Confocal Raman microspectroscopy as an analytical tool to assess the mitochondrial status in human spermatozoa†
Konrad
Meister
,
Diedrich A.
Schmidt
,
Erik
Bründermann
* and
Martina
Havenith
Ruhr-Universität Bochum, Physical Chemistry II, Bldg. NC 7/72, Universitätsstr. 150, 44780, Bochum, Germany. E-mail: erik.bruendermann@rub.de; Fax: +49 2343214183; Tel: +49 2343224239
First published on 13th April 2010
Abstract
Confocal Raman microspectroscopy was used to investigate human sperm cells. Raman mapping with a 532 nm excitation laser allowed to unambiguously characterize the nucleus, the neck, and, in particular, the mitochondria-rich middle piece of a human sperm cell. The effect of ultraviolet radiation on different organelles of the sperm was quantified by localized spectral Raman signatures obtained within milliseconds. Chemical changes within the sub-cellular structure of the sperm cells were recorded as a function of ultraviolet light exposure time, showing the proof-of-principle that Raman microspectroscopy can be a fast diagnostic method for detecting the mitochondrial and motility status of human spermatozoa.
Introduction
Spermatozoa are terminally differentiated cells with organelles specialized in particular functions. The human sperm cell is usually divided into a head, a connecting piece (the neck), a middle piece and the flagellum.Low numbers of morphologically normal and motile spermatozoa are often associated with male infertility.1 But, even though it is generally accepted that immotile or poorly motile sperm are incapable of fertilization, there is still very little knowledge about the molecular mechanism that controls the function and movement of normal human sperm.2
In the latest issue of the WHO Manual for Andrology Laboratories, strict criteria are presented for the morphological appearance of normal spermatozoa.1 In addition, chemical criteria related to motility or proper functional organelles such as mitochondria are desirable. So far, only morphological abnormalities or defects of the corresponding regions (middle piece, neck) are described.1 Since the sperm count of men around the world has dropped to 50% of what it was more than 50 years ago,3 there is an urgency to investigate organelles. Especially, those organelles which control or participate in cellular movement of the sperm are of particular interest. The understanding of motility may improve the current standards for testing male fertility.
In addition, detailed knowledge of a fully functional sperm may allow us to disrupt normal, crucial functions of the sperm, which could lead to the development of an effective male contraceptive. The mobility is an especially attractive target to control male fertility as recently shown by Kirichok et al.4
The investigation and imaging of mammalian spermatozoa have been carried out using optical or electron microscopy, X-ray imaging, or secondary ion-mass spectroscopy. These techniques provide high spatial resolution, but may damage the sample during the analysis, cannot be used to examine living cells, or do not simultaneously reveal structure and chemical composition. Infrared microspectroscopy can, at the same time, provide both structural and chemical information,5,6 giving further insight into the chemical composition of the entire cell and the physio-pathological mechanism involved.
Confocal Raman microspectroscopy (CRM) is a non-invasive technique which combines Raman spectroscopy with a confocal optical microscope. Site specific images of the cell's shape can be obtained with a chemical analysis of the cellular components. CRM is frequently used as a powerful tool to investigate different biological tissues and living cells. Recent studies included the investigation of different cell types, sub-cellular organelles, and cellular uptake processes.7–10
Among the sub-cellular organelles studied, mitochondria are of special interest.11–13 Raman bands associated to mitochondria are under discussion, whether they indicate mitochondrial activity in living cells, and therefore are a spectroscopic signature of life.11–14
In the 1980s a single cell Raman spectrum was measured by Kubasek et al. using a salmon sperm cell.15 Although Raman microspectroscopy has matured, investigations on human sperm cells are rare. Recently, Huser et al. investigated human sperm cells,16 showing autofluorescence images and corresponding single point Raman spectra in selected regions of the sperm. They found that Raman microspectroscopy can distinguish DNA packaging of normal and abnormal human spermatozoa.16
In this work, we present Raman images of human sperm cells in which different sub-cellular organelles can clearly be identified. Furthermore, we used ultraviolet radiation to damage spermatozoa and investigate the effect of radiation on different organelles of the sperm and their spectral Raman signatures. Ultraviolet radiation was chosen since it is known to have a direct impact on cells, causing damage in the DNA and gene mutation that will eventually lead to cancer. UVA was used since it can penetrate the skin more deeply, and therefore can cause severe damage on an even deeper level such as in connective tissue. We could distinguish untreated and irradiated cells, in particular, the affected sub-cellular structures.
Experimental
Confocal Raman microspectroscopy and imaging
Raman spectra were acquired using a WITec (Ulm, Germany) Alpha300 R/A/S confocal Raman microscope. The excitation wavelength of 532 nm is provided by a frequency doubled Nd:YAG laser. Laser radiation was reduced to 10 mW to exclude cellular damage.The laser radiation is coupled into a Zeiss microscope through a wavelength-specific single-mode polarization-preserving fiber. The laser beam is collimated via an achromatic lens and passes a holographic bandpass filter. The beam is focused on the sample using a Nikon Fluor (100×/0.9NA) objective. The sample is located on a piezoelectrically driven microscope scanning stage, which has an x,y-resolution of 3 nm and a reproducibility of ±5 nm. The z-resolution is 0.3 nm with a reproducibility of ±2 nm. The reflected laser and Rayleigh-scattered light are blocked by a holographic bandpass and edge filter. The Raman-shifted scattered light is focused into a multimode optical fiber, which serves as the entrance slit for the spectrometer. The 3.5 µm diameter single-mode input fiber and the 50 µm diameter multimode output fiber provide the optical apertures for the confocal arrangement. Detection is made by a back-illuminated 1024 × 127 pixel CCD (charge-coupled device) camera operating at −60 °C. The diffraction grating provides a spectral resolution of <4 cm−1 per CCD pixel, and scattered light can be measured for a Raman-shift from 140 cm−1 to 3750 cm−1.
Sample preparation
Human spermatozoa were obtained from a healthy donor. Sperm cells were prepared according to a known swim-up protocol.17 A drop of 50 µL solution was transferred to a glass or CaF2 coverslip and smeared out. The cells were allowed to attach and remained immobilized during the measurements.4 An optical microscope was used to confirm that sperm cells were evenly distributed and well isolated.In order to study the influence of UV radiation, cells were exposed to defined periods of UVA light (365 nm), using an LF-206.LS UV hand lamp (Uvitec). The illuminance of the radiation was 0.6 mW cm−2. Measurements of illuminated cells were taken after a total exposure time of 0 min, 15 min, 30 min, 45 min, or 60 min, respectively. The same time series was used for cells without UV exposure as a control.
Data recording
Confocal Raman measurements were taken approximately 2 µm above the cell and coverslip interface so that the depth of focus lies always within the cell during scanning.Imaging involved the scanning of the laser beam over a whole sperm cell. The image size is in the range of up to 80 × 80 pixels and 30 µm × 30 µm, depending on the shape and size of the cells studied. At each pixel a full Raman spectrum was measured from 140 cm−1 to 3750 cm−1. The integration time per pixel was 400 ms to 500 ms. This leads to an average scanning time of 30 to 40 minutes per cell. Line scans of up to 50 single point measurements over a specific region of interest were also recorded. Ten spectra were accumulated at each position leading to an average scanning time of up to 5 minutes per line and cell. For single point measurements the integration time was varied in a wider range from 0.05 to 10 s. For the work presented here, a total of 38 sperm cells were studied, of which 34 were used for Raman mapping and 10 for UV exposure experiments.
Data processing
Data analysis was performed using the WITec Project Plus software. Raman images were constructed either by integrating the raw data over the vibrational bands of interest (method 1) or by k-means cluster analysis (method 2). Integration over selected spectral regions was performed by summing up the CCD counts between two frequencies. For example, the C–H stretching band between 2850 cm−1 and 3000 cm−1 to illustrate the overall structure of the cell.The unsupervised k-means cluster analysis was used to create a chemical map of the cell resolving sub-cellular structure as previously shown for fibroblast cells.7 This cluster analysis sorts data in groups in such a way that differences within each cluster are minimized while simultaneously maximizing the difference between clusters.18,19 An Euclidian distance metric was selected and in addition a data reduction scheme reducing the number of points by a factor of 5 was used. Prior to clustering, a linear background was subtracted from each spectrum in the dataset. The analysis started by choosing various random cluster initializations for spectra from 400 cm−1 to 3400 cm−1. The analysis ended when the minimum number of clusters in a stable solution was reached and the differences of the corresponding Raman spectra were maximized. Then, colour-coding for each cluster was used to create a chemical map. All spectra used for quantifying changes due to UV exposure were normalized using the Raman signal from the glass coverslip in the region from 140 cm−1 to 200 cm−1.
Results
Fig. 1A shows the optical image of a human spermatozoon attached to a coverslip. The sperm cell has a typical streamlined shape and size. Clearly distinguishable are the head structure, a middle piece and a long tail. Fig. 1B shows the chemical image resulting from the k-means cluster analysis (method 2). In combination with the corresponding Raman spectra, each of the three main clusters can, unambiguously, be assigned to certain cellular compartments of the sperm cell. The green cluster is assigned to the nucleus, the yellow cluster to the mitochondria-rich middle piece, and the red cluster belongs to the neck region. The blue colour scale corresponds to the integrated intensity of the C–H stretching vibration and shows the overall structure of the cell.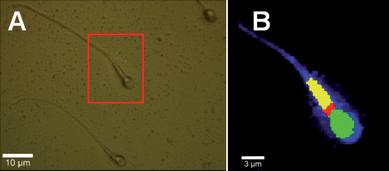 | ||
| Fig. 1 Panel A shows a bright-field optical image of human sperm cells with a scale bar of 10 µm. Panel B shows the chemical map constructed from Raman measurements taken on a single cell within the red square as indicated in panel A. The chemical image is based upon method 2 (see text); a k-means cluster analysis from 400–3400 cm−1. The nucleus (green), neck (red) and the middle piece (yellow) can unambiguously be identified. The blue colour scale corresponds to the integrated intensity of the C–H stretching vibration (2850–3000 cm−1). | ||
Single point Raman spectra from the nucleus, the middle piece, and the neck region are compared in Fig. 2. All three spectra clearly differ due to their chemical composition. Spectrum a of the neck region shows similarities to the other regions, but more pronounced, protein bands, while spectrum b is dominated by nucleic acid bands, for example, around 788 cm−1. The latter band is a result of the breathing mode of pyrimidine bases as previously assigned.16 Around 1575 cm−1 the breathing mode of the adenine and guanine bases contributes both to spectra b and c.
 | ||
| Fig. 2 Single point Raman spectra for each of the three clusters as seen in Fig. 1. Spectra a, b, and c correspond to the neck, nucleus, and middle piece, respectively. Dashed lines indicate 751 cm−1, 788 cm−1, and 1575 cm−1. | ||
Spectrum c of the middle piece shows additional bands previously assigned to structures typically found within mitochondria, for instance, at 751 cm−1.
Ultraviolet radiation
Ultraviolet radiation was used to induce specific damage to different organelles of the sperm cells. Raw data intensity maps (i.e. method 1) (see Fig. 3) were calculated from the resulting spectra using the same frequency positions as indicated in Fig. 2 to visualize the effect on the cell organelles. The upper panels (A–C) and middle panels (D–F) correspond to the middle piece and the nucleus. The lower panels (G–I) show both middle piece and nucleus. The contrast in the raw data images reduces with increasing UV exposure time and morphological structure is less visible. | ||
| Fig. 3 Shown is the optical image of a human sperm cell before UV radiation treatment and corresponding Raman images via method 1 before (A, D, and G) and after 30 min (B, E, and H) and 60 min of UVA exposure (C, F, and I). Panels A–C, D–F, and G–I show the integration over the Raman band at (751 ± 2) cm−1, (788 ± 2) cm−1, and (1575 ± 2) cm−1 as indicated in Fig. 2. The scale bar for the Raman images is 3 µm. | ||
Fig. 4 quantifies specific Raman bands at (751 ± 2) cm−1 and (788 ± 2) cm−1 as a function of UV exposure time, varied from 0 to 60 min. These bands predominantly exist in the middle piece and the nucleus. To represent the effect on the overall cell, the band from 2850 cm−1 to 3000 cm−1 corresponding to the C–H stretching modes was calculated. Reference cells, which were not exposed to UV radiation, were measured after 15 min, 30 min, 45 min, and 60 min.
 | ||
| Fig. 4 Quantitative evaluation of the characteristic Raman bands which mainly exist in the middle piece, A: (751 + 2) cm−1; in the nucleus, B: (788 + 2) cm−1; and defining the overall cell structure (C: 2850 cm−1 to 3000 cm−1), after 0, 15, 30, 45 and 60 min and for control cells without UVA exposure. | ||
After 30 minutes of UV irradiation the band at 751 cm−1 is already dramatically decreased by 70%, while the band at 788 cm−1 is slightly reduced by 20%. The overall cell structure was almost unaffected showing a reduction of a few percent. After 45 minutes of exposure, all bands diminish and later no further significant changes are observed.
To test the speed of this technique to analyse changes in spermatozoa due to UVA exposure, Raman maps were taken for 0, 30, and 60 min exposure times, each requiring 30–40 min per scan. Additional experiments at 15 and 45 min were analysed using line-scan measurements which further reduced the time to 5 min per scan. As a final test, single-point measurements with integration times of 1 s were sufficient to measure the 788 cm−1 band above the noise level (Fig. S5, see ESI†), indicating this technique can be used to assess thousands of individual spermatozoa within an hour.
Discussion
As shown in Fig. 1 (and Fig. S2, see ESI†), high resolution confocal Raman mapping allows to clearly distinguish sub-cellular components of human sperm cells identifying nucleus, neck, and middle piece. From these regions single point Raman spectra, highly localized within a specific organelle, were extracted (Fig. 2). Therefore, it is possible to focus on a specific region of a human sperm cell, such as the middle piece. The chemical map can be further extended into three dimensions as shown in Fig. S1 (see ESI†).The localized spectra in the middle piece exhibit a dominant mitochondrial feature at 751 cm−1 (Fig. 2c). This band can be mapped to find the spatial distribution and identify the mitochondrial rich region in the middle piece of sperm cells (Fig. 3A and Fig. S3†) using only raw data without requiring additional processing. There, the axoneme is surrounded by a densely packed winding helix of mitochondria.20 Mitochondria are small organelles ranging from 0.5 µm to 5 µm in diameter. They produce adenosine triphosphate (ATP) for the cell through aerobic respiration and are encased in highly folded membranes, which consist mainly of phospholipids. A high concentration of ATP is expected to be in the middle piece region since it serves as fuel for the flagellum and the overall sperm mobility. Therefore, the feature around 1575 cm−1 is also present in the middle piece (Fig. 3G).
In order to develop diagnostic methods to distinguish between normal and damaged spermatozoa, it is important to understand the spectral features of the individual components of the sperm as well as spectral differences between a damaged and an intact cell. For this reason, UV radiation was chosen since it is known to affect and to harm cells. Fig. 3 and 4 show not only the damaging effects on the whole cell but also different rates of destruction, localized in specific regions of the sperm, and especially in the middle piece.
Previous studies on calf thymus DNA and herring sperm DNA in solution showed that many base groups are damaged by UV radiation which is also capable of cutting bonds between the bases.21 The spatially resolved images of the sperm in Fig. 3G–I and the decrease of specific DNA signals (Fig. S4†) show the same trend. This further supports the idea of a loss of conformation, packing, and structure in DNA which is reflected in the decreasing image contrast with increasing UV exposure.
Severe UV damage can be found in the mitochondrial rich middle piece within 15 min exposure. The high rate of destruction implies that mitochondrial structures are first affected by environmental factors such as radiation. As a consequence, motility can decrease. These changes can be measured quickly, on the order of one second, and with sufficient signal-to-noise ratio (Fig. S4†) reducing measurement and data acquisition time for a single sperm cell.
We anticipate additional improvements can be made to reduce the time to analyse spermatozoa such that many thousands can be measured in an hour by: (1) incorporating automated optical imaging and Raman point-measurements, (2) using pulsed lasers for higher signal-to-noise ratio with decreased integration times, (3) developing Raman 2-D array detectors to directly image several spermatozoa simultaneously, and (4) using techniques such as coherent anti-Stokes Raman spectroscopy (CARS) to excite specific bands and improve the signal-to-noise, as has previously been demonstrated for epithelial,22 fibroblast,23 and NIH3T3 cells.24
Conclusion
Confocal Raman microspectroscopy was used to investigate intact human sperm cells. In combination with multivariate analysis methods, three dimensional Raman images of sperm cells clearly show different organelles such as the nucleus and mitochondria, localized in the middle piece.Large amounts of ultraviolet radiation damage structures within this middle piece first, before affecting the nucleus. The general shape of the cell is primarily unaffected, stressing the importance of monitoring degradation of chemical and sub-cellular constituents.
Damaged and intact sperm cells can easily be distinguished by characteristic Raman bands. Since a single point, localized Raman spectrum can be obtained within 400 to 500 ms, this technique has the potential for a fast fertility test to check the proper function of important organelles such as mitochondria or DNA packing in the nucleus.16 Ultimately, high throughput screening assays may be envisioned.
Non-invasive, chemically specific Raman mapping is a promising future diagnostic tool with further potential to investigate effects of stress markers, interactions with environmental factors, or drugs on human sperm cells. Future studies could involve the comparison of non-motile and motile sperm to derive improved standards based on chemical changes for distinguishing infertile and fertile sperm cells.
Acknowledgements
We acknowledge financial support for the Raman microscope under grant BMBF 05KS7PC2 and financial support by the DFG HA2394/12-2. We thank U. Schatzschneider for providing us with the UV hand lamp.References
- WHO, WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, Cambridge University Press, Cambridge, UK, 4th edn, 2003 Search PubMed.
- R. M. Turner, J. Androl., 2003, 24, 790–803.
- E. Carlsen, A. Giwercman, N. Keiding and N. E. Skakkebaek, Br. Med. J., 1992, 305, 609–613 CrossRef CAS.
- P. V. Lishko, I. L. Botchkina, A. Fedorenko and Y. Kirichok, Cell, 2010, 140, 327–337 CrossRef.
- E. Bründermann, A. Bergner, F. Petrat, R. Schiwon, G. Wollny, I. Kopf, H. Groot and M. Havenith, Analyst, 2004, 129, 893–896 RSC.
- M. Diem, M. Romeo, S. Boydston-White, M. Miljkovi and C. Matthäus, Analyst, 2004, 129, 880–885 RSC.
- C. Krafft, T. Knetschke, R. H. W. Funk and R. Salzer, Vib. Spectrosc., 2005, 38, 85–93 CrossRef CAS.
- C. Matthäus, T. Chernenko, J. A. Newmark, C. M. Warner and M. Diem, Biophys. J., 2007, 93, 668–673 CrossRef CAS.
- F. Draux, P. Jeannesson, A. Beljebbar, A. Tfayli, N. Fourre, M. Manfait, J. Sule-Suso and G. D. Sockalingum, Analyst, 2009, 134, 542–548 RSC.
- K. Meister, J. Niesel, U. Schatzschneider, D. A. Schmidt, N. Metzler-Nolte and M. Havenith, Angew. Chem., 2010 DOI:10.1002/anie.201000097.
- Y. S. Huang, K. Takeshi, Y. Masayuki, O. Takashi and H. Hiro-o, J. Raman Spectrosc., 2004, 35, 525–526 CrossRef CAS.
- V. V. Pully and C. Otto, J. Raman Spectrosc., 2009, 40, 473–475 CrossRef CAS.
- H. Tang, H. Yao, G. Wang, Y. Wang, Y.-q. Li and M. Feng, Opt. Express, 2007, 15, 12708–12716 CrossRef.
- Y.-S. Huang, T. Nakatsuka and H.-o. Hamaguchi, Appl. Spectrosc., 2007, 61, 1290–1294 CrossRef CAS.
- W. L. Kubasek, Y. Wang, G. A. Thomas, T. W. Patapoff, K. H. Schoenwaelder, J. H. Van der Sande and W. L. Peticolas, Biochemistry, 1986, 25, 7440–7445 CrossRef CAS.
- T. Huser, A. O. Christine, W. H. Christopher, H. C. Michele and B. Rod, J. Biophotonics, 2009, 2, 322–332 CrossRef CAS.
- R. Sanchez, E. Toepfer-Petersen, R. J. Aitken and W. B. Schill, Andrologia, 1991, 23, 197–203 CAS.
- J. B. McQueen, Proceedings Fifth Berkely Symposium on Mathematical Statistics, 1967, 1, 281–297 Search PubMed.
- T. Kanungo, D. M. Mount, N. S. Netanyahu, C. D. Piatko, R. Silverman and A. Y. Wu, IEEE Trans. Pattern Anal. Mach. Intell., 2002, 24, 881–892 CrossRef.
- L. Zamboni, J. Electron Microsc. Tech., 1991, 17, 412–436 Search PubMed.
- W. Ke, D. Yu and J. Wu, Spectrochim. Acta, Part A, 1999, 55, 1081–1090 CrossRef.
- A. Volkmer, J.-X. Chang and X. S. Xi, Phys. Rev. Lett., 2001, 87, 023901–023904 CrossRef.
- X. Nan, J.-X. Cheng and X. S. Xi, J. Lipid Res., 2003, 44, 2202–2208 CAS.
- J.-X. Cheng and X. S. Xi, J. Phys. Chem. B, 2004, 108, 827–840 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional high resolution Raman mapping and detection sensitivity. See DOI: 10.1039/b927012d |
| This journal is © The Royal Society of Chemistry 2010 |
