Exploiting particle shape in solid stabilized emulsions
Basavaraj
Madivala
a,
Steven
Vandebril
a,
Jan
Fransaer
b and
Jan
Vermant
*a
aDepartment of Chemical Engineering, K. U. Leuven, W. de Croylaan 46, B-3001, Leuven, Belgium. E-mail: jan.vermant@cit.kuleuven.be
bDepartment of Metallurgy and Materials Engineering, K. U. Leuven, Kasteelpark Arenbergpark 44, B-3001, Leuven, Belgium
First published on 2nd March 2009
Abstract
Pickering–Ramsden emulsions and other forms of particle stabilized soft materials have received quite some attention recently because of the relative ease of formulation and the possibility to create novel materials. There is, however, a clear need for approaches that are versatile and efficient. In the present work the effect of aspect ratio of particles on the stability of both water-in-oil and oil-in-water emulsions is investigated experimentally. Two types of non-spherical particles are used. Hydrophobic prolate ellipsoids with aspect ratios ranging from 1 to 9 are obtained by stretching polystyrene latex particles. Hydrophilic spindle type hematite particles have been synthesized with aspect ratios ranging from 1 to 6. A strong dependence of emulsion stability on the aspect ratio of the particles is observed. Optical as well as cryogenic scanning electron microscopy are used to visualize the droplet morphology and particulate structure and reveal fairly densely packed monolayers of ellipsoids, consistent with the mechanism of limited coalescence. Yet stable emulsions are only obtained for particles with a sufficient aspect ratio. Surface rheology on planar monolayers demonstrates the pronounced effect of aspect ratio on the surface moduli. The magnitude of the interfacial viscoelastic properties is shown to strongly depend on the aspect ratio at a given surface coverage. This is most probably due to an increased effective coverage and the occurrence of strong attractive shape induced capillary interactions. The dependence of the surface rheological properties on the aspect ratio of the particles rationalizes the observed emulsion stability as the surface rheological properties play a role in the coalescence process. The results demonstrate that interfaces with controlled surface rheology, as obtained by using shape induced capillary forces and packing effects, can be used for the rational design of Pickering emulsions and other types of high interface materials.
1 Introduction
There has been a revived interest in the past decade into the use of solid particles as an alternative to surfactants and block-copolymers to stabilize interfaces in liquid–liquid (l/l) or liquid–gas (l/g) systems. The potential of solid particles has been demonstrated for use and structure control in a wide range of material classes, for example ‘Pickering–Ramsden’ emulsions,1,2 foams,3,4 bi-continuous particle gels,5–7 polymeric membranes for separation technology,8 and functional polymer–polymer nanocomposites.9–11 However, whereas several factors contributing to the properties of such particle laden systems and their interfaces have been identified, several obstacles remain for the rational creation of such composite materials and formulations (see e.g.ref. 12). There is a clear need for a method that is both versatile and efficient, i.e. the required particle volume fraction should be small and the method should be relatively robust with respect to surface chemistry.Recent work has shown that both highly stable foams13 and colloidosomes14 can be obtained using long polymer microrods. In the present work, we extend on this approach by using model colloidal particles of different shapes, and it will be investigated if an adequate combination of particle shapes and wetting characteristics provides a rational and robust method for stabilization of interfaces in liquid–liquid systems. Particles with an anisotropic shape fundamentally alter geometric aspects such as the percolation threshold and packing issues, as well as capillary interactions specific to interfaces. The percolation threshold is lowered by using anisotropic particles,15 which is crucial when trying to impart mechanical rigidity to an interface. The latter has been suggested to be an important factor in determining the stability of emulsions obtained through its effect on limited coalescence.16 Secondly, the maximum packing density and hence the co-ordination number of spheroidal particles will differ. For 3D systems, the maximum packing density is a non-monotonic function of aspect ratio, reaching a maximum of 0.78 for an aspect ratio of 1.5, as shown by recent simulations as well as experiments.17 A similar non-monotonic evolution of the packing fraction as a function of the aspect ratio is also observed experimentally in 2D.18 As a consequence, as many as 10 particle–particle contacts are required to obtain a stable ellipsoidal packing compared to just 6 for spheres, which may lead to stronger materials.
Most importantly and specifically for particles at liquid interfaces, the use of non-spherical particles leads to interface mediated capillary forces.19–21 Young's equation requires that at the three phase contact line the angle between the interface and the colloid surface is equal to the contact angle θ. For particles with a curved particle surface, this condition cannot be met when the interface remains flat, and hence an undulation of the contact line emerges. As a consequence, spatially anisotropic interface mediated capillary interactions are induced.19–21 For ellipsoidal particles, these interactions are attractive and will affect the strength of interfacial gels. It should be stressed that a small deviation of the order of a few nanometres from spherical shape suffices to induce significant capillary attraction.22 Capillary attractions typically overrule electrostatic repulsion and lead to specific self-assembly with striking two-dimensional networks.23
In the present work, we demonstrate how both water-in-oil and oil-in-water emulsions can be formulated using non-spherical particles. Hydrophilic colloidal hematite particles with an aspect ratio from 1 to 6 are used to formulate emulsions by mixing of equal amounts of suspension and decane. The relative volume fraction size, surface chemistry and wetting conditions are kept constant and only the aspect ratio is altered. Emulsions that cannot be stabilized by spherical particles yield very stable emulsions when particles of the same surface chemistry and size range, but with a sufficiently large aspect ratio, are used, even at low particle volume fractions. Surface shear rheology is used to demonstrate that the shape anisotropy leads to monolayers with pronounced viscoelastic properties and high interfacial moduli, both at air–water and oil–water interfaces. To demonstrate the generic applicability of the mechanism, oil-in-water emulsions are produced by using even micrometre sized polystyrene spheroids. These larger sized particles are used to directly visualize the structure at the droplet surface by microscopy. For smaller sized particles, cryogenic scanning electron microscopy was used to visualize and characterize emulsion morphology. The mechanical properties of particle-laden interfaces constituting these oil-in-water emulsions are also measured by surface rheology, using both a sensitive magnetic rod rheometer and a bi-cone geometry on a standard rotational device. We will report on the effect of particle shape in controlling the stability of emulsions in relation to their surface rheological properties.
2 Experimental
2.1 Preparation of hematite spindles
| Major axis (nm) | Minor axis (nm) | Aspect ratio (AR) |
|---|---|---|
| 100 | 100 | 1.00 ± 0.1 |
| 175 | 66 | 2.6 ± 0.8 |
| 218 | 62 | 3.5 ± 0.7 |
| 325 | 72 | 4.5 ± 0.9 |
| 330 | 65 | 5.1 ± 0.8 |
| 350 | 55 | 6.3 ± 1.0 |
 | ||
| Fig. 1 Scanning electron micrographs of hematite particles of aspect ratio (a) AR = 2.6 ± 0.7 (b) AR = 3.5 ± 0.8 (c) AR = 4.5 ± 0.9 (d) AR = 6.3 ± 1. | ||
In addition to the hematite particles synthesized in the laboratory, commercially available hematite and goethite (α-FeOOH) particles of 250 nm major axis and an aspect ratio of about 5 supplied by Toda Kogyo Corporation (Germany) are also used as received, to demonstrate the robustness of the emulsification by non-spherical particles.
2.2 Preparation of polystyrene ellipsoids
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), de-ionized double distilled water, isopropyl alcohol (IPA) (VWR International, Belgium), and spherical polystyrene latex particles (Interfacial Dynamics Corporation, USA).
000), de-ionized double distilled water, isopropyl alcohol (IPA) (VWR International, Belgium), and spherical polystyrene latex particles (Interfacial Dynamics Corporation, USA).
An aqueous PVA solution is obtained by dissolving 8.75 g of PVA in 250 g of de-ionized, double distilled, water. After most of the PVA is dissolved, the solution is allowed to stand overnight to allow undissolved PVA to sediment. The clear PVA solution is then decanted. About 4 g of polystyrene latex suspension (15 wt%) is added and stirred to get a homogenous PVA–particle suspension. This dispersion is spread on a flat glass plate (15 × 45 × 1 cm). Any air bubble formed during pouring is removed with the help of a spatula and the film is air-dried. After 2–3 days, a particle-embedded PVA film is formed upon water evaporation. These films are cut into small pieces (4 × 5–7 cm depending on the desired aspect ratio after stretching) and stretched using a film-stretching apparatus similar to the one described in ref. 30, 31. Due to the manual stretching, the aspect ratio can not be controlled a priori; but for a given batch about 0.2 g of monodisperse ellipsoids with an aspect ratio between 1.25 and up to a maximum of about 10 is obtained. Up to 6 films can be stretched at once in the stretching apparatus. The film is stretched at 140 °C, above the Tg (105 ± 5 °C) of polystyrene.31 An immersion time in the order of only a few seconds has been used to avoid diffusion of oil into the film. The temperature is kept lower compared to the procedure used by Hoet al.31 to avoid changing the particle surface. The stretching is done manually and the embedded spherical particles undergo plastic deformation to yield a polymer film containing ellipsoids. The particle-laden polymer film is cleaned several times with tissue paper to remove oil from the film surface. Due to the non-uniform stretching of the film near the clamps, the edges of the stretched film are discarded and only the center part is selected for further processing. It is cut into small pieces of approximately 1 cm × 1 cm size and then the particles are recovered from the films.
The particles need to be thoroughly cleaned to remove all PVA from the surface. The pieces are soaked in isopropyl alcohol (IPA) under vigorous stirring for 1 to 2 h to remove traces of oil. The IPA-solution is then decanted and replaced with fresh IPA. This procedure is repeated 5 to 6 times. Film strips are then soaked in a 3 : 7 (by volume) IPA–water mixture for 10 to 12 h with magnetic stirring. At the end of this period, the sample is heated to about 85 °C for 45 to 60 min to dissolve the PVA matrix completely. The dispersion is centrifuged to separate the particles and the clear high viscous PVA solution at the top is poured off. This procedure is repeated two times. In the final washing step, particles are dispersed in water at about 90 °C for 1 h, to remove traces of PVA. The dispersion is stirred magnetically during this period and then centrifuged one last time to recover the particles. Ellipsoidal particles thus obtained are re-dispersed in an appropriate amount of de-ionized double distilled water depending on the particle concentration needed. To verify the robustness of our cleaning procedure we submitted a batch of spherical particles to the same thermal and cleaning treatment without stretching and found the zeta potential to be identical to the one of the untreated particles. The particle size and aspect ratio is estimated by counting about 30 to 40 particles on an SEM image of each batch, some examples of the particles are shown in Fig. 2. The aspect ratios (AR) of the particles synthesized are listed in Table 2.
| Major axis (µm) | Minor axis (µm) | Aspect ratio (AR) |
|---|---|---|
| 0.32 | 0.32 | 1.00 ± 0.1 |
| 0.68 | 0.22 | 3.00 ± 0.4 |
| 0.84 | 0.20 | 4.20 ± 0.6 |
| 3.00 | 3.00 | 1.00 ± 0.1 |
| 7.50 | 2.00 | 3.70 ± 0.3 |
 | ||
| Fig. 2 Scanning electron micrographs of polystyrene ellipsoids of aspect ratio (a) 3.7 ± 0.3 (b) 4.2 ± 0.6 (average major axis – 840 nm, average minor axis – 200 nm) made from spheres of initial diameters of 3000 and 320 nm, respectively, by mechanical stretching. | ||
2.3 Preparation of emulsions
A known amount of particles are dispersed in de-ionized, double distilled water to obtain an aqueous suspension of 1 wt% based on the aqueous phase. All the concentrations mentioned hence forth in the text are based only on the aqueous phase. Decane (99%, ACROS Organics) is used as received. The conductivity of the water used is 2 µS/cm and the surface tension is measured to be 72 ± 1 mN/m. The emulsion is formulated by vigorous manual shaking of the glass bottle containing the suspension and decane for about 1 min. A series of aqueous hematite particle suspensions having a particle concentration of 1 wt%, but of different aspect ratios are prepared and mixed with decane in a 1 : 1 volume ratio to determine the effect of aspect ratio on emulsion formulation. Two concentration series experiments are performed. In the first, a hematite suspension (1 wt%) and the oil phase are mixed in different volumetric ratios—1 : 9, 1 : 2, 1 : 1, 2 : 1 and 9 : 1. In the second, the volume ratio of hematite suspension and decane is fixed (1 : 1), but the particle concentration is varied from 1 to 10% by weight.2.4 Light transmission
The optical transparency and eventual creaming of the emulsion is measured with a Turbiscan MA 2000,32,33 especially to investigate the long term stability of the emulsions. It consists of a pulsed near infrared light source (λ = 850 nm) and two synchronous detectors. A transmission detector measures the light transmitted through the sample at an angle of 0° and the back scattering detector measures back scattered light at an angle of 135°. The sample is placed in a cylindrical glass tube of 1.6 cm diameter having a length of 12.5 cm. The transmission and back scattering data are acquired every 40 µm along the tube. The hematite suspension and decane are first added to a tube and light transmission is measured along the length of the tube. The tube is then shaken vigorously to create an emulsion and the optical transmission is measured as a function of time.2.5 Electrical conductivity
Electrical conductivity measurements are used to identify the continuous phase of the emulsion. A substantial conductivity indicates an emulsion containing water as a continuous phase i.e., oil-in-water emulsions and no detectable conductivity indicates that the continuous phase is oil i.e., water-in-oil emulsion.34 To enhance the conductivity of the aqueous phase, approximately 1 × 10−4 M NaCl is added to the aqueous suspension. The conductivity of the emulsions soon after their formulation is measured by inserting a conductivity probe into the emulsion phase and recording the conductivity as a function of time. The conductivity measurements are done with a bench top conductivity meter (Model 150 A plus, Thermo Orion, USA) equipped with platinum electrodes.2.6 Optical microscopy
Direct observation of the emulsion droplets and the structure of the particles at the interface is difficult due to the turbidity of the sample. Transferring a droplet of the emulsion phase to microscopy slides leads to a confinement of the droplets which would entail mechanical deformations. To visualize the droplets, the mechanical integrity of the particle coated droplets could be exploited, and we were able to deposit them onto a planar air–water interface, making characterization of the floating droplets possible by simple light microscopy. A monolayer of particle coated oil droplets is pipetted from the emulsion and gently deposited at a water–air interface. The resulting monolayer of droplets is stable up to few weeks when evaporation is prevented. Bright field video microscopy is used to visualize and determine the size distribution of the emulsion droplets. For emulsion droplets created with micrometre sized ellipsoids, their packing and organization can then be visualized. An OLYMPUS (BX51WI) fixed stage microscope equipped with a CCD camera (Hamamatsu, C8800-21C) is used. With this camera, 12-bit grey scale images of 1000 pixels × 1000 pixels resolution can be obtained. The whole set-up is placed on a vibration-isolated table. A 5× and 50× long distance objectives with a numerical aperture of 0.10, 0.45 and a working distance of 19.6 mm, 15.0 mm, respectively, are used to visualize the droplets and the particles at the droplet interface, respectively.2.7 Cryo-SEM
Cryogenic scanning electron microscopy (cryo-SEM) is used to visualize the two types of particle covered droplets i.e., polystyrene covered water droplets and hematite covered decane droplets. Cryo-SEM measurements are done using a Philips XL30 FEG ESEM equipped with an Emitech K1250 cryogenic system. The sample is frozen by quenching it in liquid nitrogen for about 2 min. The sample is then transferred to the sample preparation chamber where the sample is freeze fractured. Subsequently, a controlled sublimation of the sample is carried out at a temperature of −70 °C for about 30 min, after which the sample is coated with a thin gold layer. The sample is then transferred to the ESEM chamber maintained at a pressure smaller than 10−6 mbar and a controlled sublimation is carried out again for 15 to 20 min to remove any ice formed during the sample transfer. A fairly low acceleration voltage of 5 kV is used to avoid sample damage. The inverse water-in-oil emulsions are easier to image by cryo-SEM, because the matrix phase, i.e.decane, is removed during sublimation which renders visualization of the particles at the interface possible. This is not possible when water is the continuous phase due to the formation of large chunks of ice with the cooling method used.2.8 Interfacial rheology
A bi-cone attached to a controlled stress rheometer (MCR 501, Anton Paar Physica, Austria) is used to measure the interfacial rheology of monolayers containing hematite particles of aspect ratio 4.3 ± 0.6. For each measurement, a fresh monolayer is prepared by depositing particles from a dispersion containing a known weight of hematite particles at the water–decane interface. The spreading dispersion consisted of 0.2 wt% particles in water and equal volumes of ethyl alcohol. Typically, 10 to 200 µL suspension is used in each experiment. Before each measurement, a waiting time of 30 min is used after creating the monolayer to allow for alcohol evaporation from the interface. As with all surface rheological experiments, a careful separation of the contributions of flow in the bulk and at the interface is required. For the bi-cone, the analysis of Slattery et al.,35 and Erni et al.,36 was used which allows the determination of the complete flow field. For lower surface coverages, the sensitive magnetic rod interfacial stress rheometer is used.37,38 Monolayers of ellipsoids and spheres were prepared in a 100 mm diameter petri dish. At the centre of this dish, a rectangular channel of width, 2R = 20 mm and length, L = 70 mm was placed. For both geometries the adequate correction procedures to separate bulk and surface contributions are used.35,36,37Since the hematite particles are smaller than 1 micron, it is not possible to visualize the monolayer structure and to determine the surface coverage by optical microscopy. During the spreading process, a number of particles are lost to the sub-phase, and hence the use of a mass balance to determine surface coverage is not accurate. To determine the amount of particles at the interface and in the sub-phase, a known amount of dispersion is spread on to a water–decane interface in a petri dish. The particles that escape to the bulk during the spreading process are allowed to settle over a period of two weeks. The mass of the particles thus settled is determined by careful decantation of oil and water. From the difference between the mass of the particles in the dispersion used for spreading, and the mass of the particles accumulated at the bottom, the surface coverage is estimated. As an alternate method, the efficiency of spreading the particles at the interface can be estimated by scooping up the particles at the interface by filter paper or microscopy slides. The filter paper and glass slides are dried in a vacuum oven at 50 °C overnight to determine the amount of particles adsorbed. By using both methods, for an average of six experiments, estimates of the surface coverage within ±10% are possible.
3 Results and discussion
3.1 Emulsions stabilized by hematite particles
 | ||
| Fig. 3 Effect of particle aspect ratio on the behaviour of shaken equal volume mixtures of decane and an aqueous suspensions containing 1 wt% of suspensions. The aspect ratio of the particles in the aquous phase is continuously changed (a) AR = 1.0 (b) AR = 2.8 ± 0.7 (c) AR = 3.6 ± 0.8 (d) AR = 4.6 ± 0.9 (e) AR = 5.3 ± 0.8 and (f) AR = 6 ± 1. | ||
During the vigorous shaking of the aqueous suspension and the decane, droplets of decane are generated. The particles in the aqueous phase are trapped by the interface that is generated as they reduce the total surface energy, by taking away a part of the interface between water and oil. After shaking, the droplets coarsen and their specific area is decreased and they become covered by a densely packed monolayer of hematite particles. The coalescence process therefore halts due to what is described as ‘surface saturation’,39 the process is referred to as limited coalescence.16,39–41 The oil water droplets which would coalesce and lead to macroscopic phase separation in the absence of particles are now stable, as coalescence and potentially also Ostwald ripening are arrested due to the particle monolayer. The mechanism of limited coalescence is similar to that observed in the stabilization of phase separating binary liquid mixtures using colloidal particles to obtain so called ‘bijels’.5,6 The coarsening is supposed to stop when all the droplets are covered with a densely packed monolayer. However, what has been described as ‘surface saturation’ only seems to play a role provided the aspect ratio of the particles is sufficiently large, as the particles have the same wetting properties and more or less the same size. Hence either the capturing efficiency or the way in which the non-spherical particles affect the stiffness of the interface are dependent on aspect ratio (see below).
It is interesting to note that the volume of emulsion that is generated also depends on the aspect ratio of the particles. As the aspect ratio is increased as shown in Fig. 3(d) to 3(f), the amount of emulsified phase increases. Similar results are observed with an increase in particle concentration at a constant aspect ratio. This suggests that an increase of aspect ratio is an efficient manner to stabilize the interface at a low particle loading, which could play an important role in formulation of a wide range of high interface materials. When a small volume of the phase containing the emulsion droplets is injected into a petri dish containing water, all droplets immediately cream to the top, indicating that the emulsion consisted of sufficiently large decane droplets coated with hematite ellipsoids. This is consistent with the hydrophilic nature of the hematite particles, which are expected to give rise to stable oil-in-water emulsion.2,42 It should be noted that when other, more energy intensive emulsification techniques are used and smaller droplets will be formed, they may actually sediment as the average density of the hematite covered decane droplets increases as the surface to volume ratio increases, keeping in mind the high density of the hematite.
To get an idea of the droplet size polydispersity, a microscopy image of the emulsion droplets deposited onto the water–air interface is shown in Fig. 4. The emulsion droplets are polydisperse and the size varies between 20 and 475 µm with an average diameter of 185 µm. The nature of the continuous phase was confirmed by conductivity measurements, using a small amount of electrolyte of 1 × 10−4 M NaCl added to the aqueous phase. Immediately after emulsification, the electrode of the conductivity probe is inserted into the emulsion phase and the conductivity is measured as a function of time (not shown here). There is an initial decrease in conductivity most probably due to film drainage, but the conductivity eventually leveled off after about 2 h and essentially remained unchanged thereafter. The conductivity value of 15 µS/cm confirms that the continuous phase is water, not oil.
 | ||
| Fig. 4 Optical micrograph of oil droplets coated with hematite particles when deposited at an air–water interface. | ||
The data in Fig. 3 and 4, clearly demonstrate that sufficiently long particles are efficient emulsifiers of the two immiscible fluid phases, even when spherical and lower aspect ratio particles with the same wetting properties do not produce an emulsion. These results suggest that the observed stabilization of emulsions and the phenomenon of limited coalescence by particles, are not only controlled by the wetting and surface coverage properties. As mentioned in the introduction, two shape related factors that may contribute are effects related to packing and the occurrence of shape induced capillary attractions. The maximum random jammed packing density in three-dimensions17 and also the packing in dense monolayers of ellipsoids18 depends non-monotonically on the aspect ratio, reaching a maximum for slightly anisotropic particles (AR ∼ 1.8). The observation that no emulsion droplets could be generated with the particles of low to moderate aspect ratios suggests that maximum packing per se is not the determining issue. The effective surface coverage may play a role, but then a more gradual effect of the aspect ratio would be expected. More importantly, the shape induced capillary interactions do increase significantly with increasing aspect ratio. These forces arise because Young's equation requires that at the three phase contact line, the angle between the interface and the colloid surface is equal to the contact angle θ. This condition can not be met when the interface remains flat, hence the contact line is not elliptical, but an undulated saddle-like ellipse that is elevated at the sides and depressed at the tip. Such a deformation profile has been calculated numerically and confirmed experimentally.20,21 Lehle et al. calculated the maximum change in the meniscus elevation (Δumax) by solving Young's equation locally.21 These calculations show that the meniscus deformation is a strong function of both aspect ratio and contact angle. For particles with the same wetting properties (as is the case here), the magnitude of this maximum meniscus elevation increases with increasing aspect ratio. It has been shown that these attractive capillary forces result in aggregated structures in planar monolayers containing ellipsoids.23 For planar monolayers of polystyrene particles, very dense particle networks were observed, having as a typical building block ellipsoids connected at their tips in triangular or flower-like configurations. These networks responded elastically in shear and compression,23 and further surface rheological properties will be reported below to confirm this. The formation of such highly elastic solid shells around the droplets prohibit the coalescence of these drops, as also suggested by Arditty et al.16 This mechanical integrity of the droplets is also evidenced from the fact that they can be manipulated and transferred to a petri dish to float on water as described above.
| Particle conc. (wt%) | Suspension (vol. frac.) | Decane (vol. frac.) | Emulsion (vol. frac.) |
|---|---|---|---|
| 1.0 | 0.5 | 0.5 | 0.60 |
| 5.0 | 0.5 | 0.5 | 0.74 |
| 10.0 | 0.5 | 0.5 | 0.90 |
 | ||
| Fig. 5 A digital image of vials obtained by mixing amounts of suspensions of hematite ellipsoids of AR = 4.6 ± 0.9 at 1% by weight and decane at ratios of (a) 1 : 1 (b) 2 : 1 and (c) 9 : 1 volume ratio. | ||
 | ||
| Fig. 6 A digital image of the emulsions obtained by mixing a suspension of hematite ellipsoids (AR = 4.6 ± 0.9) of (a) 1%, (b) 5% and (c) 10% by weight particle concentration with decane and shaking vigorously. | ||
 | ||
| Fig. 7 Microscopy image of emulsion droplets obtained by mixing suspension of hematite ellipsoids (AR = 4.6 ± 0.9) of (a) 1%, (b) 5% and (c) 10% by weight particle concentration with decane, subsequently spread onto a water–air interface. | ||
3.2 Emulsions stabilized by polystyrene and goethite particles
To confirm the generic nature of the emulsification by exploiting particle shape effects using non-spherical particles, a variety of other systems were tested. First, commercially available hematite spindles and iron oxide particles (goethite, α-FeOOH) with different surface chemistry were used. It was observed that both suspensions readily formed very stable emulsions, images of the droplets are shown in Fig. 8(a). The size of goethite coated decane droplets obtained by simple shaking was found to be in the size range of 21–860 µm with an average diameter of 200 µm. The hydrophilic goethite particles formed oil-in-water emulsions, similar to the emulsions stabilized by hematite particles. | ||
| Fig. 8 Optical microscopy images of (a) oil-in-water droplets stabilized by spindle-like goethite particles (at 5× magnification) (b) inverse, water-in-oil droplets stabilized by ellipsoidal polystyrene particles prepared from 320 nm spheres (at 5× magnification) (c) inverse, water-in-oil emulsions stabilized by ellipsoidal polystyrene particles prepared from 3000 nm spheres (at 5× magnification) (d) same as (c) but at 50× magnification revealing the particle packing. | ||
Polystyrene spheres and ellipsoids enable one to verify that the effect of aspect ratio is also valid for inverse emulsions. When emulsions are formulated using a suspension of 320 nm diameter or 3 µm diameter spheres, no emulsification is obtained when shaking equal volume mixtures of decane and 1 wt% suspensions. With both submicrometre particles of AR = 3.0 ± 0.4 and micrometre sized particles 4.3 ± 0.6 prepared from 320 nm spheres and of AR = 3.7 ± 0.3 prepared from 3 µm spheres, a stable emulsion can be obtained. The emulsions obtained with hydrophobic polystyrene latex particles are of water-in-oil type, consisting of water droplets coated with polystyrene ellipsoids. This is confirmed by measurements of the conductivity of the continuous phase and also by the fact that a drop of emulsion phase injected into water sediments to the bottom. The droplets can be visualized as in Fig. 8(b) and (c). The average size of water droplets coated with nano-sized ellipsoids is about 210 µm. In the microscopy images, some of the water droplet coated micron-sized ellipsoids appeared to be non-spherical (spheroidal) in shape with an average major axis of 500 µm and an average minor axis of 450 µm. Similar non-spherical particle coated ‘armored’ bubbles and liquid drops that support inhomogeneous external stresses have been observed recently.43 It should be noted that the emulsions are already obtained at lower aspect ratios with polystyrene particles compared to the hematite. This suggests that the critical aspect ratio depends on the surface chemistry and wetting and is probably related to the dependence of the interaction forces between the particles in the monolayer covering the droplets on the contact angle. A clear increase in average droplet size with an increase in particle dimension is observed.
The micrometre sized PS ellipsoids offer the possibility to visualize the structure of the droplets and the monolayers by optical microscopy as in Fig. 8(c). A higher magnification image showing the surface of a droplet covered with a dense monolayer of ellipsoids is shown in Fig. 8(d). It reveals a complex packing of ellipsoids in the monolayer, which is not random. Some degree of translational order could be found in the form of ellipsoids stacked in a side-side configuration. A few ellipsoidal particles appear with a circular cross section which represent ellipsoids that are flipped upright, as has only been observed in dense planar monolayers, under compressional stress.18 Again the limited coalescence can explain the observation as it will lead to a reduction of available surface area per volume as time goes on and hence also leads to an—albeit potentially complicated—compression of the particles at the interface which seems to induce ‘flippers’. If the number of particles in an upright configuration could be increased, porcupine droplets could be envisaged. Especially at high aspect ratios, flipped particles could provide mechanical stability by steric stabilization or lead to bridging of droplets at a distance, thereby also preventing coalescence.
The structure of the monolayer around the droplets coated with small polystyrene and hematite nanoparticles can not be visualized with optical microscopy, hence cryo-SEM is used. The micrographs of water droplets coated with polystyrene ellipsoids are shown in Fig. 9. In Fig. 9(a), a single water droplet of about 30 micrometre diameter, covered by a monolayer of ellipsoids, is shown. A higher magnification image of this water droplet shows that packing of ellipsoids in the monolayer is again not completely random (Fig. 9(b)) and a limited number of out-of-plane flipped particles can be observed. For the in-plane structures, a translational order in the form of ellipsoids stacked in a side-side configuration could be found. The cryo-SEM images of oil-in-water type emulsions also shows oil droplets densely covered with particles but they will not be reported here as the presence of ice formation during the cooling hampers a good visualization and may even have altered the structure of the monolayer.
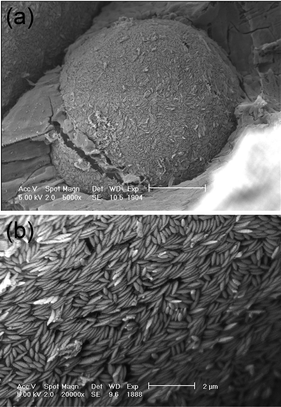 | ||
| Fig. 9 (a) Cryo-SEM image of a water droplet covered with polystyrene ellipsoids of 4.3 ± 0.6 aspect ratio. (b) Detail of the packing. | ||
3.3 Surface rheology
Due to the increase of the effective surface coverage with aspect ratio and the presence of the strong attractive interface mediated capillary forces, the surface rheological properties of the interface can be expected to be different from the bare, Newtonian surface. The low frequency plateau modulus for monolayers of polystyrene spheres and ellipsoids (data from ref. 23 and 44) as a function of surface coverage are given in Fig. 10(a) for PS spheres and ellipsoids of aspect ratio 5.5, at the air–water interface. Two significant features emerge: firstly, the threshold at which a mechanical response can be measured decreases with increasing aspect ratio as a significant elastic response is obtained at much lower surface coverages. Secondly, although we only have limited data points, a characteristic power law dependence is obtained for the modulus as a function of surface coverage, and when the aspect ratio is increased, most probably due to the presence of the capillary interactions, higher surface moduli are obtained. | ||
| Fig. 10 (a) G′s,0 as a function of surface coverage for spherical particles (from ref. 44) and spheroidal particles of AR = 5.5. (b) Effect of aspect ratio on the surface storage and loss modulus for monolayers of hematite particles with aspect ratios of 2.8 and 4.6 deposited using suspensions with the same bulk concentrations. | ||
Fig. 10(b) shows the evolution of the surface moduli as a function of the strain amplitude for two 2D-suspensions of hematite particles at the oil–water interface as a function of the strain amplitude, prepared and deposited in the same manner and corresponding to a surface coverage of about 0.7, but with a different aspect ratio (factor 1.6). The linear viscoelastic moduli differ by a factor 5 or more. The effect of aspect ratio on the surface therefore seems to be more pronounced than a mere increase of the effective surface coverage, which is proportional to aspect ratio. The scaling with an effective volume fraction is observed for hematite suspensions in water in 3D.29 The difference between the hematite particles at the interface and in bulk lies in the microstructure. In bulk, Solomon and Boger show how the screened electrostatic interactions dominate and an effective volume fraction takes into account shape and double layer thickness.29 At the interface, the attractive shape induced capillary interactions has been shown to dominate even over very strong electrostatic interactions for polystyrene ellipsoids and leads to aggregated suspensions in 2D.23 Dense particle networks are observed, having as a typical building block ellipsoids connected at their tips in triangular or flower-like configurations. These networks responded elastically in shear and compression.23 For the hematite particles no direct observations are possible at planar or curved interfaces, but the nonlinear rheological response is consistent with an aggregated microstructure. In Fig. 10(b), as the strain amplitude is increased, the surface storage modulus is observed to decrease monotonically, whereas the surface loss modulus is observed to decrease, already at small values of the strain amplitude. In 3D, this behaviour is consistent with a structure of a flocculated dispersion. The increase of the loss modulus with strain is caused by rearrangements in the network leading to enhanced dissipation.45 Surface rheological properties of colloidal gels of spheres in 2D have been shown to be very similar in nature to their 3D counterparts, similar properties and scaling behavior have been observed.44 Hence the strain sweep results can be seen as a indication of an aggregated microstructure. This should be tested more rigorously using measurements that asses the thixotropic nature of the interface and address the possible issues associated with polydispersity, but this lies beyond the scope of the present work. The rheological results, combined with the observations of the structures at the droplet interface and observations indicate that shape effects can be exploited to control the surface rheological properties in a manner which is robust and predictable for both air–water and oil–water interfaces.
In order to evaluate the effect of surface coverage on the rheological properties for the hematite suspensions, oscillatory shear experiments are performed on monolayers of hematite particles (AR = 4.3 ± 0.6) at the water–decane interface. The monolayer is allowed to stand for 30 min before each experiment to allow alcohol used in the spreading aliquot to diffuse away from the system. Time dependent measurements (not shown here) showed only minor and very slow changes over time scales of up to six hours. The surface elastic and viscous modulus measured 30 min after spreading the particles, are plotted in Fig. 11(a) as a function of strain amplitude at a fixed frequency of 6.3 rad/s, for two different surface concentrations. The monolayers exhibit dominant elastic behaviour at low strains in both cases. As the strain is increased, the typical nonlinear response for colloidal gels is again observed. The surface storage modulus decreases monotonically as a function of strain amplitude, whereas the loss modulus first increases before decreasing. Fig. 11(a) demonstrates that the limiting strain amplitude for which the response remains linear is smaller than 0.05% and decreasing with surface coverage, which is similar to the values observed in 3D flocculated gels46 and in 2D aggregated systems of spherical particles.44 Stable dispersions or glassy systems typically yield at larger strain amplitudes. The strain sweeps in Fig. 11(a) confirm the trends observed in Fig. 10(b) and indicate that a fairly brittle surface gel is obtained from the non-spherical particles. Correspondingly, the linear viscoelastic surface moduli at a strain amplitude of 0.01% are found to be independent of frequency, for both volume fractions studied (Fig. 11(b)). Surface rheological measurements at other surface coverages also show similar trends. Overall, it can be concluded that the rheological behaviour is consistent with the presence of an aggregated 2D microstructure and the moduli show a strong dependence on aspect ratio and surface coverage. Control over the rheological properties of these monolayers can be obtained by both the surface coverage and the aspect ratio, enabling one to tune the surface rheological response.
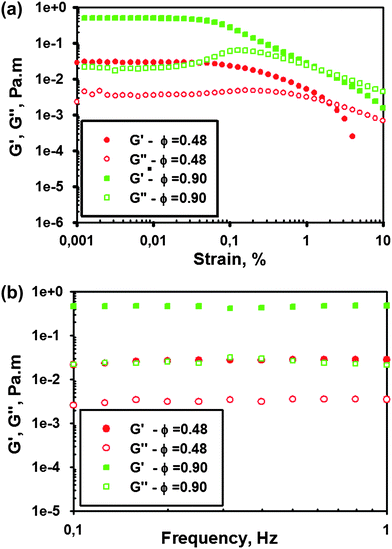 | ||
| Fig. 11 (a) Strain sweep and (b) frequency sweeps at a fixed strain of 0.01% on the monolayers of hematite ellipsoids of an aspect ratio 4.3 ± 0.6 at two different surface coverages. | ||
The ellipsoidal particles used in this work form dense aggregated structure at planar interfaces23 yielding linear viscoelastic moduli that are orders of magnitude higher than aggregated networks of spheres, even when accounting for the increase of the effective surface coverage. A significant difference between the systems of different aspect ratios is the occurrence of the strong capillary attractions. This may be the main reason why sufficiently long ellipsoidal particles can stabilize emulsions efficiently. The results presented here therefore point to the important role of surface rheology in stabilizing Pickering emulsions. The importance of surface rheology, and the presence of a high modulus rigid interface, as for example induced by the presence of a dense aggregated 2D suspension, may also rationalize why in spherical particles, mixtures of salt, surfactant and particles stabilize emulsions more efficiently.47,48 A second factor contributing to the formation of stable droplets is the efficiency of particle capture during shaking. In the surface rheology experiments it was observed that the efficiency of particle deposition at the interface depends on the particle aspect ratio. For the same spreading protocol, the higher aspect ratio particles deposited more readily at the interface. Understanding the mechanisms by which particles are trapped at the interface could be an important step in further designing efficient emulsification processes.
4 Conclusions
Non-spherical particles can be used to efficiently form stable oil-in-water and water-in-oil emulsions. Using a series of hematite particles of the same surface chemistry and roughly the same size range, it was demonstrated that above a critical aspect ratio, stable droplets are obtained at fairly low volume fractions when producing emulsions by simple shaking. The robustness of the effect was shown using a range of non-spherical particles. Inverse emulsions are obtained using ellipsoidal polystyrene particles. Direct observation for micrometre sized particles and cryo-SEM observations for sub-micron ellipsoids demonstrate a dense packed layer on the particle surface with some local ordering and a number of out-of-plane oriented particles. Surface rheological measurements of these surface coverages show that monolayers are highly elastic. Due to the increased effective surface coverage and the occurrence of shape induced capillary forces, strong but brittle elastic surface gels are obtained. The control over the emulsion stability seems to be closely linked to the surface rheology, and can be affected by aspect ratio and surface coverage. The aspect ratio dependent emulsion stabilization has potential in many applications where, for example, destabilization can be achieved just by shape changes. Also, the size of emulsion droplets can be tuned by changing the particle concentration, particle size and energy input.Acknowledgements
We would like to thank Rudy Devos (MTM, K.U. Leuven) for his assistance in cryo-SEM imaging and Dr S. Reynaert for help with the measurements using the ISR. JV and JF acknowledge financial support from a research program of the Research Foundation – Flanders (FWO-Vlaanderen, project G.00469.05). JV and MGB acknowledge the EU for support through the NoE Softcomp (6th FP).References
- S. U. Pickering, J. Chem. Soc., 1907, 91, 2001 RSC.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Coll. Int. Sci., 2003, 100, 503 CrossRef.
- W. Ramsden, Proc. Roy. Soc. London, 1903, 72, 156 CAS.
- B. P. Binks and T. S. Horozov, Ang. Chem., Int. Ed., 2005, 44, 3722 Search PubMed.
- K. Stratford, R. Adhikari, I. Pagonabarraga, J. C. Desplat and M. E. Cates, Science, 2005, 309, 219.
- E. M. Herzig, K. A. White, A. B. Schofield, W. C. K. Poon and P. S. Clegg, Nature Mat., 2007, 6, 966–971 CrossRef CAS.
- M. E. Cates and P. S. Clegg, Soft Matter, 2008, 4, 2132 RSC.
- P. Vandezande et al., Chem. Mater., 2008, 20, 3457 CrossRef.
- F. Gubbels et al., Macromolecules, 1995, 28, 1559–1566 CrossRef CAS.
- I. Mironi-Harpaz and M. Narkis, J. Appl. Polym. Sci., 2001, 81, 104 CrossRef CAS.
- J. Vermant, G. Cioccolo, K. G. Nair and P. Moldenaers, Rheol. Acta, 2004, 43, 529 CrossRef CAS.
- Colloidal Particles at Liquid Interfaces, ed. B. Binks and T. S. Horozov, Cambridge University Press, Cambridge, 2006 Search PubMed.
- R. G. Alargova, D. S. Warhadpande, V. N. Paunov and O. D. Velev, Langmuir, 2004, 20, 10371 CrossRef CAS.
- P. F. Noble, 0.J. Cayre, R. G. Alargova, O. D. Velevand and V. N. Paunov, J. Am. Chem. Soc., 2004, 126, 8092 CrossRef CAS.
- T. Schilling, S. Jungblut and M. A. Miller, Phys. Rev. Lett., 2007, 98, 108303 CrossRef CAS.
- S. Arditty, V. Schmitt, F. Lequeux and F. Leal-Calderon, Eur. Phys. J. B, 2005, 44, 381 CrossRef CAS.
- A. Donev, I. Cisse, D. Sachs, E. A. Variano, F. H. Stillinger, R. Connelly, S. Torquato and P. M. Chaikin, Science, 2004, 303, 990 CrossRef CAS.
- M. G. Basavaraj, G. G. Fuller, J. Fransaer and J. Vermant, Langmuir, 2006, 22, 6605 CrossRef CAS.
- J. C. Loudet, A. M. Alsayed, J. Zhang and A. G. Yodh, Phys. Rev. Lett., 2005, 94, 018301 CrossRef CAS.
- J. C. Loudet, A. G. Yodh and B. Pouligny, Phys. Rev. Lett., 2006, 97, 018304 CrossRef CAS.
- H. Lehle, E. Noruzifar and M. Oettel, Eur. Phys. J. E, 2008, 26, 151 CrossRef CAS.
- E. A. Van Nierop, N. A. Stijnman and S. Hilgenfeldt, Europhys. Lett., 2005, 72, 671 CrossRef CAS.
- M. G. Basavaraj, J. Fransaer and J. Vermant, Langmuir, 2009 DOI:10.1021/la803554u.
- E. Matijevic, Annu. Rev. Mater. Sci., 1985, 15, 483 CAS.
- M. Ocana, M. P. Morales and C. J. Serna, J. Colloid Interface Sci., 1995, 171, 85 CrossRef CAS.
- M. Ocana, M. P. Morales and C. J. Serna, J. Colloid Interface Sci., 1999, 212, 317 CrossRef CAS.
- D. M. E. Thies-Weesie, A. P. Philipse and S. G. J. M. Kluijtmans, J. Colloid Interface Sci., 1995, 171, 211 CrossRef.
- M. Ohmori and E. Matijevic, J. Colloid Interface Sci., 1992, 150, 594 CrossRef CAS.
- M. J. Solomon and D. Boger, J. Rheol., 1998, 42, 929 CrossRef CAS.
- K. M. Keville, E. I. Franses and J. M. Caruthers, J. Colloid Interface Sci, 1991, 144, 103 CrossRef CAS.
- C. C. Ho, A. Keller, J. A. Odell and R. H. Ottewill, Colloid Polym. Sci., 1993, 271, 469 CrossRef CAS.
- O. Mengual, G. Meunier, I. Cayre, K. Puech and P. Snabre, Talanta, 1999, 50, 445 CrossRef CAS.
- O. Mengual, G. Meunier, I. Cayre, K. Puech and P. Snabre, Colloids Surf., A, 1999, 152, 111 CrossRef CAS.
- S. Fujii, S. P. Armes, B. P. Binks and R. Murakami, Langmuir, 2006, 22, 6818 CrossRef CAS.
- S. G. Oh and J. C. Slattery, J. Colloid Interface Sci., 1978, 67, 516 CrossRef CAS.
- P. Erni, P. Fischer, E. J. Windbah, V. Kusnezov, H. Stettin and J. Lauger, Rev. Sci. Instrum., 1998, 69, 4916.
- C. F. Brooks, G. G. Fuller, C. W. Frank and C. R. Robertson, Langmuir, 1999, 15, 2450 CrossRef CAS.
- S. Reynaert, C. F. Brooks, P. Moldenaers, J. Vermant and G. G. Fuller, J. Rheol., 2008, 52, 261 CrossRef CAS.
- S. Arditty, C. P. Whitby, B. P. Binks, V. Schmitt and F. Leal-Calderon, Eur. Phys. J. E, 2003, 11, 273 CrossRef CAS.
- R. M. Wiley, J. Colloid Interface Sci., 1954, 9, 427 Search PubMed.
- T. M. Whitesides and D. S. Ross, J. Colloid Interface Sci., 1995, 169, 48 CrossRef CAS.
- E. J. Stancik, M. Kouhkan and G. G. Fuller, Langmuir, 2004, 20, 90 CrossRef CAS.
- A. B. Subramaniam, M. Abkarian, L. Mahadevan and H. A. Stone, Nature, 2005, 438, 930 CrossRef.
- S. Reynaert, P. Moldenaers and J. Vermant, Phys. Chem. Chem. Phys., 2007, 9, 6463 RSC.
- W. J. Frith, T. A. Strivens and J. Mewis, J. Colloid Interface Sci., 1990, 139, 55 CrossRef CAS.
- R. Buscall, P. D. A. Mills, J. W. Goodwin and D. W. Lawson, J. Chem. Soc. Faraday Trans., 1988, 84, 4249 Search PubMed.
- B. P. Binks, J. A. Rodrigues and W. J. Frith, Langmuir, 2007, 23, 3626 CrossRef CAS.
- B. P. Binks and J. A. Rodrigues, Langmuir, 2007, 23, 7436 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
