Correlation of atomic force microscopy and Raman micro-spectroscopy to study the effects of ex vivo treatment procedures on human red blood cells
Mehdi
Asghari-Khiavi
a,
Bayden R.
Wood
a,
Adam
Mechler
b,
Keith R.
Bambery
a,
Donna W.
Buckingham
c,
Brian M.
Cooke
c and
Don
McNaughton
*a
aCentre for Biospectroscopy and School of Chemistry, Monash University, 3800, Victoria, Australia. E-mail: don.mcnaughton@sci.monash.edu.au; Fax: +61 3 9905 4597; Tel: +61 3 9905 4525
bDepartment of Chemistry, School of Molecular Sciences, La Trobe University, 3086, Victoria, Australia
cDepartment of Microbiology, Monash University, 3800, Victoria, Australia
First published on 29th January 2010
Abstract
The effects of fixation and dehydration on the distribution of heme-based molecules inside red blood cells and the structural integrity of the cells have been investigated using Raman mapping and AFM topographic imaging. A strong correlation was observed between the thickness of the cells as determined from AFM images and the intensity of the characteristic heme bands in the Raman maps, demonstrating that heme compounds are relatively evenly distributed inside dried and fixed cells in the majority of cases. The exception occurred when cells were dried in phosphate buffered saline, where more hemichrome appears close to the periphery of the cell despite the AFM image showing a plateau like topography. Using neat formaldehyde solution as a fixative is inadequate for a complete structural preservation and results in diffusion of hemoglobin into the surrounding area. However, a mixture of formaldehyde (3%) and glutaraldehyde (0.1%) in buffer was found to be sufficient to retain the structural integrity of cells with minimal autofluorescence. This protocol was also suitable for red blood cells infected with Plasmodium falciparum parasites, and preserved the characteristic knob-like structures on the infected red blood cell surface.
1. Introduction
Raman spectroscopy is used in almost all disciplines of natural sciences, and it has recently emerged as the tool of choice for various biochemical applications.1 Although the technique can be used to analyze tissues and cells in their native state,2–7 when it is applied in conjunction with techniques such as scanning confocal microscopy, scanning near-field optical microscopy, tip-enhanced Raman microscopy, or when transport or prolonged storage precedes analysis, dehydration and fixation of cells and tissue are almost always necessary.The aim of fixation is to preserve the structural integrity of the cell or tissue in a state as close as possible to its natural morphology. However, fixation may introduce artifacts by altering or distorting the natural architecture of the tissue. Shrinkage or swelling of the tissue during fixation may cause compression, stretching, or movement of unfixed compounds so that sub-cellular structures or organelles may be relocated at sites different to their usual location.8 Some fixatives may also produce a very high level of fluorescence which can either totally swamp or quench Raman bands (particularly when using excitation sources in the range 450–650 nm). Conventional tissue preparation protocols for pathological examination such as dehydration with organic solvents9 and cross-linking with aldehydes,10–13 therefore, may not be useful for preparing cells for spectroscopic examination and there is a need for determining appropriate tissue preparation protocols that minimize experimental artifacts and reduce spectral contamination.14
In a recent communication,15 we reported the resonance Raman spectra of hemichrome and hemochrome, low spin components in which the sixth coordination site of the iron is occupied by the imidazole group of the distal histidine. In that work we found that in the process of air-dehydration and fixation of red blood cells (RBCs) in precipitating fixatives, hemoglobin changes to hemichrome while cross-linking fixatives initially maintain hemoglobin in its native conformational state (mainly oxy-Hb). Polymerization with cross-linking fixatives, however, increases auto-oxidation kinetics of hemoglobin and gradually oxidizes it to the met-Hb form whilst dehydration under nitrogen converts hemoglobin to hemochrome.
In this study, we investigate the effect of different preparation procedures on the morphology of RBCs and the distribution of heme complexes by correlating Raman maps and atomic force microscopy (AFM) topographic images of RBCs. Various fixatives including the precipitating agents, methanol, acetone, acetic acid, and the cross-linking agents, formaldehyde, glutaraldehyde, and acrolein were examined. Micro-Raman maps of heme content inside dried and fixed RBCs were recorded and compared with the corresponding AFM height images. This hybrid system combines the important features of two non-destructive analysis methods namely the 3D surface morphology high spatial resolution of AFM with the chemical identification capabilities of Raman micro-spectroscopy enabling the characterization of materials with respect to their chemical composition. We also use the same approach to evaluate the fixation protocol in Plasmodium falciparum infected RBCs (iRBCs) and determine the extent of cellular change/disruption upon parasite infection.
2. Experimental section
Blood samples, RBCs and iRBCs were prepared as previously described.7,16Blood smear
2 µL of blood was smeared on an aluminium coated glass plate to obtain a single layer of cells and then air-dried for 60 min.Air-dried RBCs
2 µL of packed RBCs (obtained by centrifugation of a suspension of RBCs in phosphate buffered saline, PBS) were deposited onto an Al coated glass substrate. To reduce crystallization of material from the PBS and to prepare a single layer of cells, after 1 min, most of the deposited solution was aspirated by micropipette prior to air drying for 60 min.Fixed cells
For fixation of RBCs using dehydrating agents, methanol, acetone, and acetic acid, 2 µL of packed cells were smeared on an Al coated glass disk, rapidly air-dried then immersed in pre-chilled fixative at −20 °C for 10 min. The fixed cells were extensively washed with distilled water after air drying. To fix RBCs in the cross-linking agents, formaldehyde, glutaraldehyde, and acrolein, 100 µL of packed cells were added to 1.5 mL of fixative solution in PBS (pH = 7.4). After fixation, the cells were washed with PBS and 4 µL of the suspension of fixed RBCs then deposited onto an Al coated glass substrate, and left to adsorb for 1 min. The residual solution was then removed with filter paper, and the attached cells on the substrate washed with distilled water before air drying. The fixation time was 30 min for RBCs fixed in glutaraldehyde and acrolein, and 4 h for the cells fixed in formaldehyde and formaldehyde–glutaraldehyde mix.Instrumentation
For combined Raman mapping and AFM imaging an NT-MDT NTegra AFM coupled to a Renishaw inVia Raman system was used. The Raman microprobe is attached to an optical microscope with a modified microscope stage to accommodate the AFM head. AFM and scanning Raman mapping were performed consecutively on the same area. A 100× objective (NA 0.7) and a 532 nm frequency doubled Nd:YAG laser excitation line were used in all Raman experiments. The laser power at the sample was ∼60 µW and the acquisition time for each spectrum was 5 s. Raman maps were carried out in a matrix of spots with a step size of 250 nm in both x and y directions, and a complete Raman spectrum collected at every mapping point.Non-contact mode silicon AFM cantilevers (NSG10, NTMDT) with a typical spring constant of 11.5 N m−1 and a nominal tip radius of 10 nm were used for AFM imaging. All samples were imaged in ambient conditions using semi-contact (tapping) mode with settings of 512 pixels per line and 0.5 Hz scan rate.
UV-vis absorbance spectra of supernatants were run on a Cary UV-visible spectrometer at a resolution of 1 nm using a 1 cm quartz cuvette.
Data analysis
The data processing of Raman maps and AFM images was carried out in Cytospec™ version 1.4 and Gwyddion 2.9 (http://gwyddion.net) program packages. The spectra used for Raman mapping were not treated using any form of data manipulation. The averaged spectra presented in the figures were baseline corrected using the concave rubber-band algorithm in OPUS software. Unsupervised hierarchical cluster analysis (UHCA) was performed by applying the D values spectral distance measure based on Pearson's correlation coefficient, and the clustering was carried out using Ward's algorithm. Heme (hemichrome, hemozoin) concentration maps were constructed by using the intensity of the oxidation state marker band, ν4, a strong band that appears at ∼1373 cm−1 for these compounds as can be observed in Fig. 1. | ||
| Fig. 1 Raman spectra of hemichrome (A) and extracted hemozoin (B) at 532 nm excitation; the laser power at the sample was ∼60 µW and the acquisition time was 10 s; hemichrome and extracted hemozoin were prepared by the methods described in ref. 15 and 16. | ||
3. Results and discussion
3.1. Effect of buffer and dehydration
Raman maps of heme content were recorded and correlated with AFM topographic images of dried cells in two different RBC samples: a blood smear from whole blood and a smear of packed RBCs that had been suspended in PBS. Fig. 2 depicts AFM height mode images of a dried RBC in a blood smear and RBC dried in PBS, and the corresponding false colour 3D-Raman maps of the same cells using the intensity of the band at 1373 cm−1 to image the hemichrome distribution. There is a good correlation between the AFM height images and the intensity of characteristic heme band in the dried RBC in the blood smear sample suggesting that hemichrome is localized almost evenly inside the cell. However, comparing the Raman data to the AFM images of the dried RBC in PBS shows that there is more hemichrome distributed at the edge of the cell rather than at its centre (Fig. 2B). | ||
| Fig. 2 AFM height mode images of an air-dried RBC in a blood smear or of a dried RBC that had been first re-suspended in PBS (left column), and the corresponding 3D-Raman maps of the same cells using the band at 1373 cm−1 to image the hemichrome distribution (right column). The scanning step size for Raman mapping was 250 nm. | ||
From the AFM images it is apparent that the morphology of RBCs in blood smears was similar to that of live cells (biconcave discoid) although drying on a substrate flattens the RBC onto the surface and decreases its height while increasing its lateral size. The mean height and lateral size of dried RBCs are ∼0.5 µm and ∼7.6 µm, respectively, (Table 1) while these are ∼1.5 µm and ∼7 µm, respectively, for ‘live’ cells in human blood.17 These values demonstrate that RBCs lose ∼60% of their volume when dried. This is also the case for dried cells in PBS. However, the morphology of RBCs in the latter sample is changed and bi-concavity is lost, suggesting initially that treatment with isotonic salt solutions before smearing results in deformed cells. The Raman maps, however, indicate that the morphology of the RBC is not perturbed by isotonic buffer. This retention of shape is supported by the fact that the natural shape of the RBC is preserved in a ‘buffered’ RBC fixed in glutaraldehyde or glutaraldehyde–formaldehyde mixture (see below). The similarity between Raman maps of dried RBCs in a blood smear and RBCs dried in PBS corroborates this argument and suggests that the structural integrity of the RBC may be preserved in the latter sample but it is buried under a layer of buffer material. This layer may also account for the larger size of the cells dried in PBS (Table 1). The sharp edge of these cells is another indication that the crystallization of buffer salts does not result in a uniform salt coating for the cell.
| Treated sample | Height/µm | Diameter/µm |
|---|---|---|
| Dried RBC in blood smear | 0.5 ± 0.1 | 7.6 ± 0.5 |
| Dried RBC in buffer | 0.6 ± 0.1 | 7.8 ± 0.4 |
| RBC fixed in methanol | 1.0 ± 0.2 | 6.7 ± 0.5 |
| RBC fixed in acetic acid (0.1%)–methanol | 0.8 ± 0.2 | 6.8 ± 0.4 |
| RBC fixed in acetone | 1.2 ± 0.3 | 6.1 ± 0.7 |
| RBC fixed in methanol–acetone (50–50) | 1.1 ± 0.2 | 6.2 ± 0.6 |
| RBC fixed in glutaraldehyde | 1.4 ± 0.1 | 6.9 ± 0.3 |
| RBC fixed in formaldehyde | 1.4 ± 0.1 | 6.4 ± 0.4 |
| RBC fixed in a mixture of formaldehyde–glutaraldehyde | 1.4 ± 0.1 | 7.0 ± 0.5 |
| RBC fixed in acrolein | 1.2 ± 0.2 | 6.7 ± 0.5 |
| iRBC fixed in a mixture of formaldehyde–glutaraldehyde | 1.8 ± 0.3 | 6.0 ± 0.5 |
3.2. Effect of precipitating fixatives
To study the effect of the fixatives methanol, acetone, and acetic acid on the morphology of RBCs and on the distribution of hemichrome, Raman maps of fixed cells were recorded and compared with the corresponding AFM topographic images. Fig. 3 shows AFM height mode images of RBCs fixed in pure methanol, pure acetone, acetone–methanol mix (50–50), and acetic acid (0.1%) in methanol along with the corresponding 3D-Raman maps of the same cells using the intensity of the ν4 Raman band of heme to image its distribution. | ||
| Fig. 3 AFM topography images of RBCs fixed in precipitating fixatives (left column), and the corresponding 3D-Raman maps of the same cells using the band at 1373 cm−1 to image the hemichrome distribution (right column). The scanning step size for Raman mapping was 250 nm. | ||
The correlation of the AFM height images with the characteristic micro-Raman maps of heme in Fig. 3 demonstrates the high resolution of Raman maps and suggests that the in-depth distribution of hemichrome inside fixed RBCs is almost homogeneous. Fig. 3 shows that fixation by precipitation does not preserve the three-dimensional organization of the RBCs and that the morphology of the fixed cells is essentially different from that of live, unfixed cells. The precipitating fixatives do not form cross-links thus lipid and cell membranes are not fixed in place and are lost in subsequent washing steps or may be drastically relocated within the cell. Methanol fixation causes shrinkage of RBCs and the shrinkage is often greater in the z-direction than in the x–y direction, presumably due to the adherence of the cells to the underlying substrate. Acetic acid on the other hand is associated with tissue swelling.8 Adding very small amounts of acetic acid (0.1%) to methanol prevents the shrinkage of RBCs; however, it lyses the RBC as is evidenced by a considerable decrease of the volume of the fixed cell (Fig. 3B), and the appearance of strong absorption bands of hemoglobin in the UV-vis spectra of the supernatant solution of the fixed cells. Moreover, acetone alone or in combination with methanol precipitates proteins and washes away most of the lipids, causing collapse of the membrane of the cells (Fig. 3C and D). As is shown in Table 1 the mean height and lateral size of RBCs fixed by precipitation are respectively ∼0.8–1.2 µm and ∼6.1–6.8 µm demonstrating a relative decrease in the volume of fixed cells when compared to live RBCs.
3.3. Effect of cross-linking fixatives
Fig. 4 depicts AFM surface topography images of RBCs fixed in glutaraldehyde (2%), formaldehyde (3%), acrolein (2%), and a mix of formaldehyde (3%)–glutaraldehyde (0.1%), and the corresponding three-dimensional chemical maps of the same cells using the band at 1373 cm−1 to image the hemichrome distribution. For RBCs fixed in glutaraldehyde, only the AFM image is reported because using glutaraldehyde alone results in a strong autofluorescence background, which precludes the collection of Raman data when using 532 nm excitation. | ||
| Fig. 4 AFM surface topography images of fixed RBCs in cross-linking fixatives (left column), and the corresponding 3D-Raman maps of the same cells using the band at 1373 cm−1 to image the hemichrome distribution (right column). The scanning step size for Raman mapping was 250 nm. For glutaraldehyde fixation autofluorescence precluded Raman spectroscopy. | ||
Fig. 4 shows a close correlation between the AFM height mode images and the corresponding Raman maps of fixed RBCs demonstrating that hemoglobin is laterally distributed evenly inside these cells. Fig. 4 also indicates that glutaraldehyde results in the best structural integrity preservation; however, cross-linked fixed cells have a very high level of autofluorescence (at 532 nm excitation). It is reported that the treatment of glutaraldehyde-fixed tissue with sodium borohydride lowers autofluorescence levels by chemically reducing free aldehydes and keto groups to the respective alcohols.18,19 However, incubation of glutaraldehyde-fixed RBCs in sodium borohydride (1 mg mL−1 PBS) contributes little to the suppression of autofluorescence in our experiments (unpublished data). Formaldehyde on the other hand is excellent in reducing autofluorescence; however, it does not provide a very rigid or tightly linked system and is inadequate for a permanent ultrastructural preservation (see below). When using low levels of glutaraldehyde (0.1%) in conjunction with formaldehyde (3%), the amount of autofluorescence is acceptable while the structural integrity of the cells is also preserved (Fig. 4C). Moreover, formaldehyde penetrates tissue quickly, stabilizing the sub-cellular structures, while glutaraldehyde produces a more rigid fixation but penetrates more slowly.20,21 Fast penetrating formaldehyde, therefore, may serve as a temporary stabilizer for a more permanent fixation by glutaraldehyde.
Using formaldehyde alone as a fixative results in loss of hemoglobin as revealed by UV-vis spectroscopy. Fig. 5 compares UV-vis absorption spectra of a supernatant solution of the RBCs fixed in formaldehyde 3%, glutaraldehyde 2%, and a mix of formaldehyde 3%–glutaraldehyde 0.1% after storage of the cells in fixative solution for 24 h. The absorbance of met-hemoglobin characteristic bands (particularly the Soret band at ∼406 nm) is dramatically higher in the supernatant solution of the former sample demonstrating that structural fixation with formaldehyde is not as strong as with glutaraldehyde which results in leaky erythrocytes. The observed leakage of hemoglobin may be explained by an increase in osmolality and hence depression of the intracellular water activity and in the presence of formaldehyde leading to a rapid entry of water and disruption of the incompletely fixed material. In the case of glutaraldehyde, the cross-linking reactions are rapid and the cell is essentially fixed before any cellular disruption by an osmotic mechanism can occur.22
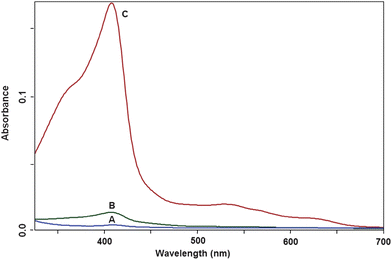 | ||
| Fig. 5 UV-vis absorption spectra of supernatant solution (after centrifugation at 2500 rpm for 5 min) of the RBCs fixed in glutaraldehyde 2% (A), mix of formaldehyde 3%–glutaraldehyde 0.1% (B), and formaldehyde 3% (C) following storage of the cells in fixative solution for 24 h. | ||
As can be seen in Fig. 4, fixation of RBCs in acrolein and formaldehyde results in decreased cell volumes compared with the cells fixed in glutaraldehyde and formaldehyde–glutaraldehyde mix.
3.4. Malaria parasite-infected RBCs
As outlined above, amongst fixatives investigated, a mix of formaldehyde (3%) and glutaraldehyde (0.1%) in buffer was found to be the best protocol for fixation of RBCs for spectroscopic examination. To evaluate the efficiency of this protocol for fixation of RBCs infected with P. falciparum malaria parasites, AFM images were recorded and compared with the corresponding Raman maps. These AFM images show that in iRBCs the symmetrical shape is lost and there is considerable variation in morphology depending on the stage of maturation of the parasite and the accumulation of the malaria pigment hemozoin. Often iRBCs contain a large protuberance, usually in the cell periphery but sometimes in the cell centre with several deep invaginated areas around it. Fig. 6 depicts an AFM topographic image of a RBC infected with a mature parasite (trophozoite) fixed in formaldehyde–glutaraldehyde mix and a false colour 3-cluster Raman map of the same cell generated by performing UHCA on the spectral region 1520–1680 cm−1 where the major spectral differences between hemichrome and hemozoin occur. Mean cluster spectra from two of the clusters are shown in Fig. 6D and by comparison with the spectra of hemichrome and extracted hemozoin in Fig. 1, the outer cluster correlates with hemichrome while the inner cluster correlates mainly with hemozoin. Hence the latter cluster depicts localization of malaria pigment corresponding with a large protuberance in the AFM topography image. The middle cluster correlates with a mixture of hemozoin and hemichrome. In this case it appears that the protocols for normal RBCs are also appropriate for iRBCs. As is clear from the AFM image in Fig. 6, the applied protocol can preserve structural integrity of the iRBC including the characteristic knob-like structures on the surface of the cell. The size of knobs is 20–50 nm in height and 100–150 nm in width which is similar to the values reported in the literature as measured by SEM, TEM, and AFM in unfixed and fixed iRBCs.23–27 | ||
| Fig. 6 AFM height mode image of a RBC infected with a mature P. falciparum malaria parasite, fixed in formaldehyde (3%)–glutaraldehyde (0.1%) mix (A) and a false colour 3-cluster Raman map of the same cell generated by performing UHCA on the region 1520–1680 cm−1 (B). Cross-sectional view of knobs along the specified line (C) and mean extracted spectra from the inner and outer clusters (D). The scanning range is 10 × 10 µm and the difference between the max- and the min-height (in AFM image) is 2 µm. The scanning step size for Raman mapping was 250 nm. | ||
4. Conclusion
The effects of fixation and dehydration on the structural integrity of RBCs and the distribution of heme inside RBCs have been studied by correlating AFM topographic images with Raman maps of the cells. There is a close correspondence between Raman maps and AFM images in fixed cells demonstrating a homogenous lateral distribution of heme components inside these cells. However, Raman maps of dried RBCs from PBS show more hemichrome at the periphery of the cell while AFM images exhibit a more even plateau-like topography. Using formaldehyde alone as a fixative is inadequate for a complete structural preservation and results in the diffusion of hemoglobin. Using a mix of formaldehyde (3%) and glutaraldehyde (0.1%) in buffer was found to be a good compromise between the level of autofluorescence and the structural integrity of the cells. The hybrid AFM–Raman technique has thus allowed us to find the optimum fixative technique to both retain the internal structure of the cell and allow for spatially resolved spectroscopic analysis. The protocol has also been found to provide a map of hemozoin and hemichrome distribution within malaria infected RBCs with the knowledge that the fixation process is highly unlikely to affect this distribution. The techniques and information presented in this paper will be extremely useful for future structure/function studies in both normal and abnormal RBCs and show great promise as a general technique in understanding and monitoring cellular chemistry.Acknowledgements
This work is funded by an Australian Research Council (ARC) Discovery Grant. AM acknowledges his Monash Fellowship and BRW an ARC QEII Fellowship. MAK acknowledges support by Monash University scholarships MGS and MFRS. BMC is a National Health and Medical Research Council (NHMRC) Senior Research Fellow. We thank Professor Leann Tilley (La Trobe University) for valuable comments.References
- M. Schmitt and J. Popp, J. Raman Spectrosc., 2006, 37, 20–28 CrossRef CAS.
- P. R. Carey, in Biochemical Applications of Raman and Resonance Raman Spectroscopies, Academic Press, New York, 1982, ch. 4 and 5 Search PubMed.
- G. J. Puppels, F. F. M. de Mul, C. Otto, J. Greve, M. Robert-Nicoud, D. J. Arndt-Jovin and T. M. Jovin, Nature, 1990, 347, 301–303 CrossRef CAS.
- K. Ramser, E. J. Bjerneld, C. Fant and M. Kall, Proc. SPIE-Int. Soc. Opt. Eng., 2002, 4614, 20–27 CAS.
- T. Frosch, M. Schmitt, K. Schenzel, J. H. Faber, G. Bringmann, W. Kiefer and J. Popp, Biopolymers, 2006, 82, 295–300 CrossRef CAS.
- J. Kneipp, H. Kneipp, M. McLaughlin, D. Brown and K. Kneipp, Nano Lett., 2006, 6, 2225–2231 CrossRef CAS.
- B. R. Wood, P. Caspers, G. J. Puppels, S. Pandiancherri and D. McNaughton, Anal. Bioanal. Chem., 2007, 387, 1691–1703 CrossRef CAS.
- W. E. Grizzle, J. L. Fredenburgh and R. B. Myers in Theory and Practice of Histological Techniques, ed. J. D. Bancroft and A. Stevens, Churchill Livingstone, Edinburgh, 6th edn, 2008, ch. 4, pp. 53–74 Search PubMed.
- M. A. Hayat, in Fixation for Electron Microscopy, Academic Press, New York, 1981, pp. 267–270 Search PubMed.
- E. Ó. Faoláin, M. B. Hunter, J. M. Byrne, P. Kelehan, M. McNamara, H. J. Byrne and F. M. Lyng, Vib. Spectrosc., 2005, 38, 121–127 CrossRef.
- C. P. Tarnowski, S. Stewart, K. Holder, L. Campbell-Clark, R. J. Thoma, A. K. Adams, M. A. Moore and M. D. Morris, J. Biomed. Opt., 2003, 8, 179–184 CrossRef CAS.
- J. G. Riess, Chem. Rev., 2001, 101, 2797–2919 CrossRef CAS.
- A. I. Alayash, A. G. Summers, F. Wood and Y. Jia, Arch. Biochem. Biophys., 2001, 391, 225–234 CrossRef CAS.
- E. Gazi, J. Dwyer, N. P. Lockyer, J. Miyan, P. Gardner, C. Hart, M. Brown and N. W. Clarke, Biopolymers, 2005, 77, 18–30 CrossRef CAS.
- M. Asghari-Khiavi, A. Mechler, K. R. Bambery, D. McNaughton and B. R. Wood, J. Raman Spectrosc., 2009, 40, 1668–1674 CrossRef CAS.
- B. R. Wood, S. J. Langford, B. M. Cooke, J. Lim, F. K. Glenister, M. Duriska, J. K. Unthank and D. McNaughton, J. Am. Chem. Soc., 2004, 126, 9233–9239 CrossRef CAS.
- N. Mohandas and E. Evans, Annu. Rev. Biophys. Biomol. Struct., 1994, 23, 787–818 CrossRef CAS.
- W. Baschong, M. Dürrenberger, A. Mandinova and R. Suetterlin, Methods Enzymol., 1999, 307, 173–189 CAS.
- W. Baschong, R. Suetterlin and R. H. Laeng, J. Histochem. Cytochem., 2001, 49, 1565–1571 CAS.
- N. Chungsamarnyart, T. Fujioka and J. Kitoh, Cell Tissue Res., 1981, 217, 471–477 CAS.
- J. Kuo, in Methods in Molecular Biology, ed. N. Hajibagheri, Humana Press, New Jersey, 2nd edn, 2007, vol. 117, pp. 35–45 Search PubMed.
- D. Hopwood, Histochem. J., 1972, 4, 267–303 CrossRef CAS.
- M. Aikawa, K. Kamanura, S. Shiraishi, Y. Matsumoto, H. Arwati, M. Torii, Y. Ito, T. Takeuchi and B. Tandler, Exp. Parasitol., 1996, 84, 339–343 CrossRef CAS.
- E. Nagao, O. Kaneko and J. A. Dvorak, J. Struct. Biol., 2000, 130, 34–44 CrossRef CAS.
- T. Arie, R. M. Fairhurst, N. J. Brittain, T. E. Wellems and J. A. Dvorak, J. Struct. Biol., 2005, 150, 163–169 CrossRef CAS.
- M. Rug, S. W. Prescott, K. M. Fernandez, B. M. Cooke and A. F. Cowman, Blood, 2006, 108, 370–378 CrossRef CAS.
- A. Li, A. H. Monsoor, K. S. W. Tan and C. T. Lim, J. Microbiol. Methods, 2006, 66, 434–439 CrossRef.
| This journal is © The Royal Society of Chemistry 2010 |
