Phase and self-assembly transition induced by glycerol–borax interaction in an aqueous surfactant two-phase system†
Ying
Zhao
a,
Yun
Yan
*a,
Lingxiang
Jiang
a,
Jianbin
Huang
*a and
Heinz
Hoffmann
b
aBeijing National Laboratory for Molecular Science (State Key Laboratory for Structural Chemistry of Unstable and Stable Species), College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, People's Republic of China. E-mail: yunyan@pku.edu.cn; jbhuang@pku.edu.cn; Fax: +86-10-62751708; Tel: +86-10-62765058
bUniversity of Bayreuth, BZKG, Bayreuth, 95448, Germany
First published on 26th August 2009
Abstract
We report that the phase and self-assembly transition in an aqueous surfactant two-phase (ASTP) system can be induced by a small amount of glycerol. The ASTP was formed from a cationic Gemini surfactant, C12C6C12(Et), and an anionic surfactant, sodium laureate (SL), in a borax solution. Upon addition of 0.3–2 vol% glycerol to the system, the ASTP system underwent a striking phase transition: three phases at 0.3–0.5% glycerol, a single birefringent phase at 0.7–1.2% glycerol, then again two phases, with the upper one like ice-cream. FF-TEM, CSLM and polarized microscopy revealed that the lamellae in the original upper phase were transformed into multilamellar vesicles. This phase and the microstructure transition were attributed to neutralization of sodium laureate to lauric acid by the formation of protons from the reaction of borax with glycerol. The variation of the charge density on the bilayer assemblies and the formation of LA were confirmed by fluorescence quenching and ATR-IR experiments. Our results demonstrate that a small amount of glycerol can be used to tailor the phases and microstructures in surfactant systems containing pH-sensitive components.
Introduction
The influence of glycerol on the self-assembly or aggregation of amphiphiles in aqueous solution has attracted increasing attention both in biochemistry and colloid science. On the one hand, glycerol is active in maintaining the structure of biological macromolecules and in promoting protein self-assembly,1,2 so that it is extensively used in lyophilization and low-temperature preservation protocols.3,4 On the other hand, as an environmentally friendly polar solvent, glycerol is widely employed in many formulations, especially in pharmacy and cosmetic products,5,6 where amphiphilic molecules are present. In these systems low amounts of glycerol (less than 20–30%) are considered to have no significant influence on the aggregation properties of the amphiphilic molecules, except that the CMC increases slightly and the aggregation number decreases slightly.7–9 For instance, the CMC of TTAB (tetradecyltrimethylammonium bromide) increased from 3.45 mM in water to 4.14 mM in 20% glycerol, while the aggregation number decreased from 60 to 46. As a consequence, the radius of the micelles decreased from 18 to 16.4 Å.10 Clearly, this amount of glycerol did not change the morphology and the size of the micelles considerably. This situation was similar in vesicle and liquid crystal systems. It has been reported that the size of the vesicles formed from the siloxane surfactant in 20% glycerol is almost the same as that in water;11 the crystalline structures of pluronic surfactants were not affected by 20% glycerol.12 For this reason, glycerol is used as a viscosity or sweetness enhancer in many industrial formulations.5,6 In fundamental research, 20–30% glycerol (w/w) is widely employed as a cryoprotectant to suppress the formation of ice crystals in the freeze-fracture replica technique.13–17However, this situation may be different if glycerol coexists with borax in the amphiphile solution. Borax itself is often used as a buffer by virtue of its hydrolysis in aqueous solution.18–22 One of the hydrolysis products is boric acid, which behaves as a weak acid upon binding one hydroxide ion from water. The acidity of boric acid may be greatly enhanced in glycerol or other polyols owing to the ‘didiol reaction’.23–25 It has been reported that the pH of the boric acid may be lowered by 2 units in the presence of glycerol.26 In this sense, the glycerol–borax reaction may be used as an additional variable to tailor the self-assembly or aggregation of pH-sensitive surfactants, such as alkyl carbonates and alkyl amine oxides.
The objective of this work was to study the effect of glycerol on the phase and self-assembled structures in an aqueous surfactant two-phase (ASTP) system formed by a catanionic surfactant mixture. ASTP systems are an interesting phenomenon in surfactant systems, which have been found to have attractive potential in membrane separation and other biotechnology.27,28 Furthermore, the sensitivity of the phases with subtle disturbance also makes ASTP systems convenient to study the factors that govern the phases and microstructures. The ASTP system we are interested is formed by a cationic Gemini surfactant, hexanediyl-α,ω-bis(dodecyldiethylammonium bromide (C12C6C12(Et)), and an anionic surfactant, sodium laureate (SL), in the presence of 10 mM borax which has been reported by us in a previous report.29 In this work we report the variation of the macrophases and microstructures upon addition of glycerol. We found that the reaction between glycerol and borax acts as a new paradigm to control the charge density on the surfactant bilayers, and can be used to tailor the phase behavior and the morphology of the self-assembled structures.
Experimental section
Materials
Sodium laureate (SL) was prepared by neutralizing lauric acid with NaOH in ethanol. Cationic Gemini surfactants hexanediyl-α,ω-bis(dodecyldiethylammonium bromide), abbreviated as C12C6C12(Et), were synthesized and purified according to literature methods.30 The purity of all the surfactants was examined, and no surface tension minimum was found in the surface tension curve. The water used was doubly distilled from potassium permanganate containing deionized water to remove traces of organic compounds.Sample preparation
The solutions of the mixed systems were prepared by dissolving the powder of C12C6C12(Et) and SL at 3:7 molar ratio in different glycerol–water mixed solvents in the presence of 10 mM borax. Samples were vortex-mixed and equilibrated in a thermostatic bath for periods of weeks before investigation. All the reported measurements of the mixtures of C12C6C12/SL were performed at 30 °C.Macro and polarization microscopic observation
The macroscopic features of the samples were photographed with and without polarizers. The microscopic images of the birefringent samples were taken by a polarization microscope (Leica – DMLP).Transmission electron microscopy (TEM)
Micrographs were obtained with a JEOL – 100 CX II transmission electron microscopy by freeze-fracture replica technique (FF-TEM). Fracturing and replication were carried out in an EE-FED.B freeze-fracture device equipped with a JEE-4X vacuum evaporator.Confocal laser scanning microscopy (CLSM)
Hydrophobic Nile red-containing samples were prepared through the following process: 15 µL stock solution of Nile red in acetone (1 mg/mL) was added to a test tube, followed by volatilization of the acetone. Then a desired surfactant solution was added to the tube. All samples were allowed for equilibrium for 24 hours before CLSM experiments. The solutions containing Nile red were dropped onto a pre-cleaned glass surface, which was covered by a slide. The edge of the slide was sealed to avoid evaporation. CLSM observation was conducted on a Leica Tcs-sp confocal laser scanning microscope.Fluorescence quenching
Pyrene was used as the fluorescence probe whereas Cs+ and S2O32− as the quencher.31 A desired amount of pyrene in ethanol was added to a test tube, followed by volatilization of the ethanol. Then a certain amount of C12C6C12(Et)/SL solution was added to the tube, which was vigorously stirred and allowed for equilibrium for 24 hours before experiments. The final concentration of pyrene was kept low enough (5 × 10−7 M) to prevent excimer formation. All of the fluorescence quenching measurements were performed on an Edinburgh FLS920 lifetime and steady-state fluorescence spectrophotometer (excitation at 335 nm and range emission from (350–450). The final concentration of the quencher was kept at 30 mM.ATR-IR
IR spectra of solution were collected with a NICOLET iN10 MX spectrometer (Thermo Scientific, America) in the range 4000–650 cm−1. The spectrometer was equipped with an attenuated total reflection (ATR) accessory with a SMART Itr(diamond). The ATR accessory is specifically suited for aqueous solution because the large O–H water band is attenuated, and sample bands at longer wavelengths become enhanced.Results and discussion
Phase behavior of C12C6C12(Et)/SL system in 10 mM borax buffer (without glycerol)
At 30.0 °C, ASTP is formed at mixing ratios of C12C6C12(Et):SL close to charge-neutral mixing in the presence of 10 mM borax.29 In this study, 3:7 ASTP with the C12C6C12(Et)/SL system was used. It can be seen in Fig. 1a that the upper to lower phase volume ratio is about 1 to 7. The upper phase is rich in surfactant, which was verified by solubilization of hydrophobic dye of Sudan III (Fig. 1b). Although no obvious birefringence was observed macroscopically, liquid crystal-like textures were observed under polarized microscopy (Fig. 1c). Freeze-fracture replica TEM observation revealed the presence of lamellar structures (Fig. 1d). The absence of birefringence in the phase is probably caused by the low lamella concentration or alignment of the lamellae parallel to the interface. | ||
| Fig. 1 (a, b): Photographs of the mixed system of C12C6C12(Et)/SL (3:7, Ctotal = 50 mM, 10 mM borax) (a) without and (b) with Sudan III. (c): Microscopic image of the upper phase observed by a polarization microscope (d): FF-TEM image of the lamellae formed in the upper phase. | ||
Phase behaviour upon addition of a small amount of glycerol
The ASTP in the 3:7 C12C6C12(Et)/SL mixed system was changed dramatically upon addition of small amount of glycerol. As shown in Fig. 2, a striking bluish middle phase occurs at 0.3–0.5% glycerol, which is probably an indicative of vesicle formation.32 The volume of this middle phase is almost doubled as the content of glycerol increases from 0.3% to 0.5%. Meanwhile, the volume of the upper phase in the 0.3–0.5% glycerol system increases slightly when compared with the original upper phase where no glycerol is present. It is noteworthy that the upper phases become strongly birefringent, which probably results from the stiffening of the lamellae.33,34 Upon addition of 0.7–1.2% glycerol, the system turns into one homogenous bluish phase, which is birefringent between crossed-polarizers. At glycerol concentrations above 1.2%, the samples separate again into two phases with the upper phase being white and ice-cream-like and the lower one transparent. Clearly, addition of less than 2% glycerol induces a significant phase transition in the ASTP system of C12C6C12(Et)/SL/borax. | ||
| Fig. 2 Photos for C12C6C12(Et)/SL (3:7, Ctotal = 50 mM) in 10 mM borax containing different amount of glycerol. The numbers above the photos are the volume percent (vol%) of glycerol. Upper: photos without polarizers. Lower: photos with crossed-polarizers. | ||
Microstructures in the phases
FF-TEM and CLSM experiments were carried out to investigate the microstructures in the phases induced by glycerol addition. Firstly, lamellar structures were observed by FF-TEM in the upper phase of the 0.5% glycerol system (Fig. 3a) which is similar to what is observed in the original upper phase where no glycerol is present (Fig. 1d). This demonstrates that the bilayer structure is not affected very much by the addition of 0.5% glycerol. However, the occurrence of the bluish middle phase indicates that vesicles gradually form.32 This can be verified by the occurrence of a homogenous bluish birefringent phase at 0.7–1.2% glycerol. CSLM images for the microstructures in the 0.5% glycerol system are given in Fig. 3b and c, where many dispersed spheres with diameters less than 5 μm and some irregular large patches can be observed. The phase-contrast image (Fig. 3c) reveals that the spheres are multilamellar vesicles whereas the irregular large patches are membrane fragments. The presence of vesicles and membrane fragments in the bluish middle phase indicates that the lamellae in the upper phase are either transformed into spherical vesicles or broken into fragments upon addition of glycerol. Similar vesicles and membrane fragments were also observed in the glycerol content range of 0.7–1.2%, where the system has transformed into a homogeneous bluish phase with stationary birefringence. In the latter phases the size of the vesicles is more polydisperse, and the number density of these structures is much higher than those in the 0.5% glycerol system. In addition, deformation of vesicles was observed (see ESI†), which is probably the origin of the stationary birefringence.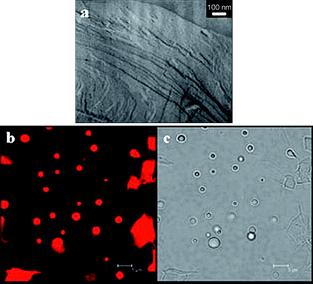 | ||
| Fig. 3 (a): FF-TEM of the lamellae formed in the upper phase of C12C6C12(Et)/SL (3:7, Ctotal = 50 mM, 10 mM borax) in 0.5% glycerol–water mixed solvent. (b): CLSM images of vesicles formed in the middle phase of C12C6C12(Et)/SL (3:7, Ctotal = 50 mM, 10 mM borax) in the presence of 0.5% glycerol. (c): Phase contrast images of (b). Bars in (b) and (c) represent 5 μm. | ||
Mechanism of this phase transition
The above results clearly show that addition of less than 2% glycerol to the ASTP system induces significant phase and microstructure transition, which is clearly different from what has been extensively reported;7–12 the influence of such a small amount of glycerol on surfactant systems has not been found so far. This significant effect of less than 2% glycerol on the ASTP phases in our research can be attributed to the borax–glycerol interaction. In an aqueous borax system, the following equilibria exist:| [B4O5(OH)4]2− + 5H2O ⇌ 2H3BO3 + 2B(OH)4− | (1) |
| H3BO3 + H2O ⇌ B(OH)4− + H+ | (2) |
Without glycerol addition, reaction (2) is disadvantageous due to the formation of a large amount of B(OH)4− in reaction (1). However, reaction (2) is greatly promoted in the presence of glycerol owing to the consummation of B(OH)4− ions by the didiol reaction:23–25
 | (3) |
As a result, a considerable number of protons are produced, which directly results in discharging of SL in the C12C6C12(Et)/SL system due to capture of protons by the laureate ions to form lauric acid (LA). Since the borax concentration is 10 mM in the 50 mM 3:7 C12C6C12(Et)/SL system, the maximum concentration of protons produced by the borax–glycerol reaction is 20 mM, which is less than the total concentration of SL (35 mM). Consequently, at most 20 over 35 SL molecules can be discharged into LA given that enough glycerol is added. The discharge of SL leads to a decrease of the negative charge density in the bilayer. As a result, an increase of the positive charge density in the phase occurs, which brings up the increase of the average head group area of the surfactants. According to Israelachvili,35 the critical packing parameter P, which is defined as P = v/al, with v the volume of the hydrophobic tail of the surfactant molecule, a the head group area at the aggregate–water interface, and l the length of the hydrophobic chain, is decreased. This suggests that high-curvature self-assemblies are preferred upon addition of glycerol to the ASTP system, in good agreement with the experimental results observed in Fig. 3c and d. If the positive charge in one domain is very high, the lamellae could be broken due to strong repulsive force between the neighboring molecules. This is probably the reason for the occurrence of lamellar fragments. Since LA is insoluble at room temperature, both the vesicle membrane and the lamellar fragments collapse as the amount of LA exceeds the capacity of the bilayers, so that the system demixes into two phases again.
The increased charge density in the C12C6C12(Et)/SL mixed system upon addition of glycerol was confirmed by fluorescence quenching experiments. In the presence of hydrophilic quenchers like Cs+ and S2O32−, fluorescence intensities of pyrene in aggregates depend on the local concentrations of the quenchers in the palisade and electrical double layers of the aggregates. For charged aggregates, the local concentration of the oppositely charged quenchers increases with increasing aggregate surface charge density. Therefore, the quenching efficiencies (i.e., the ratio of fluorescence intensity without and with the quencher, I0/Iq) reflect not only the sign of the surface charges but also the variation of the charge density on the aggregates. As shown in Table 1, the quenching efficiencies of Cs+ to pyrene in the bilayers formed in zero and 1% glycerol systems are almost equal to one, which indicates no quenching occurred by Cs+. However, the quenching efficiency of S2O32− increases considerably upon addition of 1% glycerol. This demonstrates that the bilayers carry positive charges before any glycerol is added, and the charge density increases upon addition of glycerol, which is in line with our analysis above.
| Quenching efficiency, I0/Iq | ||
|---|---|---|
| 0 glycerol – upper phase | 1% glycerol | |
| Cs+ | 0.98 | 0.96 |
| S2O32− | 1.24 | 1.83 |
The formation of LA upon addition of glycerol to the ASTP system is reflected in the ATR-IR spectra. As shown in Fig. 4, striking changes of the vibration of carbonate groups are observed. Before addition of glycerol, the carbonate groups have a strong band at 1556 cm−1 and a much weaker one at 1654 cm−1, corresponding respectively to the absorption of the carboxyl group and the carbonate group taking part in the hydrogen bond.36 Upon addition of 1% glycerol, the strength of the band at 1654 cm−1 is almost doubled, demonstrating the significant increase of LA concentration. Moreover, the characteristic vibration of COOH at 1700 cm−1 in D2O is doubled as well (see ESI†), confirming the increase of the amount of LA in the system upon addition of glycerol.
 | ||
| Fig. 4 ATR infrared spectra of C12C6C12(Et)/SL (3:7, Ct = 50 mM.) in 10 mM borax. Black line: the spectra for the surfactant-rich upper phase without addition of glycerol; red line: after addition of 1% glycerol to the system. | ||
So far we have known that the change of the macro-phase behaviours and the microstructures upon addition of glycerol in the C12C6C12(Et)/SL/borax system is caused by the reaction between glycerol and borax that leads to formation of LA. It can be inferred that addition of HCl to the ASTP system would result in a similar phase sequence. Fig. 5a shows the phase photos in the C12C6C12(Et)/SL/water system induced by HCl of different concentrations: three phases at 1–2 mM HCl, a single birefringent phase at 3 mM HCl, then again two phases at higher HCl concentration, with the upper one like ice-cream. The quantitative discrepancy of the amount of acid required to produce similar phases can be ascribed to the different strength of these two acids.
 | ||
| Fig. 5 (a): Comparison of the samples of the C12C6C12(Et)/SL/water (3:7, Ctotal = 50 mM) mixed system with an increasing concentration of hydrogen chloride. The HCl concentration in mM is indicated above the sample tubes. Upper: photos without polarizers. Lower: photos with crossed-polarizers. (b) Photos for C12C6C12(Et)/SL (3:7, Ctotal = 50 mM, 10 mM borax) in the presence of 2% glycerol (b1) at 30 °C, and at 50 °C, (b2) without and (b3) with crossed-polarizers. | ||
Since the formation of the ‘ice-cream’ phase is a result of collapse of bilayers induced by LA, it can be expected that it will disappear when the system is treated with NaOH solution or heated to improve the solubility33 of LA. This is indeed the case: upon addition of dilute NaOH, the ‘ice-cream’ phase was dissolved; after the system was heated to 50 °C, the system was transformed into a single birefringent phase, indicating the formation of multilamellar vesicles (Fig. 5b).
On the basis of the above experimental results, the phase and self-assembly transition induced by a small amount of glycerol in the C12C6C12(Et)/SL/borax system can be briefly illustrated as Fig. 6. Before addition of glycerol, planar bilayers, which are slightly positively charged, are present in the upper phase of the ASTP. Upon addition of a small amount of glycerol, part of the negatively charged SL molecules in the bilayer are transformed into neutral LA due to the release of protons from the glycerol–borax reaction. As a result, the positive charge density is increased and self-assemblies of higher curvature are preferred. Therefore, the planar lamellae in the original upper phase are transformed into multilamellar vesicles.
 | ||
| Fig. 6 Illustration of the aggregate transition induced by glycerol–borax interaction in C12C6C12(Et)/SL (3:7, Ctotal = 50 mM, 10 mM borax) system. For the sake of clarity, only a few borax molecules in the bilayers are shown. | ||
Conclusions
The aqueous two-phase system containing an alkyl carbonate surfactant in the presence of borax can be modified by addition of a small amount of glycerol. The reaction between borax and glycerol produces protons which neutralise the carbonate groups of the surfactants by formation of carbonate acid. This leads to an increase of the positive charge density in the bilayers and transforms the lamellae into vesicles. Our results demonstrate that the glycerol–borax reaction can be used as a paradigm to tailor the phase and self-assembly of surfactant systems that contain pH-sensitive components.Acknowledgements
This research was supported by the National Natural Science Foundation of China (20873001, 20633010, and 50821061) and the National Basic Research Program of China (Grant No. 2007CB936201).References
- S. L. Bradbury and W. B. Jakoby, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 2373 CAS.
- R. V. Rariy and A. M. Klibanov, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 13520 CrossRef CAS.
- G. W. Buchko, N. J. Hess, V. Bandaru, S. S. Wallace and M. A. Kennedy, Biochemistry, 2000, 39, 12441 CrossRef CAS.
- F. H. Gleason, S. E. Mozley-Standridge, D. Porter, D. G. Boyle and A. D. Hyatt, Mycological Res., 2007, 111, 129 Search PubMed.
- Physical Pharmacy, ed. M. Martin, J. Swaebrick and K. P. Ananthapadamanabhan, Lea & Febiger Press: Philadelphia, PA, 1983 Search PubMed.
- M. Sjöberg and T. Warnheim, Surfactant Sci. Ser., 1997, 67, 179 Search PubMed.
- L. Cantú, M. Corti, V. Degiorgio, H. Hoffmann and W. Ulbricht, J. Colloid Interface Sci., 1987, 116, 384 CrossRef CAS.
- L. G. Ionescu, V. L. Trindade and E. F. de Douza, Langmuir, 2000, 16, 988 CrossRef CAS.
- G. D'Errico, D. Ciccarelli and O. Ortona, J. Colloid Interface Sci., 2005, 286, 747 CrossRef CAS.
- C. C. Ruiz, L. Díaz-López and J. Aguiar, J. Colloid Interface Sci., 2007, 305, 293 CrossRef.
- Y. Yan, H. Hoffmann, A. Leson and C. Mayer, J. Phys. Chem. B, 2007, 111, 6161 CrossRef CAS.
- P. Alexandridis, R. Ivanova and B. Lindman, Langmuir, 2000, 16, 3676 CrossRef CAS.
- B. Disanayaka, C. L. Zhao and M. A. Winnik, Langmuir, 1990, 6, 162 CrossRef CAS.
- J. I. Escalante, M. Gradzielski, H. Hoffmann and K. Mortensen, Langmuir, 2000, 16, 8653 CrossRef CAS.
- M. Brard, W. Richter, T. Benvegnu and D. Plusquellec, J. Am. Chem. Soc., 2004, 126, 10003 CrossRef CAS.
- C. Giordani, A. Molinari, L. Toccacieli, A. Calcabrini, A. Stringaro, P. Chistolini, G. Arancia and M. Diociaiuti, J. Med. Chem., 2006, 49, 4581 CrossRef CAS.
- J. Gummel, F. Cousin, J.-M. Verbavatz and F. Bou, J. Phys. Chem. B, 2007, 111, 8540 CrossRef CAS.
- R. A. Moss, T. F. Hendrickson, R. Ueoka, K. Y. Kim and P. K. Weiner, J. Am. Chem. Soc., 1987, 109, 4363 CrossRef CAS.
- S. Creutz, J. V. Stam, F. C. De Schryver and R. Jrme, Macromolecules, 1998, 31, 681 CrossRef CAS.
- S. Creutz and R. Jerome, Langmuir, 1999, 15, 7145 CrossRef CAS.
- A. Morel, H. Cottet, M. In, S. Deroo and M. Destarac, Macromolecules, 2005, 38, 6620 CrossRef CAS.
- J. Yao, Y. Feng, Y. Zhao, Z. C. Li, J. B. Huang and H. L. Fu, J. Colloid Interface Sci., 2007, 314, 523 CrossRef CAS.
- R. K. Schultz and R. R. Myers, Macromolecules, 1969, 2, 281 CrossRef CAS.
- C. Y. Chen and T.-L. Yu, Polymer, 1997, 38, 2019 CrossRef CAS.
- S. J. Gao, J. M. Guo and K. Nishinari, Carbohydr. Polym., 2008, 72, 315 CrossRef CAS.
- M. G. Mellon and V. N. Morris, Ind. Eng. Chem., 1925, 17, 145 CrossRef CAS.
- G. X. Zhao and J. X. Xiao, J. Colloid Interface Sci., 1996, 177, 513 CrossRef CAS.
- H. Q. Yin, M. Mao, J. B. Huang and H. L. Fu, Langmuir, 2002, 18, 9198 CrossRef CAS.
- T. Lu, Z. H. Li, J. B. Huang and H. L. Fu, Langmuir, 2008, 24, 10723 CrossRef CAS.
- T. Lu, F. Han, Z. C. Li, J. B. Huang and H. L. Fu, Langmuir, 2006, 22, 2045 CrossRef CAS.
- L. X. Jiang, M. L. Deng, Y. L. Wang, D. H. Liang, Y. Yan and J. B. Huang, J. Phys. Chem. B, 2009, 113, 7498 CrossRef CAS.
- E. W. Kaler, A. K. Murthy, B. E. Rodriguez and J. A. N. Zasadzinski, Science, 1989, 245, 1371 CAS.
- M. Jonströmer and R. Strey, J. Phys. Chem., 1992, 96, 5993 CrossRef.
- H. Hoffmann, C. Thunig, P. Schmiedel and U. Munkert, Langmuir, 1994, 10, 3972 CrossRef CAS.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninhem, J. Chem. Soc., Faraday Trans. 2, 1976, 525 Search PubMed.
- M. T. Islam, N. Rodríguez-Hornedo, S. Ciotti and C. Ackermann, AAPS J., 2004, 6 Search PubMed , article 35.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S4. See DOI: 10.1039/b911564a |
| This journal is © The Royal Society of Chemistry 2009 |
