Ahmed M. Zaed, Michael D. Swift and Andrew Sutherland
Org. Biomol. Chem., 2009,7, 2678-2680
DOI:
10.1039/B907341H,
Communication
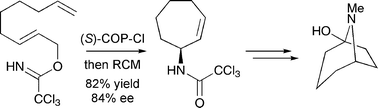
A stereoselective synthesis of (+)-physoperuvine, a