Antibody functionalized interdigitated μ-electrode (IDμE) based impedimetric cortisol biosensor†
Sunil K.
Arya
*a,
Ganna
Chornokur
a,
Manju
Venugopal
b and
Shekhar
Bhansali
*a
aBioMEMS and Microsystems Lab, Department of Electrical Engineering, University of South Florida, 4202 E. Fowler Avenue, ENB 118, Tampa, FL 33620, USA. E-mail: sunilarya333@gmail.com; bhansali@usf.edu
bGuided Therapeutics Inc., 5835 Peachtree Corners East, Suite D, Norcross, GA 30092, USA
First published on 29th June 2010
Abstract
This paper reports on an ultrasensitive, disposable, impedimetric biosensor for cortisol detection. C-Mab (a Cortisol specific monoclonal antibody) was covalently immobilized via amide bond on the surface of the interdigitated μ-electrodes (IDμEs) functionalized with dithiobis(succinimidyl propionate) (DTSP) self-assembled monolayer (SAM). After C-Mab binding, unreacted active groups of DTSP were blocked using ethanolamine (EA) and glycine (Gly) mixture. The disposable sensors were exposed to solutions with different cortisol concentrations and a label-free electrochemical impedance (EIS) technique was used to determine the cortisol concentration. EIS results confirm that the EA-Gly/C-Mab/DTSP/IDμE based biosensor exhibited the sensitivity of 2.855 kohms M−1 and could accurately detect cortisol in the range of 1 pM to 10 nM in saliva. This work establishes the feasibility of using an impedance based biosensor as a disposable cortisol detector, capable of working with complex bodily fluids (e.g., saliva). The architecture enables the use of cortisol sensors at point-of-care.
1. Introduction
Increasing need for a fast, real-time and reliable medical diagnosis has led to growing interest in new point-of-care biological sensors capable of the sensitive and specific detection of biomolecules.1–3 Among the various biosensors available today, electrochemical biosensors, capable of a direct transduction of the biomolecular recognition event into an electronic signal, enable lower detection limits in chemical analysis.4,5 Electrochemical sensors continue to be popular for their low cost, mass fabrication, technical simplicity, fast response, high sensitivity and their ability to be integrated into microsystems and decentralized in-field analysis.6–8 Amongst this class of sensors, the electrochemical impedance spectroscopy (EIS) based biosensors continue to be a method of choice due to their ability of recording the information directly at the electrode surfaces, thus enabling label-free biosensing devices.9–12 EIS is a powerful and sensitive technique that can be utilized for the characterization of surface-modified electrodes and for the investigation of electrochemical processes occurring on electrode surface.10,13 It analyzes the resistive and capacitive properties of materials in response to the periodic small amplitude AC signal over a wide frequency range.9,14 EIS responds to the change caused by binding of analyte to bio-recognition elements immobilized on the surface of the electrodes. Yet another advantage of EIS is that it uses a small perturbation (usually sinusoidal), thus reducing the matrix interferences in analytical systems.10,13,15Interdigitated microelectrode (IDμE)-based EIS sensors possessing a series of parallel micro-band electrodes with alternating micro-bands connected together, offer advantages such as rapid reaction kinetics, improved sensitivity, large electrode aspect ratio (w/l) and increased signal-to-noise ratio.10,16–21 Additionally, IDμE is a two-electrode system and it does not require a third (reference) electrode for EIS response measurement. Furthermore, these systems attain steady-state current response faster than three- or four-electrode systems, enabling easier measurements.22–24
Accurate measurement of cortisol, a stress biomarker, is gaining increasing clinical significance. Increased cortisol levels have been linked to stress-related diseases, including chronic fatigue syndrome (CFS),25 irritable bowel syndrome (IBS)26 and post-traumatic stress disorder (PTSD).27 In addition, cortisol is responsible for maintaining blood pressure, glucose levels, and carbohydrate metabolism within the physiological limits.28,29 In the body, the cortisol serum levels fluctuate between 100 and 500 nM under normal daily conditions, usually reaching the maximum levels in the morning.29,30 Historically cortisol measurements were made in blood serum. It has recently been established that cortisol levels in saliva accurately correlate to cortisol levels in serum, thus enabling the non-invasive estimation of cortisol.31 However, salivatory cortisol levels are nearly two orders of magnitude lower than serum cortisol (maximum (morning) cortisol salivary levels range between 1 and 8 ng/ml in healthy adults (2.78–22.2 nM)),29 requiring a detection technique that can accurately estimate cortisol in the sub-nanomolar or picomolar range.
There are a number of potential techniques that can be used for such cortisol estimation, e.g. high performance liquid chromatography (HPLC),32 fluorometric assay,33 reverse phase chromatography,34 equilibrium dialysis, ultra filtration techniques,35,36 radioimmunoassay (RIA), flow immunoassay and enzyme-linked immunosorbent assay (ELISA).28,37–40 However, these techniques tend to be laborious, lengthy, expensive or involve the use of radioisotopes, making them inappropriate for point-of-care applications. Various biosensors based on surface plasmon resonance (SPR),29,41 electrochemical,2,42 and optical technique28 have been reported recently for cortisol detection. The fabrication of these sensors is a bit involved, and in some cases the analyte needs to be modified which is undesirable.
In this work, we report the successful fabrication of an interdigitated μ-electrode (IDμE)-based ultrasensitive, label-free impedimetric biosensor for cortisol testing. Monoclonal antibody immobilization involves the use of dithiobis(succinimidyl propionate) (DTSP) self-assembled monolayer (SAM) on IDμEs. The sensor was tested with saliva samples and obtained data were compared with ELISA results for the same samples. The novel biosensor enabled cortisol detection down to 1 pM within the 40 min analysis time.
2. Materials and methods
2.1. Chemicals and reagents
Dithiobis(succinimidyl propionate) (DTSP) and sodium borohydride (NaBH4) were purchased from ThermoFisher Scientific. Monoclonal cortisol antibody (anti-cortisol, C-Mab) 2330-4809 was procured from Abd serotec. Phosphate buffered saline (PBS) tablets and hydrocortisone (cortisol) were purchased from Sigma Aldrich. SU-8 resist was purchased from Microchem Corp. All other chemicals were of analytical grade and were used without further purification. PBS solution (10 mM, pH 7.4) was prepared by dissolving 1 tablet in 200 ml of deionized water. Working solutions of hydrocortisone were prepared by dilution in PBS (10 mM, pH 7.4). Deidentified saliva samples were provided by Guided Therapeutics Inc. The results of University of South Florida (USF) measurements were correlated with saliva sample analysis which was performed by the Systems Laboratory (Dr Clemens Kirschbaum) in Dresden, Germany.2.2. Measurement and apparatus
Electrochemical impedance spectroscopy (EIS) was utilized to characterize the EA-Gly/C-Mab/DTSP/IDμE bio-electrodes and to estimate cortisol concentration. EIS measurements were carried out at equilibrium potential without external biasing in the frequency range of 0.5–105 Hz with a 5 mV amplitude using Autolab Potentiostat/Galvanostat (Eco Chemie, Netherlands). EIS measurements were carried out using 65 μl of PBS solution (10 mM, pH 7.4) containing a mixture of 5 mM Fe(CN)64− (ferrocyanide) and 5 mM of Fe(CN)63− (ferricyanide), i.e. 5 mM Fe(CN)63−/4− as a redox probe. Using the redox probe (5 mM Fe(CN)63−/4−), change in charge transfer resistance (Rct) at electrode/electrolyte interface has been investigated in electrochemical impedance.2.3. Test chip fabrication
The IDμE chips were fabricated on an oxidized 4′′ silicon wafer using standard photolithography techniques.2 Briefly, Cr/Au (200/2000 Å) layers deposited using e-beam evaporation and were patterned through liftoff. IDμEs with 5 μm wide electrode fingers at a pitch of 15 μm were used for the experiments. As a final step, SU8 chamber patterned around the electrodes using the SU8 50 to create a sample well around these electrodes (Fig. 1a). SU8 was hard baked at 200 °C to improve its resistance against hard solvents like acetone. | ||
| Fig. 1 Schematic for (a) interdigitated μ-electrodes (IDμEs), (b) optical microscopic image enlarged, and (c) EA-Gly/C-Mab/DTSP/IDμE bio-electrode fabrication. | ||
2.4. SAM preparation and antibody immobilization
The IDμE chips were pre-cleaned with acetone, isopropyl alcohol, and de-ionized water, and exposed to 2 mg ml−1 solution of DTSP in acetone for 1 h for SAM formation. DTSP solution was first reduced using NaBH4 and then dispensed on the pre-cleaned chips at room temperature. The DTSP SAM modified electrodes were then rinsed with acetone to remove any unbound DTSP followed by water rinsing and utilized for antibody immobilization. Cortisol antibodies were covalently attached to the DTSP SAM by incubating the electrode in 65 μl of 1 μg ml−1 antibody in PBS solution (10 mM, pH 7.4), for 1 h. Covalent binding (amide bond formation) results from the facile reaction between the amino group of the antibody and the reactive succinimidyl group of the DTSP on the SAM surface. The sensor (C-Mab/DTSP/IDμE) was washed thoroughly with PBS (10 mM, pH 7.4) to remove any unbound biomolecules followed by a 10 min washing with ethanolamine (EA) (1%) and glycine (Gly) (1%) solution in PBS (v/v 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). EA-Gly solution was used to block unreacted succinimidyl group on the DTSP SAM and to remove extra unbound antibodies on the electrode surface. Fig. 1 schematically illustrates (a) the IDμE chip, (b) optical microscopic image enlarged, and (c) EA-Gly/C-Mab/DTSP/IDμE bio-electrode fabrication. The fabricated bio-electrodes were characterized using the electrochemical impedance technique.
1). EA-Gly solution was used to block unreacted succinimidyl group on the DTSP SAM and to remove extra unbound antibodies on the electrode surface. Fig. 1 schematically illustrates (a) the IDμE chip, (b) optical microscopic image enlarged, and (c) EA-Gly/C-Mab/DTSP/IDμE bio-electrode fabrication. The fabricated bio-electrodes were characterized using the electrochemical impedance technique.
3. Results and discussion
3.1. Electrochemical impedance studies
Nyquist plots of impedance spectra were plotted to study (i) the change in charge transfer resistance (Rct) at sensor–solution interfaces after DTSP SAM formation, C-Mab binding and EA-Gly blocking, (ii) the change of charge resistance with changing concentration of cortisol. All EIS spectra were recorded in PBS (10 mM, pH 7.4) containing 5 mM Fe(CN)63−/4− as a redox probe.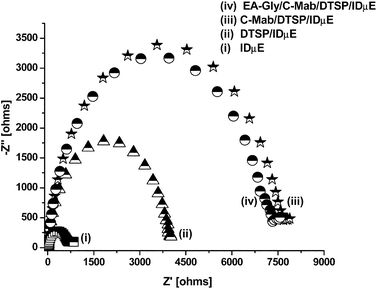 | ||
| Fig. 2 Nyquist plots of (i) blank IDμE electrode, (ii) DTSP/IDμE electrode, (iii) C-Mab/DTSP/IDμE bio-electrode and (iv) EA-Gly/C-Mab/DTSP/IDμE bio-electrode. | ||
 | ||
| Fig. 3 (a) EIS spectra of EA-Gly/C-Mab/DTSP/IDμE bio-electrode for cortisol concentration (i) buffer, (ii) 1 pM, (iii) 10 pM, (iv) 100 pM (v) 1 nM and (vi) 10 nM; and (b) normalized data curve for data obtained from EIS studies for different cortisol concentrations. | ||
Though the impedance of all electrodes fabricated in the same batch should be the same, however, lack of industrial scale process control in the lab leads to variations in impedance of individual electrodes and C-Mab modified electrodes. In order to confirm that the observed change in impedance was due to surface modification and not due to superimposed effects, all data from an electrode set were normalized. For linear range detection, normalization was achieved by plotting [charge transfer resistance for the desired concentration (Rct(Ci))]/[charge transfer resistance of the blank EA-Gly/C-Mab/DTSP/IDμE bio-electrode (Rct(Co))] versus logarithm of cortisol concentration (Fig. 3b). After normalization, all electrodes with different impedances for the EA-Gly/C-Mab/DTSP/IDμE bio-electrode exhibited similar response within 4% error for each concentration. Fig. 3b shows the normalized data curve that can be characterized using Rct(Ci)/Rct(Co) = 7.14 + 0.441 log Ccortisol. Fig. 3b reveals the linear range of 1 pM to 10 nM, sensitivity 0.441 M−1 with a correlation coefficient of 0.993 and a standard deviation of 0.685. Similar normalization was carried out for imaginary impedance data and a curve was plotted between [−Z′′ for the desired concentration (−Z′′(Ci))]/[−Z′′ of the blank EA-Gly/C-Mab/DTSP/IDμE bio-electrode (−Z′′(Co))] versus the logarithm of cortisol concentration (supplementary Fig. 1b†). A curve (supplementary Fig. 1b†) from normalized data can be characterized using −Z′′(Ci)/−Z′′(Co) = 7.33 + 0.449 log Ccortisol and reveals linearity for cortisol in the range of 1 pM to 10 nM with a sensitivity of 0.449 M−1, a correlation coefficient of 0.991 and a standard deviation of 0.953. It is clear that after normalization, both Rct and −Z′′ give similar response and can be utilized for sensing.
To account for the variation in initial impedance values for individual electrodes, all experiments were carried out using a step-by-step approach to increasing cortisol concentration. Similar step-by-step concentration studies have been reported by other researchers and help to avoid superimposed effects of multi-electrode measurement.29,43–47
 | ||
| Fig. 4 EIS spectra of EA-Gly/C-Mab/DTSP/IDμE for (i) buffer, (ii) 17-α-hydoxyprogesterone and (iii) cortisol. | ||
To compensate for the non-specific effects of other molecules present in human saliva, a new standard curve for cortisol estimation was generated using a saliva sample. For this study, a saliva sample with a known cortisol value of 3.0 nM was first diluted 1000 times to 3 pM in PBS (10 mM, pH 7.4) and then different concentrations of cortisol were prepared by adding a standard solution. Use of the diluted saliva sample as a diluent and EA-Gly as a blocker enables masking of major non-specific issues. Fig. 5a shows the Nyquist plots for various cortisol concentrations from 3 pM to 10 nM in samples using saliva as a diluent. For each concentration, the EA-Gly/C-Mab/DTSP/IDμE bio-electrode was incubated in cortisol solution for 30 min, followed by PBS washing and EIS spectra recording in PBS (10 mM, pH 7.4) containing 5 mM Fe(CN)63−/4− as a redox probe. It is clear that Rct increases with increasing cortisol concentration (Fig. 5a). The change in Rct value with cortisol concentration reveals that the EA-Gly/C-Mab/DTSP/IDμE bio-electrode senses cortisol linearly in the 3 pM to 10 nM range and follows a linear equation ΔRct (kohms) = 22.78 + 1.636 log Ccortisol (kohms). The linearity curve (Fig. 5b), plotted after the data normalization, follows Rct(Ci)/Rct(Co) = 5.03 + 0.294 log Ccortisol and exhibits linear behavior in the 3 pM to 10 nM range with a sensitivity of 0.294 M−1, a correlation coefficient of 0.98 and a standard deviation of 0.911. It was found that the use of saliva as diluent decreases the biosensor's sensitivity for cortisol; however, it enables a more selective measurement.
| Subject | R ct base (kohms) | R ct sample (100× dilution) (kohms) | R ct sample/base | Cortisol value (standard curve value) × 100 (nM) | Cortisol values from ELISA (nM) |
|---|---|---|---|---|---|
| 1 | 11.67 | 22.92 | 1.964 | 8.78 | 7.63 |
| 2 | 16.35 | 27.71 | 1.695 | 4.62 | 5.51 |
| 3 | 10.29 | 16.12 | 1.566 | 2.61 | 1.62 |
| 4 | 6.61 | 13.7 | 2.073 | 10.46 | 9.85 |
| 5 | 11.19 | 19.71 | 1.761 | 5.60 | 6.87 |
For each saliva sample, the EIS spectrum was first recorded for a fresh EA-Gly/C-Mab/DTSP/IDμE bio-electrode in PBS buffer to get the base value. The bio-electrode, analyzed previously, was then incubated with the 65 μl sample diluted 100× in PBS buffer for 30 min, followed by PBS washings, and EIS spectra recording in PBS (10 mM, pH 7.4) containing 5 mM Fe(CN)63−/4− as a redox probe (supplementary Fig. 3a–3e†). For each bio-electrode, the diameter of the Nyquist plots was found to increase after incubation with a saliva sample. After testing, the Rct value observed for the 100× diluted sample was normalized and the cortisol level was estimated using the standard curve (supplementary Fig. 2b†). Observed values were compared with the values obtained with commercial ELISA. Both sets of data follow a similar trend, thus enabling quantitative validation of the technique (Table 1). Table 1 shows the cortisol level obtained using our electrode and with the commercially available competitive ELISA. The small difference in measured values is attributed to sample handling techniques.
4. Conclusions
IDμE functionalized with a dithiobis(succinimidyl propionate) (DTSP) self-assembled monolayer (SAM) can be used to fabricate an ultrasensitive impedimetric cortisol immunosensor. A covalently immobilized monoclonal cortisol antibody based EA-Gly/C-Mab/DTSP/IDμE electrode exhibits linear behavior in the concentration range of 1 pM to 10 nM, has a low detection limit of 1 pM and linear regression coefficient of 0.996. The bio-electrode was found to be selective against 17-α-hydoxyprogesterone and was validated using the human saliva samples.Acknowledgements
This work was partially supported through NIH award 1R43MH085474-01; Instacortisol:A Realtime and Continuous Assessment of Cortisol In ISF and USF BITT Award; An Automated Cell Health Monitoring System (CHMS) Based on Electrical Impedance.References
- S. K. Arya, P. Pandey, S. P. Singh, M. Datta and B. D. Malhotra, Analyst, 2007, 132, 1005–1009 RSC.
- K. Sun, N. Ramgir and S. Bhansali, Sens. Actuators, B, 2008, 133, 533–537 CrossRef.
- J. Wang, Biosens. Bioelectron., 2006, 21, 1887–1892 CrossRef CAS.
- M. Pumera, S. Sanchez, I. Ichinose and J. Tang, Sens. Actuators, B, 2007, 123, 1195–1205 CrossRef.
- M. Tudorache and C. Bala, Anal. Bioanal. Chem., 2007, 388, 565–578 CrossRef CAS.
- S. M. Radke and E. C. Alocilja, Biosens. Bioelectron., 2005, 20, 1662–1667 CrossRef CAS.
- E. Bakker and Y. Qin, Anal. Chem., 2006, 78, 3965–3984 CrossRef CAS.
- S. Alegret, Integrated Analytical Systems, Elsevier B.V., Amsterdam, 2003 vol. XXXIX, ch. 1 Search PubMed.
- J. Guan, Y. Miao and Q. Zhang, J. Biosci. Bioeng., 2004, 97, 219–226 CAS.
- E. Katz and I. Willner, Electroanalysis, 2003, 15, 913–947 CrossRef CAS.
- K. S. Ma, H. Zhou, J. Zoval and M. Madou, Sens. Actuators, B, 2006, 114, 58–64 CrossRef.
- M. Bart, E. C. A. Stigter, H. R. Stapert, G. J. de Jong and W. P. van Bennekom, Biosens. Bioelectron., 2005, 21, 49–59 CrossRef.
- A. J. Bard, M. Stratmann and P. R. Unwin, Instrumentation and Electroanalytical Chemistry, Wiley-VCH, New Jersey, 2003, vol. 3, ch. 1–2 Search PubMed.
- A. W. Bott, Curr. Sep., 2001, 19, 71–75 Search PubMed.
- E. Barsoukov and J. R. MacDonald, Impedance Spectroscopy: Theory, Experiment and Applications, Wiley Interscience, New Jersey, 2nd edn, 2005, ch. 1 Search PubMed.
- M. Ciszkowska and Z. Stojek, J. Electroanal. Chem., 1999, 466, 129–143 CrossRef CAS.
- K. Maruyama, H. Ohkawa, S. Ogawa, A. Ueda, O. Niwa and K. Suzuki, Anal. Chem., 2006, 78, 1904–1912 CrossRef CAS.
- C. Berggren, B. Bjarnason and G. Johansson, Electroanalysis, 2001, 13, 173–180 CrossRef CAS.
- S. Laschi and M. Mascini, Med. Eng. Phys., 2006, 28, 934–943 CrossRef.
- L. M. Hagelsieb, B. Foultier, G. Laurent, R. Pampin, J. Remacle, J. P. Raskin and D. Flandre, Biosens. Bioelectron., 2007, 22, 2199–2207 CrossRef CAS.
- I. Navratilova and P. Skladal, Bioelectrochemistry, 2004, 62, 11–18 CrossRef CAS.
- D. Liu, R. K. Perdue, L. Sun and M. Crooks, Langmuir, 2004, 20, 5905–5910 CrossRef CAS.
- E. Nebling, T. Grunwald, J. Albers, P. Schafer and R. Hintsche, Anal. Chem., 2004, 76, 689–696 CrossRef CAS.
- M. Varshney and Y. Li, Biosens. Bioelectron., 2009, 24, 2951–2960 CrossRef CAS.
- W. K. Jerjes, T. J. Peters, N. F. Taylor, P. J. Wood, S. Wessely and A. J. Cleare, J. Psychosomatic Res., 2006, 60, 145–153 Search PubMed.
- F. R. Patacchioli, L. Angelucci, G. Dellerba, P. Monnazzi and O. Leri, J. Endocrinol. Invest., 2001, 24, 173–177 Search PubMed.
- L. W. Hawk, A. L. Dougall, R. J. Ursano and A. Baum, Psychosom. Med., 2000, 62, 423–434 CAS.
- J. C. Zhou, M. H. Chuang, E. H. Lan, B. Dunn, P. L. Gillman and S. M. Smith, J. Mater. Chem., 2004, 14, 2311–2316 RSC.
- R. C. Stevens, S. D. Soelberg, S. Near and C. E. Furlong, Anal. Chem., 2008, 80, 6747–6751 CrossRef CAS.
- U. Knutsson, J. Dahlgren, C. Marcus, S. Rosberg, M. Bronnegard, P. Stierna and K. J. A. Wikland, J. Clin. Endocrinol. Metab., 1997, 82, 536–540 CrossRef CAS.
- W. S. Gozansky, J. S. Lynn, M. L. Laudenslagar and W. M. Kohrt, Clin. Endocrinol., 2005, 63, 336–341 CrossRef CAS.
- K. Oka, M. Noguchi, T. Kitamura and S. Shima, Clin. Chem., 1987, 33, 1639–1642 CAS.
- D. Appel, R. D. Schmid, C. A. Dragan, M. Bureik and V. B. Urlacher, Anal. Bioanal. Chem., 2005, 383, 182–186 CrossRef CAS.
- R. Gatti, E. Cappellin, B. Zecchin, G. Antonelli, P. Spinella, F. Mantero and E. F. De Palo, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2005, 824, 51–56 CAS.
- I. Jerkunica, J. Sophianopoulos and D. Sgoutas, Clin. Chem., 1980, 26, 1734–1737 CAS.
- M. Vogeser, P. Mojmle and J. Briegel, Clin. Chem. Lab. Med., 2007, 45, 521–525 CrossRef CAS.
- N. J. Cook, A. L. Schaefer, P. Lepage and S. D. M. Jones, J. Agric. Food Chem., 1997, 45, 395–399 CrossRef CAS.
- D. Schmalzing, W. Nashabeh, X. W. Yao, R. Mhatre, F. E. Regnier, N. B. Afeyan and M. Fuchs, Anal. Chem., 1995, 67, 606–612 CrossRef CAS.
- W. A. Kaptein, J. J. Zwaagstra, K. Venema, M. H. J. Ruiters and J. Korf, Sens. Actuators, B, 1997, 45, 63–69 CrossRef.
- M. Sarkar, B. C. Das, B. D. Bora, V. Kumar, K. Mohan, H. H. D. Meyer and B. S. Prakash, Gen. Comp. Endocrinol., 2007, 154, 85–90 CrossRef CAS.
- J. S. Mitchell, T. E. Lowe and J. R. Ingram, Analyst, 2009, 134, 380–386 RSC.
- A. Kumar, S. Aravamudhan, M. Gordic, S. Bhansali and S. S. Mohapatra, Biosens. Bioelectron., 2007, 22, 2138–2144 CrossRef CAS.
- R. Maalouf, C. F. Wirth, J. Coste, H. Chebib, Y. Saikali, O. Vittori, A. Errachid, J. P. Cloarec, C. Martelet and N. J. Renault, Anal. Chem., 2007, 79, 4879–4886 CrossRef CAS.
- X. L. Su and Y. Li, Biosens. Bioelectron., 2004, 19, 563–574 CrossRef CAS.
- Y. Huang, M. C. Bell and I. I. Suni, Anal. Chem., 2008, 80, 9157–9161 CrossRef CAS.
- Y. G. Lee and K. S. Chang, Talanta, 2005, 65, 1335–1342 CrossRef CAS.
- T. T. N. Lien, T. D. Lam, V. T. H. An, T. V. Hoang, D. T. Quang, D. Q. Khieu, T. Tsukahara, Y. H. Lee and J. S. Kim, Talanta, 2010, 80, 1164–1169 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Imaginary impedance studies for cortisol estimation in standard solutions and Saliva studies. See DOI: 10.1039/c0an00242a |
| This journal is © The Royal Society of Chemistry 2010 |

