding organic modifiers to improve their ionisation. This can be achieved by adding small amounts of an organic solvent to solutions or by introduction of a gas such as methane into the ICP.
Comparison with other techniques
Many different techniques have been applied to elemental analysis but those in most current use are atomic absorption (AAS), ICP atomic emission (ICP-AES) and X-ray fluorescence (XRF) spectrometry. The critical aspects of these techniques are compared in the table below.A schematic representation of a typical ICP-MS instrument is shown below. Further information on the instrumentation and application of ICP-MS can be found in the literature (1,2).
| Technique | Scope | Detection limit range* | Advantages | Disadvantages |
|---|---|---|---|---|
| * Detection limits are values in solution: fg L−1 = 1 part in 1012, ng L−1 = 1 part in 109, μg L−1 = 1 part in 106. | ||||
| ICP-MS | The majority of elements and their isotopes | fg L−1 and below | Rapid, very sensitive, multi-element and multi-isotope measurements, with wide dynamic range, and relatively few interferences. Flexible sample introduction, e.g. laser ablation. | Limited tolerance of dissolved solids and organic matter. Relatively expensive with high running costs. Most applications require sample dissolution. |
| ICP-OES | Most metals and some non-metals | ng L−1 to μg L−1 | Rapid, multi-element, tolerates high solids. Relatively low cost instruments available. | Insufficiently sensitive for ultra-trace analysis, numerous potential spectral interferences. High running costs. Most applications require sample dissolution. |
| AAS | Most metals | ng L−1 to μg L−1 | Sensitive, few spectral interferences. Low cost instruments with relatively low running costs. | Single element, slow, limited dynamic range. Most applications require sample dissolution. |
| XRF | The majority of elements | μg L−1 and above | Rapid analysis of solid samples with minimum preparation. Low running costs. | Matrix effects necessitate matched calibrants or correction algorithms. |
References
- Handbook of ICP-MS, ed. K. E. Jarvis, A. L. Gray and R. S. Houk, Viridian Publishing, Woking, 2003, 380 pp Search PubMed.
- ICP Mass Spectrometry Handbook, ed. S. M. Nelms, Blackwell Publishing, 2005 Search PubMed.
| Manufacturer/model number: | |||
|---|---|---|---|
| Feature | Definition and/or test procedures and guidance for assessment | Importance | Reason |
| Non-instrumental criteria | |||
| Laboratories in possession of other spectrometers should score highest for the manufacturer with the best past record based on the following sub-features: | |||
| (a) Previous instruments | |||
| (i) Innovation | Company's record for instruments with innovative features. | I | The manufacturer should be aware of new techniques in ICP-MS. |
| (ii) Reliability record | Company's record for instrument reliability. | I | Reflects the company's ability to employ good design and manufacturing practices. |
| (iii) Similarity of operation, layout & design (including software) to existing instruments in the laboratory | For routine purposes, this may be important. However, this may be less important for research applications | I | Similarity of design and operation means that operators can draw on in-house expertise, resulting in reduced costs and time for training. It may also maximise the use of spares and fittings. |
| (iv) Confidence in the supplier | Confidence gained from past personal experience. | I | The benefits arising from good working relationship already in place. |
| (b) Servicing | Score according to manufacturer's claims and past record, judged by the sub-features (i) to (iii) below: | ||
| (i) Service support | Availability of suitable service support from the supplier, agent or third party contractor. | I/NVI | Essential to ensure reliable operation over the planned working life of the instrument. Often available as trouble-shooting self-help documentation and off-site repair. |
| (ii) Availability, cost and delivery of spares | Range of stock carried by, or quickly available to, the manufacturer/agent/ contractor. | VI | Rapid delivery of spares reduces down time. |
| (iii) Effectiveness of service support | The ability of the supplier to identify and repair faults as judged from previous experience and reports of others. | VI | A rapid response reduces down time. Note that the guaranteed response may depend on the proximity of the service centre for off-site repairs and that travel/transport costs may be a factor. |
| (c) Technical support | Score according to manufacturers’ claims and past record, judged by the sub-features (i) to (vi) below: | VI (for new users)/I | Rapidly available technical help reduces errors and enhances productivity. |
| (i) Applications department | The advice and training available from the manufacturers’ applications department. | This helps in-house staff to optimise measurements for new applications. | |
| (ii) Technical literature | The range and quality of technical literature, including the operating manual. | The availability of quality technical literature helps operators optimise the use of the instrument. | |
| (iii) Telephone and internet assistance | Willingness of the manufacturer/ supplier/contractor to give advice over the telephone or internet. This can normally be evaluated by reference to existing users. | I | Rapidly available technical help reduces the number of call outs and enhances productivity. |
| (iv) Training | This includes initial training when setting up the instrumentation and subsequent support. | I | A comprehensive training scheme will ensure that operators and instrumentation are working effectively. |
| (v) User group meetings & specialist conferences | Meetings, conferences and technical briefings organised for users of instrumentation by the manufacturer or third party. | Other users are often the best source of advice on problems, solutions and applications. | |
| Instrumental criteria | |||
|---|---|---|---|
| 1. High frequency (HF) generators | Device for generating power at typically 27MHz frequency. Modern instruments use solid state generators although older instruments may use other devices. | A high frequency source is required to provide power for the inductively coupled plasma. | |
| (a) Type of oscillator | The oscillators differ mainly in the method which is used to control the frequency of oscillation and are variously known as free running, crystal-controlled, Huth–Kuhn and tuned-line oscillators. Scoring may be inappropriate (see reason). | The primary requirement of the generator is stability (see below). There is no evidence to show that any one type of generator offers superior performance for ICP-MS applications. This conclusion pre-supposes that any generator in question meets the requirements for stability demanded by the user, and complies with local and national regulations for HF shielding and filtering. Modern generators based on solid state devices have advantages in terms of physical size when compared to their crystal-controlled predecessors. | |
| (b) Radiation shielding | All oscillators and torch boxes must be screened to prevent or minimise HF leakage and such screening must be guaranteed to comply with the regulations of the country of operation. Scoring may be inappropriate. | VI | There are strict regulations with regard to the amount of energy of specified frequencies that may be radiated from an oscillator. Stray radiation is a health hazard and interferes with telecommunications. It may also affect the instrument's detector electronics and hence the signal output. |
| (c) HF filtering | There should be adequate filtering of HF signals on the power lines of the generator to prevent them from coupling with the power lines of any detection electronics. (There should be similar filters on the power lines of detector electronics.) | VI | If any HF leakage occurs within the generator unit, the power and earth lines can conduct HF signals. These signals can then couple with the detector electronics and affect the background noise. This, in turn, will affect the limits of detection attainable. Leakage can readily couple with other local instrumentation and can modulate the signal, affecting performance of the instrument. |
| (d) Frequency of operation | The usual frequency of operation of generators for ICP-MS instrumentation is 27 MHz, but some older instruments operate at higher frequencies. Scoring may be inappropriate. There is some evidence to suggest that lower frequency operation may offer some advantages in ICP-MS. Depending on regulatory requirements, permissible operating frequencies may be limited to specific bands. | NVI | In an ICP, the eddy currents induced by the magnetic field flow more closely to the outer portions of the plasma, known as the skin depth. This is defined as the depth at which the inductive current is 1/e of the surface value (e is the base of natural logarithms) and is inversely proportional to the square root of the frequency. The higher the frequency, the smaller the skin depth and consequently there is a decrease in the transfer of energy towards the central channel of the annular plasma, which results in a lower temperature and a lower electron number density. Therefore the background emission will decrease, which is advantageous in emission as the limits of detection are usually improved, but is a disadvantage in ICP-MS as the dissociation of analyte oxide species is more difficult at low temperatures. |
| (e) Power available | The power in kilowatts which can be developed in a plasma by the generator. Score according to application, bearing in mind that a high power generator may offer more flexibility if it can also be run at low power. | I | The power required will depend on the application. All commercial systems use a Fassel type torch and normally operate at around 1300 W, although higher powers of up to 1800 W may be required in the analysis of organic solvents to ensure adequate breakdown of solvent molecules. |
| (f) Selection & indication of power settings | The power developed in the plasma should be indicated by a meter or calibrated control. According to application, score maximum for the system which gives the most accurate and complete information. Score additionally for reproducibility and the ability to select several power settings. | I | For comparison purposes in research work, as well as for method development, it is important to know the power developed in the plasma. For routine use, it is sufficient to know the power developed at the work coil and to set this reproducibly. The impedance of the circuitry must be tuned to maximise power input to the plasma. Most generators have a reflected power meter, which gives the power into the impedance matched circuit. |
| (g) Coupling efficiency | The fraction of power supplied to the coil which is transferred to the plasma. The power in the plasma can be determined directly by calorimetric measurement or indirectly by use of a dummy load and calorimetry. Scoring may be inappropriate because this parameter is not easily measured by the purchaser. | I | For maximum efficiency, it is desirable to transfer the maximum amount of power available to the plasma. |
| (h) Generator stability | The degree to which the power in the plasma varies from a set value. Score maximum for the highest degree of stability for a given mains variation. This figure should always be given by the manufacturer. | VI | Fluctuations are brought about by variations in line voltage and some form of feedback control should be incorporated in the generator. The number of ions produced is strongly dependent on the power delivered to the plasma. Short term fluctuations in power are, therefore, highly undesirable. |
| (i) Cooling | In most, if not all, plasma generators, the work coil is water cooled. Score maximum for the system that requires the lowest flow and pressure of water and calls for the least treatment of the cooling water. Most generators are air, rather than water, cooled. In such instances, score maximum for the generator which achieves cooling with the lowest heat dissipation requirement. | I | Environmental requirements and the need for high pressure limit the use of mains water cooling. Recirculating water coolers may be expensive. Instruments designed with air cooling generators that have high power requirements for cooling, may be noisy and uneconomical. |
| 2. Torch boxes | |||
| (a) Ease of access | Score according to the ease with which torches can be removed and refitted to the box and the ease with which load coils can be removed and refitted. | VI | Breakage of torches can occur if it is difficult to remove or refit them. Water leakage resulting from difficulty in tightening connections on work coils can cause damage. |
| (b) Ease of observation | It is desirable to be able to observe the plasma through an observation window which must be screened to suppress HF leakage and equipped with UV filters. Score according to the degree of convenience offered. | I | This facility allows observation of any malfunction of the plasma torch, which can cause damage and will lead to unsatisfactory performance. |
| (c) Sampling depth | The distance between the load coil and the sampling cone. It is essential to be able to set the sampling depth in the plasma reproducibly. Score according to the ease with which this adjustment can be made. | There is a significant spatial variation in the density of ions in the plasma. Incorrect positioning results in reduced sensitivity and increased interferences from refractory oxides and M(2+) species. | |
| (d) Mounting of the plasma torch and alignment | Score according to the ease with which the plasma torch can be held in position centrally within the work coil of the generator. Mounting devices with orthogonal adjustment consistent with accurate positioning are preferable. Many instruments use automated, computer controlled alignment of the torch assembly. Score additionally for this feature. | It is essential to ensure that the torch tube is centrally placed in the coil if a good, well positioned plasma is to be obtained. Off-centre torches will cause off-axis plasmas and consequent melting of the torch tube. The region of maximum ion density in the plasma must be accurately aligned in relation to the sampling cone to maximise the analytical signal and minimise interfering signals If the mounting device has many degrees of movement, it becomes very difficult to ensure that off-axis sampling does not occur. Most instruments in current production use a similar type of torch. However, other torches may have advantages in some circumstances and a mounting that permits their use is advantageous. Automatic alignment when a torch is replaced after cleaning reduces down time and aids optimum performance. | |
| (e) Exhaust systems | It is necessary for the torch box to be designed to allow exhaust gases to be removed in a manner that will not disturb the plasma. Tests for gas flow and the presence of toxic substances should be augmented by visual observation of the plasma when the exhaust is turned on. Score maximum for the minimum observed movement when the extraction system is switched on and operating. | VI | Hot and corrosive gases and toxic elements must be removed from the laboratory atmosphere; at the same time disturbance of the plasma should be avoided if high precision is to be maintained. This usually necessitates installation of a separate laboratory extraction system. |
| (f) Interlocks | It must not be possible to gain access to the torch box while the HF current is switched on, or to be able to switch on the HF unless the water and gas supplies are on. The HF must also switch off automatically in the event of the plasma being extinguished. Score zero if this interlock is not completely effective. | VI | The voltages and currents involved in plasma production are dangerous! These interlocks also prevent accidental damage to the torch and load coil in the event of failure of one of the supplies. Working interlocks are essential to ensure safe operation of the instrument. |
| 3. Gas supplies | |||
| (a) Flow control | A constant mass flow of, in particular, the nebuliser gas and, to a lesser degree, the coolant (outer or plasma) gas is highly desirable. Score highest for systems using electronic mass-flow controllers, less for spring-loaded mass flow controllers and less still for systems using restrictors and up-stream pressure gauges. There should be no interaction between the gas flows where supplies are taken from a common main. Score additionally for the accuracy of calibration. | The distribution of ions in the plasma is very dependent on gas flows, particularly the nebuliser gas flow. It is, therefore, essential that these gas flows are accurately controlled. Any fluctuation in the nebuliser gas flow will affect the precision with which the analytical signal can be measured. Any interactions caused by taking a relatively high gas flow for the coolant supply from the same source as the much lower nebuliser supply must be avoided. | |
| (b) Use of additional gases | The facility to add other gases to the argon supply may be useful in some applications. Score maximum for the facility to use the widest choice of gas mixtures and for ease of changeover. | I | Alternative gas mixtures offer advantages in some applications in the avoidance of spectral interferences. |
| 4. Sample introduction | Various sample introduction devices are available for conveying the sample to be analysed into the plasma. Brief details of the more important of these devices are listed below for guidance. Score maximum for the instrument that offers the greatest choice of such devices, taking into account the intended application. Scoring of individual devices (summarised below) is inappropriate as performance depends on application. The key issues are uptake rate, tolerance to salt content, sensitivity, robustness, construction material and price. | VI | Different sample introduction devices offer particular advantages, depending on the nature of the sample and the intended applications (see below). |
| (i) Liquid samples | |||
| (a) Nebulisers | Devices for converting a sample solution into an aerosol, which is then swept into the plasma by the nebuliser gas flow. The assembly normally comprises the nebuliser itself to create the aerosol coupled to a spray chamber to remove all but the smallest aerosol droplets. In some designs the solution supply to the nebuliser is regulated by a peristaltic pump, which must be used in the analysis of viscous solutions and can offer advantages in operating the nebuliser at a lower solution uptake rate. Many different nebuliser designs have been used with the ICP; those in most common use are described below: | Nebulisation is the usual way of introducing a sample solution into the plasma and is the standard sample introduction device fitted to ICP-MS instruments. Many designs of nebuliser and spray chamber are available, as outlined below. Low uptake rates are advantageous where sample volume is limited or where solutions with high matrix content are used. | |
| The peristaltic pump should be designed to minimise pulsing of the sample uptake, e.g. by incorporating multiple rollers. | |||
| Concentric nebuliser | Device utilising a micro-bore tube for liquid transport which interacts with an argon gas stream to create an aerosol. Construction materials include glass, quartz and plastics. A widely use plastic is PFA (perfluoroalkoxy fluorocarbon) which has good chemical resistance including HF and also resists deformation under stress. Uptake rates vary from tens of μL to a few mL per minute. They can be operated with unassisted aspiration or using a peristaltic pump. | Construction material may dictate how robust the nebuliser is in routine use. Uptake rates are fixed for any individual nebuliser. Some designs are particularly resistant to salting up (crystallisation of salt on the nebuliser tip) or to resisting blockage from particulate material. | |
| Cross-flow nebuliser | Device utilising a fine capillary metal tube for liquid sample introduction fixed at right angles to the incoming gas stream. Construction materials include PFA with rhodium capillary tubes. They can be operated with unassisted aspiration or using a peristaltic pump. | A robust design for routine analysis operating at uptake rates of 0.5–1.5 mL min−1. Acid resistant. Suitable for a wide range of sample types. | |
| Babbington or V-groove nebuliser | Device utilising a solid block with a groove cut into the front face. Solution passes through a large bore tube into the groove and across the gas stream to produce an aerosol. Construction materials include PFA. Must be operated with a peristaltic pump. | A very robust design. Highly acid resistant (can be used with HF acid). High uptake rates >1 mL min−1. Suitable for liquids containing particulate material and for introduction of slurries as well as for dilute solutions. | |
| Ultrasonic nebulisers | A device that substantially increases the efficiency of solution nebulisation by incorporating an ultrasonic frequency oscillator as the nebulisation medium. The high moisture content of the aerosol must be minimised using a desolvation unit (normally using either membrane separation or cryogenic cooling), otherwise the low power plasma used in ICP-MS will be extinguished by the high load of solvent that is injected. | Ultrasonic nebulisation increases the nebulisation efficiency to an extent that, in many cases, the limits of detection obtainable are improved by an order of magnitude over those obtainable by most common nebulisers. This device has the additional advantage that the aerosol production is independent of the carrier gas flow rate. It will not handle particulates and, unless removed, the high water loading will take power from the plasma and affect such properties as electron densities, ionisation and excitation temperatures. Also, a high water content, because of its oxygen content, leads to oxide formation. | |
| (b) Flow injection | A facility which is normally used in conjunction with a solution nebuliser in which the sample solution is injected into the blank solution supplied to the nebuliser for a brief interval before reverting back to blank solution. Data acquisition must be capable of quantifying the transient analytical signal observed during flow injection. | Flow injection offers the particular advantage of allowing the direct analysis of solutions of relatively high solids content, without preliminary dilution. The technique is particularly useful where pre-concentration or matrix modification is desirable as these can be carried out on-line. | |
| (c) Electro-thermal vaporisation | Electrothermal vaporisation involves heating a sample, normally injected onto a graphite rod, tantalum filament or into a graphite tube, using an electronic controller which permits the temperature to be increased in a programmed way in a similar manner to that used in graphite furnace atomic absorption spectrophotometry. In this way, samples may be dried, ashed and then volatilized with the volatilized fraction swept by carrier gas into the plasma. Data acquisition must be capable of quantifying a transient analytical signal observed during atomisation. | Electrothermal vaporisation offers some advantages including: (i) the highly efficient transfer of sample to plasma giving improved sensitivities; (ii) the capability of analysing very small sample volumes (down to a few μL); (iii) analysis of sample solutions containing complex matrices where the drying and ashing programmes can permit the elimination of specific interferences before measurement of the analyte signal during atomisation. The rapid release of hot gas from the volatilisation device can cause some disturbance of the plasma but can be minimised with a suitable interface. | |
| (d) Hydride generation | An accessory which incorporates a reaction vessel in which the sample solution is reacted with a reagent such as sodium borohydride causing selected analytes to form volatile hydrides which are swept into the plasma for analysis. | Hydride generation offers the advantage of significantly increasing the sensitivity and simultaneously reducing interference effects (in comparison with normal solution nebulisation) for elements which react to form volatile hydrides, including As, Bi, Cd, Ge, Pb, Se, Sb, Sn, Te. Other elements, including Hg, Os and I can be determined by similar ‘cold vapour’ generating techniques. | |
| (e) Spray chamber | Device used to remove large droplets from the nebulised aerosol and to reduce the amount of solvent in the plasma. The spray chamber is thermostatically controlled to maintain a constant and often, low temperature. Score highest for the ability to accurately control spray chamber temperature from −4° to 20 °C. This may be achieved via a water jacket surrounding the inner chamber space or by the use of a peltier cooler. | Maintaining a controlled, optimal spray chamber temperature minimises signal drift and reduces the solvent in the plasma. This reduces the magnitude of polyatomic ion interferences which form due to the presence of excessive H and O. | |
| Score additionally for designs which minimise wash-out times or, if relevant, are available in materials other than glass. | Spray chamber design can severely affect washout times, that is, the time taken to remove remnant signal from the previously analysed sample. More complex internal designs which most efficiently condense out or trap large water droplets may take longer to wash-out than more simple designs. | ||
| Short wash-out times are particularly important for very low level determinations where background contamination can cause problems. Instruments that are more capable of cleaning residues from previous samples are better suited for use with batches of samples that include some low level and some high level analyses. | |||
| The material used for the spray chamber may need to be chosen to ensure compatibility with samples and solvents (e.g. glass should not be used if hydrofluoric acid is present in the nebulised solutions). | |||
| (ii) Solid samples | Methods which involve the direct analysis of solid samples are useful but, depending on the nature of the material, methods are more likely to be imprecise. Also, because of inhomogeneity effects the small portions taken for analysis may not be representative of the bulk material. The availability of matrix-matched calibration samples may be restricted. | Direct solids analysis offers the advantages of reduced sample preparation, particularly dissolution, minimization of contamination effects (e.g. from reagents) and the capability of analysing very small sample masses. | |
| (a) Laser ablation | A facility for the analysis of solid samples in which a selected area on the surface of the sample is ablated by the energy imparted by a laser. The ablated material is then swept into the plasma by a carrier gas. | Laser ablation offers the advantage of the direct analysis of solid samples. By use of a focused laser, spatially resolved information can be obtained of element distributions over the surface of the sample. Typical resolution is of the order of 20 μm. In general, sensitivity follows an inverse relationship with the amount of material ablated, so that the smaller the laser beam, the worse the detection limits that can be achieved. Quantitative analysis can usually be achieved only if matrix-matched calibration samples are available and an analyte of known concentration is present for use as an internal standard. | |
| These facilities are usually sold as separate accessories suitable for use with an ICP-MS or other instrument types and may be purchased separately from another supplier. | |||
| (b) Electro-thermal vaporisation | Similar electro-thermal vaporisation instrumentation to that described for the introduction of liquids (see above) can be used to introduce solids into the plasma. | Selective volatilisation is a problem for electrothermal vaporisation of solids, making accurate measurements difficult. | |
| (c) Slurry nebulisation | The introduction of finely powdered material suspended in liquid media using a high solids nebuliser such as the V-groove type (see section above). | Aqueous calibrants can be used if particles are small. However, it is not a general solution to the problem of solids introduction. The method may be prone to problems of density settling and consequent segregation, and the density of the liquid should be near that of the solid. In short, the particles should be very fine and uniform in size and the material under investigation should be homogeneous. Given these requirements, the physical preparation of samples (e.g. grinding to an adequate particle size) may not offer many advantages in comparison with dissolution procedures. | |
| 5. Interface | Part of instrument between plasma (operated at atmospheric pressure) and mass spectrometer (operated at high vacuum). The interface section comprises differentially pumped stages through which plasma gases are transmitted to the mass spectrometer via sampling and skimmer orifices. All instruments are fitted with this device. | The interface is the key component of ICP-MS instrumentation because it must enable the transfer of ions from a hot, atmospheric pressure plasma to a cool, high vacuum system without loss or contamination. The design and manufacturing tolerances have a critical influence on the analytical performance of the instrument. | |
| (a) Choice of material for sampling and skimmer cones | Different materials (usually copper or nickel) are used, sometimes with a Pt insert. Score maximum for the most appropriate range of material, according to the application. | I | The material used for the manufacture of the sampling cone will contribute to the background spectrum. If nickel cones are used, a platinum insert is required if low levels of Ni are to be determined. |
| (b) Ease of exchange of sampling and skimmer cones | Sampling and skimmer cones must be regularly cleaned and occasionally replaced. Score highly for a system which allows for easy removal and refitting of the cones. | VI | The cones must be removed for regular cleaning to remove build-up of material at the orifices. Erosion of the sampling orifice also necessitates removal for replacement. |
| (c) Cost of cones | Sampling cone and skimmer degrade with use and may need to be replaced. Score maximum for the availability of appropriate quality cones at the lowest cost. | I | The cost of replacing cones may account for a significant part of the instrument's consumables budget. |
| (d) Ion extraction lenses | The lenses which are positioned closest to the back of the skimmer are largely used to extract positively charged ions from the ion bean. Score maximum for easy accessibility to the ion lens assemblies, robustness of construction and for ease of cleaning. | I | These components require regular cleaning to avoid deterioration in the performance of the instrument and attention should be given to the ease of removal and replacement. |
| (e) Collision or reaction cells | An optional device used to reduce/remove isobaric interference and/or enhance sensitivity. One or more gases added to the cell and interact with the ions before they enter the quadrupole. Gases may be inert (eg He, Xe) or reactive (e.g. H or methane). Score higher for good gas flow control and facility to introduce mixtures. | These components may reduce sensitivity or enhance it. Choice of type and amount of gas is critical, as is stable flow control. Requires careful optimisation for each application. | |
| 6. Vacuum system | Older instruments used rotary pumps, either alone or in combination with an oil diffusion pump for regions requiring the highest vacuum. Most modern instruments are supplied with a combination of rotary and turbo molecular pumps. Score maximum for the fastest pump-down time. Score additionally for minimum water consumption (if water is required for cooling) and for vacuum pumps that require minimum maintenance and have lower replacement costs. | I | Rapid pump-down times reduce instrument down time after routine maintenance. Turbo-molecular pumps, which may be air- or water-cooled, require minimal maintenance because, unlike rotary or oil diffusion pumps, routine changes of contaminated oil are unnecessary. Using a rotary pump as a “roughing pump” prior to a turbo molecular pump is an optimal combination to achieve rapid pump-down and high vacuum. |
| 7. Ion detector | Device used to collect and measure the number of ions emerging from the analyser. | ||
| (a) Type of detector | Three types are currently fitted to commercial instruments. In some cases, a choice is available either at the time of purchase, or subsequent to installation. Score highest for widest choice of detector types. | Characteristics of each detector type are different, particularly with respect to sensitivity and lifetime. | |
| (i) Continuous dynode electron multiplier | Measures the number of ions by conversion to electrons, which are multiplied by interaction with a continuous dynode. Can be vented to air without serious damage, although this action is likely to reduce the lifetime. | I | This type of detector has a limited lifetime which is related to the total accumulated charge. The life-expired detector cannot normally be repaired. Cost is lower than the discrete dynode detector described below. |
| (ii) Discrete dynode electron multiplier | Measures the number of ions by conversion to electrons, which are multiplied by interaction with a series of discrete dynodes in a manner similar to a photomultiplier tube. Excellent tolerance to venting to air. | This type of detector has a long lifetime and individual dynodes can be replaced. The initial cost is higher than the continuous dynode type but they are widely used in quadrupole spectrometers. | |
| (iii) Faraday cup | Measures the number of ions by collection in a cup-shaped metal electrode which has no intrinsic gain mechanism. The Faraday cup has a long life and rarely requires replacement in normal use. | This type of detector has a relatively low sensitivity and is usually used to extend the upper dynamic range of detection. Faraday cup detectors are not suitable for fast scan rates owing to the time taken to respond. Hence they are rarely used in quadrupole spectrometers. | |
| (b) Detector lifetime | Score highest for the least sensitivity to deterioration after atmospheric exposure and to the total accumulated count rate history. | I | All detectors age with use, and they suffer varying amounts of damage when exposed to either the atmosphere during routine maintenance or to high ion count rate usage. |
| (c) Cost of replacement | Replacement costs depend on a combination of the capital cost of a replacement detector and its expected operational life. These factors vary according to the choice of detector, which must be appropriate to the type of application. Score according to the anticipated running cost. | I | The replacement cost of detectors can make a significant contribution to the running costs of an instrument. |
| (d) Detector characteristics | The choice of detector (type and/or supplier) depends on identifying a suitable compromise between several different characteristics. | ||
| (i) Linear dynamic range | Score maximum for the detection system (i.e., detector and electronics) offering the widest linear dynamic range. | I | Permits simultaneous measurement of the widest concentration range for analytes of interest. |
| (ii) Dead time | Score maximum for the detection system with the shortest dead time (in the case of electron multiplier detectors) or the shortest time constant (in the case of Faraday cup detectors). | I | For any ion counting system, the detection system requires a finite time to measure and record an ion. During this dead time subsequent ions cannot be detected, leading to dead time losses. All instruments employing electron multiplier detectors incorporate a numeric correction to account for these dead time losses. However, the shorter the dead time of the counting system, the higher the count rate that can be detected before the response of the counting system become saturated and uncertainties in the dead time corrected data become excessive. For Faraday cup detectors, the response time will depend on the time constants used in the ion current measurement circuits. |
| (iii) Detector background noise | Score maximum for the detection system offering the lowest figure across the mass range. | I | All noise decreases the signal-to- noise ratio and, therefore, degrades the detection limit. |
| (iv) Detector overload protection | Score zero if this feature is absent from electron multiplier detectors. | I | Overload of the detector and associated electronic circuits by exposure to an excessively high ion count rate can reduce the operating life of some detector types, particularly electron multiplier detectors. Furthermore, in these circumstances, the detector may take some time to recover its normal operating characteristics. |
| (v) Operation at reduced sensitivity | Reduced sensitivity may be achieved by operating electron multiplier detectors in analogue mode, switching to a Faraday cup detector or reducing the ion transmission characteristics of the ion extraction lenses. Score zero if this facility is absent. | I | This facility extends the linear dynamic range of the instrument, allowing analytes at higher concentrations to be measured. |
| 8. Instrument control and monitoring | |||
| (a) Instrument functions | Many manual controls on earlier instruments have now been replaced by computerised control systems. In addition, essential safety interlocks can be monitored by the computer. Score highest for comprehensive computer automation with ease of use, clarity of operation and quality of display. | Computer automation optimisation and control of instrument functions allows faster, easier and more reproducible operation with extensive failsafe safety interlocks. | |
| (b) On-line diagnostics | Capability of the instrument control computer to monitor the operating status of the instrument and to log faults and deviations from operating specification. Score for the availability of a user-friendly diagnostic information. Score additionally for the ability for remote connection of the instrument to the support service, to enable remote interrogation of instrument faults to be carried out. | I | Downtime is kept to a minimum if faults can be diagnosed quickly. The availability of reliable diagnostic information may make an engineer's visit more effective or even unnecessary. |
| 9. Data acquisition | |||
| (a) Scan rate of the mass spectrometer | Atomic mass units (amu) per second. Score highest for instruments with the highest scan rate applicable over the entire mass range (6–240 m/z). | VI | Transient signals from electrothermal vaporisation or coupled chromatography systems require fast scanning. Fast scan rates generally improve precision in isotope ratio determinations where data are accumulated by repetitively scanning over a restricted region of the mass spectrum. |
| (b) Peak jumping and single ion monitoring | The capability to acquire data by repetitively monitoring a single peak or a number of mass peaks selected by the operator anywhere within the instrument's mass response range. Intermediate regions of the mass spectrum are scanned at maximum rate without acquiring data. Score zero if this feature is absent. | VI | Operation in this mode maximises the rate of data acquisition on mass peak(s) of interest, and prevents detector overload and ageing by avoiding regions of the mass spectrum containing intense mass peaks of no interest. Single ion monitoring is widely used for quantitative analysis of a specific analyte or when the ICP-MS is coupled to a chromatographic system. |
| (c) Peak measurement | The ability to select the number of data points per peak for integration, the dwell time per peak and the number of repetitions which are made. | VI | The flexibility to change these parameters allows the operator to optimise sensitivity, counting times and total analysis time. |
| 10. Data correction and manipulation | Ability to process raw data and implement quality control procedures | ||
| (a) Data processing | Facilities available should include recalibration, use of internal standards, external drift correction and interference correction. Score maximum if all are present. | VI | These facilities are required to allow appropriate correction to the raw analytical signal when necessary. e.g., for drift in instrument response, suppression of enhancement caused by sample matrix effects, and spectrum overlap interference. |
| (b) Isotope ratio measurement | The ability to measure the abundance of one isotope relative to another isotope of either the same, or a different element. Score for the availability of this feature. If relevant to the type of instrument and application, score additionally for the availability of specialised software that allows data to be acquired and processed to maximise precision. | I/VI | In some applications, isotope ratios must be measured rather than elemental abundances. Quadrupole instruments acquire data in a sequential manner and can achieve precisions of typically 0.1% RSD. If more precise measurements are required (e.g. 0.001% RSD), so that small differences in isotopic composition can be distinguished with confidence measurements must be made on a magnetic sector instrument fitted with multiple collectors. |
| (c) Reporting formats | Choice of formats for the output of raw data and/or analytical results. Typical format requirements include printing of reports for specific requirements and electronic transfer of data in a form compatible with other electronic systems (in the laboratory or directly to a customer). Score for a choice of output formats that suit the application. Score additionally for the option for the user to design customised formats. | I | Specific reporting formats may be required to satisfy existing quality or customer systems. Further manipulation of data may require formatting output so that it can be read by third party software. Files using standard data formats such as ASCII or XML are particularly useful. |
| (d) Operating system | System under which the manufacturer's software operates. Score if the operating system is compatible with software that already exists in the user's laboratory. | I | Raw and processed data files may need to be transferred to other computers for off-line manipulation. Initial training is also simpler if operators are already familiar with the operating system. Previously owned instruments may have older operating systems. |
| 11. Calibration | Data can be quantified using one of several procedures (summarised below). Score zero if the procedure(s) relevant to the proposed application(s) are not readily available with the instrument's regular software. | VI | Different matrices and different analytical problems require different calibration approaches. |
| (a) External calibration | The calibration of an instrument by comparing the response of sample solutions with calibrant solutions of the analytes of interest. | The usual method of calibrating instruments for the analysis of sample solutions without significant matrix issues, e.g. at low total dissolved salts content and low acid concentration. | |
| (b) Standard additions | The calibration of an instrument by comparing the response on sample solutions with that from the same sample solution, which has been ‘spiked’ with a known concentration of a calibrant of the analyte(s) of interest. | Appropriate for the analysis of ‘difficult’ sample solutions that fall outside the above criteria because the sample and spiked solutions have essentially matched matrices. | |
| (c) Isotope dilution | The calibration of an instrument by ‘spiking’ the sample solution with a known mass of an isotopically enriched solution of the element of interest. The ratio of two specified isotopes of the analyte are then measured to very high precision and the concentration calculated from these data and a knowledge of the natural and spike isotopic abundance | Use of isotope dilution is a calibration technique that is the least susceptible to bias. However, measurements are relatively time consuming and cannot be applied to monoisotopic elements. Measurements are often made using ‘reverse’ calibration of the spike against a natural standard of the analyte; this is facilitated by appropriate software. | |
| (d) Surrogate calibration | The capability to calibrate an instrument for the determination of an isotope by external calibration using calibrants that do not contain the analyte of interest. | This calibration procedure can be used when a calibrant of the analyte is not available. Calibration is undertaken by comparison with a calibrant containing (normally) an adjacent element in the mass spectrum, account being taken of differences in natural isotopic composition, and sometimes the mass response of the instrument. | |
| (e) Semi-quantitative analysis | Calibration is achieved by measuring the instrument response from a single multi-element calibrant selected to contain a range of elements that cover the full mass range of interest, though not necessarily containing the specific analytes of interest. | By measuring the instrument response as a function of mass, the concentration of any analyte can be estimated from calibration data obtained with a single calibrant. This allows rapid multi-element analysis of a wide range of samples. Results are semi-quantitative because of differences in the matrix of standard and sample solutions and uncertainties in characterising the mass response function from a limited number of elements. | |
| 12. Overall performance characteristics | Parameters most relevant to making a judgement on the overall analytical suitability of the instrument for the intended purpose, as summarised below. | VI | Users cannot normally test the performance of individual components in isolation from the rest of the instrument and indeed it would not normally be sensible to do this because of the way all components interact with one another. |
| (a) Sensitivity | Counts per unit concentration at a specified mass. The response from a solution containing a range of elements across the mass range should be used to evaluate relative sensitivity. Score maximum for the instrument demonstrating the highest sensitivity, taking into account the proposed application. In multi-element applications, score additionally for a uniformly high sensitivity across the entire mass range. | I | High sensitivity coupled with a low and stable background will result in the lowest detection limit. A uniform mass response is useful for applications requiring multi-element determinations, particularly if semi-quantitative calibration procedures are to be used. |
| (b) Instrument stability | The stability of response over both short term (e.g. 5–10 min) and long term (e.g. 4 h) operation. One way of measuring instrument stability is to make periodic measurements on a single sample solution at suitable intervals throughout the chosen test period (typically 4 h). The sample solution should contain relevant elements covering the mass range of interest. | Any drift in instrument response will degrade the quality of analytical results. Significant drift will necessitate frequent recalibration. | |
| (c) Background signal | Signal resulting from the detection of stray ions and photons. Test by measuring the signal from high purity water, analysed under relevant operating conditions at mass numbers 6–240 m/z. Score highest for the smallest background signal at each mass number. | The magnitude of and variations in the background signal will affect detection limits. | |
| (d) Polyatomic ion interference | The signal from specific molecular species formed from the plasma gas (Ar) and elements entrained either from air, or introduced via the sample solution (e.g. H, C, N, O, S, Cl). An example is 40Ar16O, which causes a background interference on Fe at 56 m/z. Score highest for the smallest signal relative to an adjacent mass peak obtained during a full mass range scan. Some instruments now have special features or devices (e.g. a collision or reaction cell or a shield torch) which selectively reduce certain polyatomic species; however, such features may also reduce the overall instrument sensitivity. Score for the presence of such features only if the application requires the low level determination of analytes that suffer polyatomic interferences which are reduced in severity by the feature. | VI | The presence of polyatomic ions may introduce a bias and/or a degradation in detection limits for analyte peaks that suffer overlap interference. Measurements should be made using operating conditions relevant to the intended applications since the magnitude of this interference effect may change significantly at other operating conditions. |
| (e) Refractory oxide ion interference | The signal from molecular ions formed from a major component of the sample combined with oxygen. An example is 90Zr16O, which causes overlap interference on Pd at 106 m/z. Score highest for the least interference obtained during a full mass range scan under relevant operating conditions, measured using a sample solution containing the refractory element. | VI | The presence of refractory oxide ions may introduce a bias and/or a degradation in detection limits for analyte peaks that suffer overlap interference. The magnitude of this interference may change significantly with changes in instrument operating conditions which can be optimised to reduce the severity of the interference. This type of interference is also influenced by the choice of nebuliser. |
| (f) Doubly charged ion interference | Although most ions formed in the argon plasma are singly charged, those with a second ionisation energy lower than 15.8 eV (the first ionisation energy of argon) will form a proportion of doubly charged species. Doubly charged ions may be detected from elements such as Ba, Ca, Ce, Eu, Gd, La, Sc, Sm, Sr, Ti, Y, Zn, Zr. Score highest for the lowest intensity of doubly charged species measured relative to the singly charged parent for all elements from the above list relevant to the application. | VI | The presence of doubly charged ions may introduce a bias and/or a degradation in detection limits of any analyte species that suffer from partial overlap interference. The magnitude of this interference may change significantly with changes in instrument operating conditions which can be optimised to reduce the severity of the interference. |
| (g) Resolution | The mass peak width at 10% of the peak maximum, which should be measured for a number of elements selected across the mass range of the instrument. On quadrupole instruments the minimum acceptable resolution is one mass unit. Since all instruments are likely to achieve this level of performance, it may be inappropriate to score this feature. | On quadrupole instruments, it is essential that adjacent mass peaks may be distinguished and wing overlaps avoided. | |
| (h) Abundance sensitivity | A parameter that measures the overlap interference from a specific mass peak of high intensity on an adjacent low intensity mass peak 1 amu away. In ICP-MS, abundance sensitivity can be measured as the ratio of concentrations between two adjacent isotopes where no significant interference effects from tailing can be detected. Score highest for the largest figure obtained from solutions of analytes of relevance to the intended application run under routine operating conditions. Note that the magnitude of this parameter will change with instrument operating conditions. | An example of where high abundance sensitivity is important is in the determination of an analyte mass peak situated 1 amu from an intense polyatomic ion interference. High abundance sensitivity is required to minimise interference on the analyte peak. | |
| (i) Matrix effects | Effects derived from the sample matrix that suppress or enhance analyte sensitivity, causing differences in sensitivity when the signals from sample solutions are compared with simple aqueous calibrants. These effects should be absent in dilute solutions and at a minimum in solutions of more complex matrices. Score highest for the least effect in the presence of a matrix typical of the intended application, run under relevant operating conditions. Note that the magnitude of this parameter may change with changes in instrument operating conditions. | VI | Calibration using simple aqueous standards is possible if suppression or enhancement effects are not significant, so avoiding the necessity of preparing matrix-matched calibration samples. |
| (j) Sample signal stability | The change in the signal with time from a sample matrix representative of the application. Score highest for minimum drift. | VI | Drift in the analytical signal from complex matrices can be caused by deposition of un-dissociated sample material in the sampling cone so obstructing the orifice. This drift must be corrected during a run to avoid calibration errors. This test is complementary to the test for instrument stability, which would normally be undertaken using a sample at high dilution, and so not detect drift problems that may occur when real samples are run. |
| 13. Value for money (points per £) | Sum of the allocated scores divided by the purchase price of the instrument. | I | ‘Simple’ instruments are often good value for money, whereas those with unnecessary refinements are often more costly. |
Footnote |
| † Correspondence should be addressed to Dr M. Sargent, LGC. E-mail: E-mail: ms@lgc.co.uk. |
| This journal is © The Royal Society of Chemistry 2010 |