Covalent layer-by-layer assembly—an effective, forgiving way to construct functional robust ultrathin films and nanocomposites
David E.
Bergbreiter
* and
Kang-Shyang
Liao
Department of Chemistry, Texas A&M University, P.O. Box 30012, College Station, Texas 77842-3012, USA. E-mail: bergbreiter@mail.chem.tamu.edu
First published on 3rd October 2008
Abstract
Layer-by-layer (LbL) assembly is a versatile way to construct thin film nanocomposites and to modify surfaces. This technology is broadly useful because it is a simple and forgiving synthetic method. Most commonly, this approach to fabrication of an interface involves multilayer ionic assembly of polyelectrolytes. While ionic grafts are easy to prepare, ionic assemblies cannot be used in all applications. This highlight focuses on an alternative approach that uses covalent bonds to form multilayer grafts. Selected examples of this chemistry showing the scope of this methodology in the formation of ultrathin film nanocomposites and its potential are discussed below.
 David E. Bergbreiter David E. Bergbreiter | David Bergbreiter's work at Texas A&M University since 1974 has involved studies in organic, polymer and catalysis chemistry. In addition to work involving polymer surface chemistry and thin films discussed here, he and his group have studied asymmetric synthesis, and green chemistry ways to recover/recycle homogeneous transition metal catalysts. |
 Kang-Shyang Liao Kang-Shyang Liao | Kang-Shyang Liao entered Texas A&M University in the Bergbreiter group in August 2003. His research focused on designing new functional graft surfaces using covalent layer-by-layer self-assembly techniques. He received his PhD in August 2008 and is currently pursuing a post-doctoral position with Professor Seamus Curran at the University of Houston. |
1. Introduction
Modern approaches to layer-by-layer (LbL) assembly most often use ionic processes first reported almost twenty years ago by Decher.1 These LbL processes are widely used methods for surface modification in which thin film surface grafts are assembled by alternate deposition of mutually attractive molecules or particles.2–4 Most commonly these species are positively and negatively charged polymers. This self-assembly approach to a multilayer graft requires formation of a charged surface as a first step. If an anionic surface is present, the first deposition step involves entropically favored assembly of a cationic polymer onto this anionic surface. This process is entropically favored because the polyvalent attachment of a single cationic polymer releases many cations and anions in to solution. The product of this ionic assembly process is a surface with an excess of the polycation. Subsequent alternating depositions of more anionic and then cationic polymers produce additional ‘bilayers’. This process can be automated and can be continued until the graft reaches the desired thickness or the surface has the desired properties. By varying experimental conditions, graft thicknesses can be reproducibly controlled. This LbL approach is simple and works on substrates of any shape. Recent reviews discuss this chemistry.5–7While LbL assembly most often uses polyelectrolytes for graft construction, others have shown that other chemistry works too. For example, self-assembly of hydrogen bond donor and acceptor polymers is a non-ionic way to effect multilayer assembly. In this scheme, an uncharged hydrogen bond acceptor polymer like poly(ethylene oxide) or poly(N-isopropylacrylamide) and a hydrogen bond donating polymer like poly(acrylic acid) are alternately deposited one on the other to form a multilayer graft.
Though ionic or hydrogen bond self-assembly processes are experimentally simple and broadly useful, they have some drawbacks. Specifically, ionic or hydrogen bonded LbL assemblies can disassemble under conditions where the ionic or hydrogen bonds are unstable. For example, strongly acidic, strongly basic, or high ionic strength solutions can affect these grafts' stability. Most often, this is not a problem. In other cases, facile disassembly of a multilayer grafts even provides advantages (e.g. in drug release applications). Nonetheless, some applications are better served by more chemically robust multilayer grafts that withstand harsh conditions.
Covalent assembly is an alternative way to assemble multilayers grafts on diverse surfaces that provides this extra stability and is the subject of this highlight. This relatively ‘new’ way to make grafts is an approach to surface modification with ancient roots. Oriental lacquerware fabrication dates back to 4000 BC in China and Japan and has similarities to covalent multilayer assembly processes.8,9 The sap used for the lacquerware coatings is an aqueous emulsion of phenolic lipids and polysaccharide–glycoprotein complexes from lacquer trees. The process that generates the product’s high gloss, durable coating is an LbL process involving as many as 40 repeated coating–drying–polishing–rubbing–drying cycles. Enzymatic radical dimerization or polymerization of phenolics leads to covalent bonds during the drying cycle of this covalent LbL process. The numerous perfectly preserved examples of lacquerware from the Qin dynasty (around 200 BC) of China are both artistic and aesthetically pleasing evidence that substantiates the notion that covalent multilayer grafts can be durable and useful.
2. Requirements and general features for covalent LbL assembly
Covalent LbL assembly differs from ionic LbL assembly in a number of respects. First, while ionic LbL assembly only requires two mutually attractive polyelectrolytes, the polymers to be used as building blocks in covalent assembly have to possess complementary functional groups that can form stable covalent bonds in situ. Second, these reactions preferably should be reactions that can be carried out under mild conditions. Ideally, these reactions would occur under ambient atmosphere so as to minimize the need for inert atmosphere techniques in the assembly process. Third, if the reactions generate side products, the side products should be easily separated from the film so that contamination of the penultimate LbL assembly by impurities is avoided. Reactions that Sharpless has described as ‘click’ chemistry are ideal candidates for this sort of synthetic chemistry.10 General features of a covalent LbL assembly process and the procedure that is used in this covalent grafting process are summarized in Scheme 1. | ||
| Scheme 1 Steps used in the covalent LbL assembly process of an electrophile (E) and nucleophile (Nu). | ||
For applications that require functional robust thin films or nanocomposites, covalent LbL assembly has potential advantages over traditional non-covalent LbL assembly methods. First, covalent bonding affords high stability to the product thin film assembly allowing it to withstand harsh conditions such as extreme pH or extreme ionic strength.
Second, insitu covalent bond formation avoids the need for post-assembly cross-linking to change the properties of a multilayer graft. Third, given the wide variety of functional polymers, many different sorts of reactions can be used to form a multilayer graft. Fourth, the chemistry can be carried out in either aqueous or organic solutions depending on the type of reactions that are used to couple the bilayers to one another. This can be advantageous if it is desirable to incorporate materials that do not dissolve or that cannot be used in the aqueous solutions normally used in ionic or hydrogen bond based LbL assembly procedures. Fifth, covalent bond formation is usually a spontaneous energetically favorable process so a newly deposited layer cannot readily disassemble from the previous layer. This makes it possible to incorporate small mono- or bifunctional molecules during the LbL process. Ionic or hydrogen bonding LbL assembly typically requires multiple interactions between polymers or nanoparticles disfavoring incorporation of a mono- or bifunctional anion or cation. This feature of covalent assembly also makes it possible to incorporate copolymers with a higher percentage of other functionality into the multilayer grafts. Finally, a covalent LbL assembly process leaves behind excess reactive groups inside the multilayer matrix that can further react with other molecules to tailor the product functional interfaces for other functions. This can all be accomplished using various combinations of small molecules with polymers, polymers with polymers, or polymers with nanoparticles to form products of varying composition (Scheme 2). In this highlight, we describe the ideas involved in covalent LbL assembly. We discuss covalent self-assembly processes emphasizing examples where polyvalent reagents are used in the assembly process. We also briefly note applications of some of the functional thin films or nanocomposites made by these covalent LbL assembly methods.
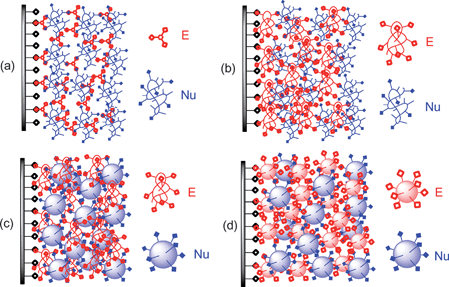 | ||
| Scheme 2 Covalent LbL assemblies of electrophiles (E) and nucleophiles (Nu): (a) small molecules and a polymer; (b) polymers or copolymers; (c) a polymer and a nanoparticle; or (d) nanoparticles. | ||
3. Covalent LbL assembly based on carbonyl chemistry
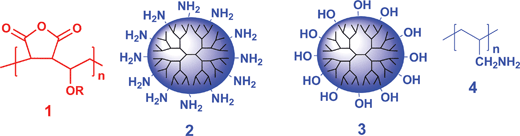
The earliest example of chemistry used for covalent LbL assemblyvia stable covalent bonds was chemistry that involved condensation reactions of nucleophiles and carbonyl derivatives. Such nucleophilic additions to carbonyl groups include reactions like amide, ester, urea, urethane, oxime, and imine bond formation using electrophilic polyfunctional or polymeric carbonyl derivatives like those shown below. Of these reactions, amide bond formation between an amine and an activated carboxylic acid is the most commonly used process for covalent self-assembly. This partly reflects the desirable stability of amide bonds but also reflects the ready availability of the reagents.11–25Bergbreiter and Crooks' groups described early examples of covalent LbL assembly using the commercially available poly(maleic anhydride)-c-poly(methyl vinyl ether) copolymer [Gantrez, 1 (R = –CH3)] as an electrophile in the reaction of nucleophilic amine- or hydroxyl-terminated generation five (G5) poly(amidoamine) or G5 poly(iminopropane-1,3-diyl) dendrimers (2 or 3).26–28LbL covalent assembly of these polyvalent anhydride polymers and polyvalent nucleophilic dendrimers on Au, Al, Si, or polymer substrates formed grafts like those in Scheme 2c that were ∼15 nm thick per bilayer. Such LbL covalent assemblies impart new chemistry to their substrates. For example, these ultrathin multilayer grafts on Au had pH-switchable permselectivity. Cyclic voltammetry studies showed that at pH 11, the graft's net negative charge due to CO2Na groups excluded electroactive Fe(CN)63− anions but Ru(NH3)63+ cations had normal redox chemistry. At pH 4, the residual amine groups in the graft were positively charged and only the electroactive Fe(CN)63− anions underwent redox chemistry.
Other work showed that the amic acid groups formed during the covalent reaction of amines and anhydrides in this covalent self-assembly could be imidized with mild heating to form impermeable monolithic films. When these imidized nanocomposites were formed on Al and a hydrophobic octadecyl layer was attached as a final step, the underlying Al was passivated against corrosion in NaOHaq or from pitting in neutral NaClaq by these ultrathin covalently assembled coatings. Similar passivation of Al was also seen in work by Bruening's group when they formed films using Gantrez (1) and poly(allylamine) (4).29 This work showed that the impedances of Al electrodes coated with a 27 nm, 3-bilayer Gantrez–poly(allylamine) film increased 10-fold after imidization of the intermediate covalent assembly at 150 °C.
The Grunlan and Bergbreiter groups have shown that covalent LbL assembly and ionic LbL assembly can together lead to conductive thin films on functionalized polyethylene (PE) powder.30 In this case, a covalent LbL assembly of PEI (5) and Gantrez (1) was prepared on oxidized PE powder. The covalent LbL multilayer graft so formed promoted good adhesion in further ionic LbL deposition of carbon black/polyelectrolyte filled bilayers. Compression molding of the product produced composite films that had conductivities of 0.2 S cm−1 with only 6 wt% of carbon black.
Bergbreiter, Batteas and coworkers recently described using aminated multiwall carbon nanotubes (MWNTs) (6) in place of PEI in reactions with Gantrez (1) to form covalent LbL nanocomposites that were superhydrophilic or superhydrophobic depending on the penultimate surface modification step.31 This work also directly compared covalent LbL assembly of MWNT–NH–PEI (6) and Gantrez (1) with an ionic assembly of 6 and poly(acrylic acid). This work showed that the covalently assembled graft was chemically more robust than its ionic counterpart that delaminated in 1 M HCl. Both the covalently and ionically assembled LbL nanocomposites exhibited micro/nano roughness and both the covalent and ionically assembled LbL nanocomposites on PE exhibited superhydrophobicity (θa = 165°) after acylation with octadecanoic acid.
Covalent LbL assembly, like ionic LbL assembly, works with objects of varying shape. Li and coworkers work leading to fluorescent nanotubes of PEI (5) and 3,4,9,10-perylenetetracarboxylic dianhydride (7) by covalent LbL assembly of this polyvalent nucleophilic polymer and dianhydride inside the pores of alumina membranes illustrates this.32 After 10 covalent bilayers, the aluminium membrane was dissolved to liberate the 350 nm diameter nanotube products. Thinner 50 nm nanotubes were formed after three bilayer depositions. The flexible covalently assembled nanotubes so formed retain their fluorescent properties for up to 10 months.
Lynn and coworkers showed that nucleophilic addition of the amines of PEI (5) to the carbonyl group of poly(2-vinyl-4,4-dimethylazlactone) (8) provided a stepwise LbL ‘click’ route to covalent multilayer assemblies coupled to each other via a diamide of 2,2-dimethylglycine.33 These grafts' thicknesses increased linearly with the number of bilayers at the rate of 6 nm per bilayer. The product’s retained unreacted azlactone groups were quantitatively and rapidly consumed throughout the bulk of the thin film after the grafting process was completed by treatment with propylamine. Such chemistry shows that these azlactone-containing LbL grafts are amenable to post-fabrication blocking, patterning, or passivation chemistry. An example of such post-fabrication chemistry was shown in a fluorescence patterning experiment that used a hydrazide-functionalized coumarin or a tetramethylrhodamine cadavarine dye on a PDMS stamp to create <100 µm fluorescent grid.
Condensation of an aldehyde-containing polymer with an alkoxyamine-containing polymer to form oximes has been used by Yu's group in covalent LbL assembly of conjugated polymers onto glass and gold substrates.34 This work showed that the functional group in the electrophilic polymer had to be an aldehyde—polymers with less reactive ketone groups are not reactive enough to ‘click’ to alkoxyamines to successfully form covalent LbL assemblies.35 Later work by this group showed that this covalent assembly process produced multilayer films on gold substrates that were highly insulating and defect free with finely tunable capacitance based on the number of deposition layers.36 Similar covalent LbL chemistry using imines was also described by Gao's group. In this case, the assembly process used glutaraldehyde and poly(allylamine hydrochloride) (PAH) and was carried out on MnCO3 particle templates.37 In this example, the template core was dissolved to yield chemically stable microcapsules—a result that suggested cross-linking of the imine-containing multilayer had occurred. Imine chemistry has also recently been shown to be useful in covalent LbL assembly to form a DNA-containing film.38
Finally, ester formation involving an inorganic acid (boronic acid) and poly(vinyl alcohol) (PVA) has been used in covalent LbL assembly of multilayer films by Yang's group.39 In this case, thiols containing a boronic acid group were assembled on gold nanoparticles (cf. 9). The pendant boronic acids rapidly formed cyclic boronate esters of PVA in a covalent LbL process to form multilayer grafts containing Au nanoparticles. A linear increase of the absorbance due to the surface plasmon band of these modified gold nanoparticles with the number of bilayers provide evidence that a stepwise and uniform assembly process had occurred. Unreacted boronic acid groups in the product grafted film in this case served as a handle to immobilize a glycosylated-protein—horseradish peroxidase—and this enzyme was subsequently shown to have been immobilized with good retention of its catalytic activity.
4. Covalent LbL assembly based on aromatic substitution chemistry
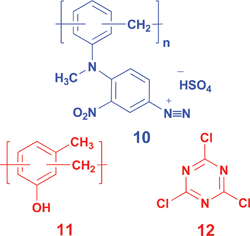
Other covalent bond forming reactions are also suitable for covalent LbL assembly. For example, electrophilic aromatic substitution by a polymeric aryl diazonium salt (10) on an electron-rich polymer formed from formaldehyde and m-cresol (11) has been shown by several groups to be suitable as a way to assemble grafts on quartz or on a sulfonated polystyrene latex.40,41 This chemistry not only served as a way to covalently self-assemble a multilayer graft that was stable to solvents like THF and DMF, it simultaneously introduced azo dye groups at each coupling event. That enabled Cao's group to follow the linear growth of the assembly process by UV-visible spectroscopy.The Bergbreiter and Simanek groups reported using nucleophilic aromatic substitution of PEI (4) and cyanuric chloride (12) as a route to LbL covalent grafts on silica.42Water, instead of organic solvents, can be used as a solvent for the PEI-grafting in this case. The step-by-step process shows a trend of linear growth based on acid–base titration of the free amine groups of the PEI-grafts. A 6-bilayer graft had a capacity of 1 mequiv. of base g−1.
5. Covalent LbL assembly based on Cu-catalyzed azide–alkyne [3 + 2] cycloadditions
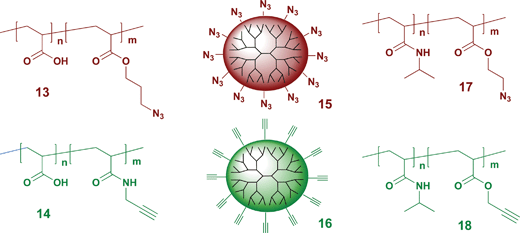
The recent development of Cu(I) catalyzed azide–alkyne [3 + 2] cycloadditions to form 1,2,3-triazoles has attracted interest in many areas of chemistry including this area of covalent self-assembly. Caruso's group was the first to use such Cu-catalyzed [3 + 2] cycloadditions in covalent LbL assembly on quartz, silicon, or gold by alternately dipping the substrates into solutions of PAA copolymerized with either azide (13) or alkyne (14) functionality in the presence of Cu(I) and sodium ascorbate.43 A subsequent study used this same chemistry to fabricate responsive polymer capsules whose size was pH dependent.44 In that example, the multilayers were readily functionalized through a post-assembly reaction with a ‘clickable’ rhodamine dye, showing again how covalently assembled LbL grafts can serve as a versatile platform for further functionalization.The Hawker group also used this [3 + 2] cycloaddition chemistry for the covalent LbL assembly of dendritic thin films on silicon wafers.45Azide and alkyne-terminated dendrimers (15 and 16) derived from hydroxyl-terminated 2,2-bis(methylol)propionic acid dendrimers from the 2nd to the 5th generation were used for the construction of the thin films. Film thickness growth was linear but depended on the dendrimer size, varying from 0.46 to 1.22 nm per triazole layer with 2nd to 5th generation dendrimers. Linear polymer analogs gave layers that were four times thicker per layer but rougher.
Bergbreiter also used azide–alkyne [3 + 2] cycloadditions to fabricate PNIPAM grafts on PE substrates.46Water-soluble PNIPAM copolymers containing pendant azide or alkyne groups (17 and 18) that can be thermally separated from aqueous solutions were used to alternately assemble a multilayer graft. As was true in Caruso's work, free azide groups inside the grafts can be readily labeled with an alkyne-terminated fluorescent reagent.
6. Conclusions and outlook
LbL assembly based on ionic interactions has proven to be a versatile route for surface modification and construction of ultrathin nanocomposites for the past two decades. Covalent LbL assembly based on facile ‘click’ covalent bond formation is an effective alternative, especially for applications where a more robust ultrathin film or nanocomposite is desired. The scope of the chemistry that can be used and the potential for tailoring the product multilayer grafts after grafting makes covalent LbL assembly even more attractive for applications in emerging areas such as nanotechnology and biomedicine.Acknowledgements
Support of this research by the Robert A. Welch Foundation (Grant A-639) is gratefully acknowledged.References
- G. Decher, J.-D. Hong and J. Schmitt, Makromol. Chem. Macromol. Symp., 1991, 46, 321–327 Search PubMed.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319–348 CrossRef CAS.
- G. Decher and J. B. Schlenoff, Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, Wiley-VCH: Weinheim, Germany, 2003 Search PubMed.
- P. Hammond, Adv. Mater., 2004, 16, 1271–1293 CrossRef CAS.
- K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319–2340 RSC.
- J. F. Quinn, A. P. R. Johnston, G. K. Such, A. N. Zelikin and F. Caruso, Chem. Soc. Rev., 2007, 36, 707–718 RSC.
- J. Kumanotani, Prog. Org. Coat., 1997, 34, 135–146 CrossRef.
- V. Otto, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4327–4335 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- P. Kohli and G. J. Blanchard, Langmuir, 2000, 16, 4655–4661 CrossRef CAS.
- P. Kohli and G. J. Blanchard, Langmuir, 2000, 16, 8518–8524 CrossRef CAS.
- J. S. Major and G. J. Blanchard, Langmuir, 2001, 17, 1163–1168 CrossRef CAS.
- J. S. Major and G. J. Blanchard, Chem. Mater., 2002, 14, 2567–2573 CrossRef CAS.
- J. S. Major and G. J. Blanchard, Chem. Mater., 2002, 14, 2574–2581 CrossRef CAS.
- J. S. Major and G. J. Blanchard, Chem. Mater., 2002, 14, 4320–4327 CrossRef CAS.
- T. Serizawa, K. Nanameki, K. Yamamoto and M. Akashi, Macromolecules, 2002, 35, 2184–2189 CrossRef CAS.
- T. Serizawa, Y. Nakashima and M. Akashi, Macromolecules, 2003, 36, 2072–2078 CrossRef CAS.
- T. Serizawa, D. Matsukuma, K. Nanameki, M. Uemura, F. Kurusu and M. Akashi, Macromolecules, 2004, 37, 6531–6536 CrossRef CAS.
- F. Zhang, Z. Jia and M. P. Srinivasan, Langmuir, 2005, 21, 3389–3395 CrossRef CAS.
- S. R. Puniredd and M. P. Srinivasan, Langmuir, 2005, 21, 7812–7822 CrossRef CAS.
- F. Zhang and M. P. Srinivasan, Colloids Surf., A, 2005, 257-258, 509–514 CrossRef.
- S. R. Puniredd and M. P. Srinivasan, Langmuir, 2006, 22, 4092–4099 CrossRef CAS.
- S. R. Puniredd and M. P. Srinivasan, J. Colloid Interface Sci., 2007, 306, 118–127 CrossRef CAS.
- S. R. Puniredd and M. P. Srinivasan, Ind. Eng. Chem. Res., 2007, 46, 464–471 CrossRef CAS.
- Y. Liu, M. L. Bruening, D. E. Bergbreiter and R. M. Crooks, Angew. Chem., Int. Ed. Engl., 1997, 36, 2114–2116 CrossRef CAS.
- Y. Liu, M. Zhao, D. E. Bergbreiter and R. M. Crooks, J. Am. Chem. Soc., 1997, 119, 8720–8721 CrossRef CAS.
- M. Zhao, Y. Liu, R. M. Crooks and D. E. Bergbreiter, J. Am. Chem. Soc., 1999, 121, 923–930 CrossRef CAS.
- J. Dai, D. M. Sullivan and M. L. Bruening, Ind. Eng. Chem. Res., 2000, 39, 3528–3535 CrossRef CAS.
- Y.-S. Kim, K.-S. Liao, C. J. Jan, D. E. Bergbreiter and J. C. Grunlan, Chem. Mater., 2006, 18, 2997–3004 CrossRef CAS.
- K.-S. Liao, A. Wan, J. D. Batteas and D. E. Bergbreiter, Langmuir, 2008, 24, 4245–4253 CrossRef CAS.
- Y. Tian, Q. He, C. Tao and J. Li, Langmuir, 2006, 22, 360–362 CrossRef CAS.
- M. E. Buck, J. Zhang and D. M. Lynn, Adv. Mater., 2007, 19, 3951–3955 CrossRef CAS.
- E. W. L. Chan, D. C. Lee, M. K. Ng, G. Wu, K. Y. C. Lee and L. Yu, J. Am. Chem. Soc., 2002, 124, 12238–12243 CrossRef CAS.
- D. C. Lee, B. J. Chang, G. M. Morales, Y. A. Jang, M. K. Ng, S. T. Heller and L. Yu, Macromolecules, 2004, 37, 1849–1856 CrossRef CAS.
- M. K. Park, D. C. Lee, Y. Liang, G. Lin and L. Yu, Langmuir, 2007, 23, 4367–4372 CrossRef CAS.
- W. Tong, C. Gao and H. Mohwald, Macromol. Rapid Commun., 2006, 27, 2078–2083 CrossRef CAS.
- Z. Lu, C. M. Li, Q. Zhou, Q.-L. Bao and X. Cui, J. Colloid Interface Sci., 2007, 314, 80–88 CrossRef CAS.
- Y. Ma, L. Qian, H. Huang and X. Yang, J. Colloid Interface Sci., 2006, 295, 583–588 CrossRef CAS.
- J. Chen, G. Luo and W. Cao, Macromol. Rapid Commun., 2001, 22, 311–314 CrossRef CAS.
- Y. Zhang, S. Yang, Y. Guan, W. Cao and J. Xu, Macromolecules, 2003, 36, 4238–4240 CrossRef CAS.
- D. E. Bergbreiter, E. E. Simanek and I. Owsik, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4654–4665 CrossRef CAS.
- G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318–9319 CrossRef CAS.
- G. K. Such, E. Tjipto, A. Postma, A. P. R. Johnston and F. Caruso, Nano Lett., 2007, 7, 1706–1710 CrossRef CAS.
- R. Vestberg, M. Malkoch, M. Kade, P. Wu, V. V. Fokin, K. B. Sharpless, E. Drockenmuller and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2835–2846 CrossRef CAS.
- D. E. Bergbreiter and B. S. Chance, Macromolecules, 2007, 40, 5337–5343 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |