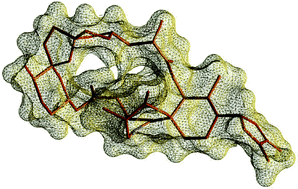
The relative stereochemistry of 13,19-didesmethyl spirolide C was determined through careful analysis of NMR parameters strongly dependent upon molecular conformations supported and extended by computational studies. This work has also shed light on the conformational behavior of spirolides in solution. An equilibrium between two possible conformers of the identified diastereoisomer was inferred, while the uncommon cyclic imine moiety of spirolides—the putative pharmacophore of this class of toxins—was interestingly found to adopt only a single dominant conformation. The insightful details provided on spirolide conformations may represent a key means to pharmacologists involved in clarifying the mechanism of action of spirolide, which is yet to be totally defined.