Stereospecific synthesis of aszonalenins by using two recombinant prenyltransferases
Wen-Bing
Yin
,
Jun
Cheng
and
Shu-Ming
Li
*
Philipps-Universität Marburg, Institut für Pharmazeutische Biologie, Deutschhausstrasse 17A, D-35037 Marburg, Germany. E-mail: shuming.li@Staff.uni-Marburg.de; Fax: +49-6421-2825365; Tel: +49-6421-2822461
First published on 3rd April 2009
Abstract
In previous studies, two prenyltransferases were overproduced and characterised biochemically. AnaPT from Neosartorya fischeri is involved in the biosynthesis of acetylaszonalenin and was shown to catalyse the C3-prenylation of (R)-benzodiazepinedione (6). CdpNPT from Aspergillus fumigatus catalysed the N1-prenylation of different tryptophan-containing cyclic dipeptides. In this report, CdpNPT was found to catalyse the C3-prenylation of 6 and its (S)-isomer (7). Interestingly, AnaPT and CdpNPT introduced prenyl moieties from opposite sides of the indoline ring system. This feature was successfully used for the chemoenzymatic synthesis of four aszonalenin stereoisomers by using 6 and 7 as substrates and AnaPT and CdpNPT as catalysts. The stereoselectivity of the one-step reactions was about 100% and the conversion rates reached 85–100%.
Introduction
Aszonalenin (1) and its diastereomer epi-aszonalenin C (2) (Fig. 1), as well as their N1-acetylated forms, have been identified in different Aspergillus and Neosartorya strains.1–5 These compounds belong to indole derivatives consisting of the amino acids tryptophan and anthranilic acid and carry a reverse prenyl moiety, i.e.3′-(3′,3′-dimethylallyl) (= 3′-DMA), at position C3 of the indoline ring. A five-membered ring is inserted between the indoline and the seven-membered ring system. The absolute configuration of 1 and 2 was confirmed by chemical synthesis of a dihydro derivative and by X-ray analysis of the acetylated form, respectively.6,7 Several natural products with similar structures are also found in different fungi,5e.g. roquefortine C in Penicillium roqueforti8 (3), amauromine in Amauroascus sp9 (4) and 5-N-acetylardeemin (5) in Neosartorya fischeri var. brasiliensis10 (Fig. 1) as well as epiamauromine in Aspergillus ochraceus9 and fructigenine A and B in Penicillium fructigenum.11 All of these substances have a cis-configuration between the two five-membered rings.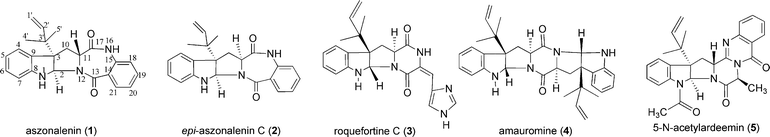 | ||
| Fig. 1 Examples of C3-prenylated indole derivatives from fungi. | ||
Due to their important biological activities,12,13 different strategies were developed for the synthesis of roquefortine C,14 isoroquefortine C,15,16 amauromine17–19 and 5-N-acetylardeemin.18,19 In these reports, many steps are essential for the desired compounds. With the exception of (−)-dihydroaszonalenin,6 no report on the synthesis of aszonalenins is available in the literature. Here, we describe a novel strategy for stereospecific synthesis of all four aszonalenin stereoisomers with a cis-configuration at positions C2 and C3 of the indoline ring by one-step reactions using purified enzymes.
Results and discussion
In a previous study,5 we identified a biosynthetic gene cluster for acetylaszonalenin from the fungus Neosartorya fischeri NRRL181 by genomic mining. A putative prenyltransferase gene from this cluster, anaPT, was cloned and overexpressed in E. coli. The overproduced recombinant protein AnaPT was purified to homogeneity and characterised biochemically. AnaPT was found to catalyse the prenylation of (R)-benzodiazepinedione (6) in the presence of dimethylallyl diphosphate (DMAPP), resulting in the formation of 1 with an absolute configuration of (2R,3S,11R) (Scheme 1). As shown in Fig. 2A, the conversion of 6 to 1 was nearly quantitative and only one enzymatic product was detected.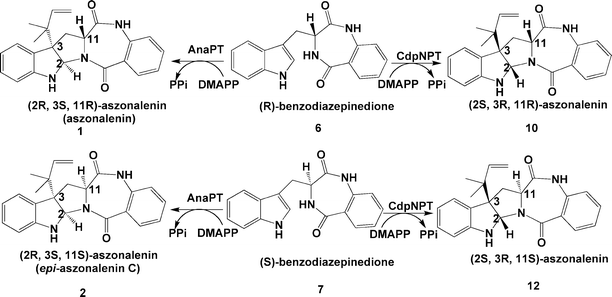 | ||
| Scheme 1 Stereospecific chemoenzymatic synthesis of aszonalenins from benzodiazepinediones. | ||
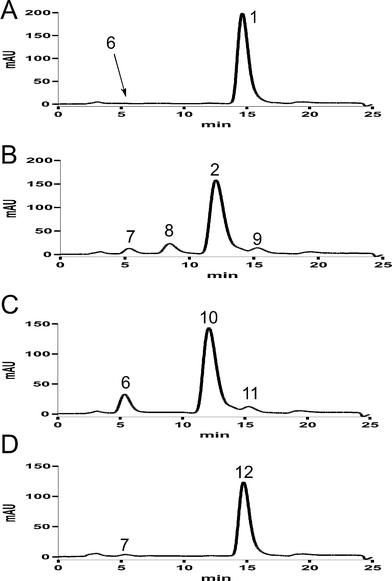 | ||
| Fig. 2 HPLC analysis of enzymatic reaction mixtures of 6 with AnaPT (A), 7 with AnaPT (B), 6 with CdpNPT (C), and 7 with CdpNPT (D). | ||
To get isomers of 1, we then synthesized the (S)-isomer 7 by using L- instead of D-tryptophan (Experimental section) and incubated it with AnaPT and DMAPP. As shown in Fig. 2B, 7 was also well accepted by AnaPT, with a conversion rate of 95.8%. Three enzymatic products with a ratio of 1.5:13.5:1 could be detected at 8.5, 12.1, and 15.3 min, respectively. After isolation, the structures of the enzymatic products were elucidated by 1H and 13C NMR (Tables 1 and 2) as well as by mass spectrometry. The NMR spectra of the major product at 12.1 min corresponded very well to those of epi-aszonalenin C (2),7 with a configuration of (2R,3S,11S).
| Compound | 6 a | 7 | 1 a | 12 | 2 | 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC |
| a The NMR data of 1 and 6 were adopted from Ref. 5. For numbering please see Fig. 1. | ||||||||||||
| C-2 | 7.13, br s | 124.82 | 7.13, br s | 124.82 | 5.58, s | 81.7 | 5.58, s | 81.7 | 5.67, s | 80.4 | 5.68, s | 80.5 |
| C-3 | – | 110.56 | – | 110.56 | – | 60.7 | – | 60.7 | – | 61.5 | – | 61.3 |
| C-4 | 7.42, d, 7.9 | 119.83 | 7.42, dd, 7.5, 1.2 | 119.83 | 7.15, d, 7.6 | 125.3 | 7.15, d, 7.3 | 125.3 | 7.33, d, 7.6 | 126.8 | 7.33, d, 7.6 | 126.8 |
| C-5 | 6.94, t, 7.6 | 118.84 | 6.93, td, 7.4, 1.2 | 118.84 | 6.72, t, 7.4 | 118.4 | 6.72, t, 7.5 | 118.4 | 6.76, t, 7.4 | 118.5 | 6.77, t, 7.4 | 118.5 |
| C-6 | 7.05, t, 7.9 | 122.29 | 7.05, td, 7.5, 1.2 | 122.29 | 7.08, t,7.6 | 128.6 | 7.08, t,7.6 | 128.6 | 7.06, td, 7.6, 1.0 | 128.5 | 7.07, td, 7.6, 1.0 | 128.5 |
| C-7 | 7.30, d, 7.2 | 112.34 | 7.30, dd, 7.5, 1.2 | 112.34 | 6.62, d, 7.9 | 109.2 | 6.62, d, 7.9 | 109.2 | 6.52, d, 7.9 | 109.3 | 6.52, d, 7.9 | 109.3 |
| C-8 | – | 138.11 | – | 138.11 | – | 133.9 | – | 133.9 | – | 135.4 | – | 135.6 |
| C-9 | – | 127.11 | – | 127.10 | – | 131.2 | – | 131.1 | – | 128.9 | – | 128.8 |
| C-10 | 3.38, dd, 15.0, 6.0 | 24.95 | 3.38, dd, 15.0, 5.7 | 24.95 | 3.46, dd, 14.2, 7.6 | 33.4 | 3.46, dd, 13.9, 7.4 | 33.4 | 3.14, d, 13.6 | 31.4 | 3.14, d, 13.6 | 31.4 |
| 3.13, dd, 15.0, 8.8 | – | 3.12, dd, 15.0, 8.4 | – | 2.42, dd, 14.0, 9.0 | – | 2.42, dd, 13.9, 9.2 | – | 2.57, dd, 13.5, 9.7 | – | 2.58, dd, 13.6, 9.5 | – | |
| C-11 | 4.09, dd, 8.8, 5.7 | 54.58 | 4.09, dd, 8.8, 5.9 | 54.57 | 3.99, br t, 8.1 | 57.0 | 3.98, dd, 8.8, 7.6 | 57.0 | 4.11, d, 9.2 | 56.9 | 4.11, d, 9.4 | 57.0 |
| C-13 | – | 170.97 | – | 170.97 | – | 166.8 | – | 166.9 | – | 166.5 | – | 166.6 |
| C-14 | – | 128.37 | – | 128.38 | – | 126.8 | – | 126.8 | – | 126.0 | – | 126.0 |
| C-15 | – | 138.08 | – | 138.08 | – | 149.0 | – | 149.0 | – | 148.8 | – | 148.8 |
| N-16 | – | - | – | – | 7.91, s | – | 7.65, s | – | 7.33, br s | – | 7.37, s | – |
| C-17 | – | 173.72 | – | 173.73 | – | 169.8 | – | 169.7 | – | 170.5 | – | 170.5 |
| C-18 | 7.12, d, 8.0 | 122.47 | 7.11, dd, 8.0, 1.2 | 122.47 | 6.90, d, 8.2 | 120.4 | 6.89, d, 7.9 | 120.4 | 6.81, d, 7.9 | 120.6 | 6.82, d, 7.9 | 120.7 |
| C-19 | 7.53, td, 8.0, 1.6 | 134.15 | 7.52, td, 8.0, 1.8 | 134.15 | 7.44, td, 7.9, 1.0 | 132.6 | 7.44, td, 7.9, 1.3 | 132.6 | 7.45, td, 7.9, 1.3 | 132.9 | 7.45, td, 7.6, 1.3 | 132.9 |
| C-20 | 7.24, td, 7.7, 1.0 | 125.82 | 7.23, td, 7.8, 1.2 | 125.82 | 7.22, t, 7.6 | 124.9 | 7.22, t, 7.6 | 124.9 | 7.26, t, 7.6 | 124.9 | 7.26, t, 7.6 | 125.0 |
| C-21 | 7.78, dd, 7.9, 1.3 | 131.67 | 7.78, dd, 8.0, 1.8 | 131.68 | 7.83, d, 7.9 | 131.2 | 7.83, dd, 7.9, 1.3 | 131.2 | 7.99, dd, 7.9, 1.3 | 131.0 | 7.99, dd, 7.9, 1.3 | 131.0 |
| C-1′ | 5.14, d, 10.7 | 114.3 | 5.15, d, 10.7 | 114.2 | 5.15, d, 10.6 | 114.4 | 5.15, d, 10.7 | 114.4 | ||||
| 5.11, d, 17.3 | 5.12, d, 17.3 | 5.12, d, 17.3 | – | 5.12, d, 17.3 | – | |||||||
| C-2′ | 6.11, dd, 17.3, 10.7 | 143.7 | 6.11, dd, 17.3, 10.7 | 143.8 | 6.08, dd, 17.3, 10.6 | 144.0 | 6.08, dd, 17.3, 10.7 | 144.1 | ||||
| C-3′ | – | 41.4 | – | 41.4 | – | 41.3 | – | 41.3 | ||||
| C-4′ | 1.06, s | 22.7 | 1.06, s | 22.7 | 1.01, s | 22.8 | 1.01, s | 22.8 | ||||
| C-5′ | 1.14, s | 22.5 | 1.14, s | 22.5 | 1.16, s | 22.3 | 1.16, s | 22.3 | ||||
| Compound | 8 | 9 | 11 | ||
|---|---|---|---|---|---|
| Position | δH, multi., J in Hz | δC | δH, multi., J in Hz | δC | δH, multi., J in Hz |
| For numbering please see Fig. 3. | |||||
| N-1 | 8.01, s | – | – | – | – |
| C-2 | – | 134.2 | 7.09, s | 127.1 | 7.09, s |
| C-3 | – | 104.8 | – | 107.6 | – |
| C-4 | 7.38, d, 7.9 | 117.7 | 7.49, d, 7.9 | 118.3 | 7.49, d, 8.0 |
| C-5 | 7.05, t, 7.4 | 120.7 | 7.08, t, 7.4 | 119.3 | 7.08, m |
| C-6 | 7.15, t, 7.6 | 121.9 | 7.20, t, 7.8 | 121.8 | 7.21, t, 6.9 |
| C-7 | 7.32, d, 8.2 | 110.8 | 7.30, d, 8.2 | 109.9 | 7.29, m |
| C-8 | – | 141.1 | – | 136.4 | – |
| C-9 | – | 129.3 | – | 127.9 | – |
| C-10 | 3.48, dd, 15.6, 6.8 | 23.5 | 3.50, dd, 15.2, 5.7 | 24.2 | 3.49, dd, 15.2, 6.4 |
| 3.44, dd, 15.6, 4.6 | 3.22, dd, 15.2, 8.2 | 3.21, dd, 15.1, 7.3 | |||
| C-11 | 4.21, dt, 9.8, 5.6 | 52.3 | 4.13, dt, 13.4, 5.5 | 52.0 | 4.12, dt, 13.4, 6.3 |
| C-13 | 6.10, d, 4.1 | 168.2 | 6.08, d, 4.8 | 168.3 | 6.02, d, 4.8 |
| C-14 | – | 125.3 | – | 123.3 | – |
| C-15 | – | 135.3 | – | 135.3 | – |
| N-16 | 7.56, s | – | 7.75, s | – | 7.75, s |
| C-17 | – | 171.8 | – | 171.5 | – |
| C-18 | 6.96, d, 8.2 | 119.9 | 6.96, d, 7.9 | 120.8 | 6.95, d, 8.0 |
| C-19 | 7.50, td, 7.9, 1.3 | 133.0 | 7.50, td, 7.6, 1.3 | 133.1 | 7.50, td, 7.6, 1.3 |
| C-20 | 7.23, t, 7.6 | 125.3 | 7.26, t, 7.3 | 125.3 | 7.26, m |
| C-21 | 7.85, dd, 7.9, 1.3 | 131.6 | 7.91, dd, 7.9, 1.3 | 131.6 | 7.91, d, 7.3 |
| C-1′ | 5.22, d, 18.0 | 112.9 | 4.65, d, 6.9 | 44.2 | 4.66, d, 6.7 |
| 5.19, d, 10.4 | |||||
| C-2′ | 6.18, dd, 17.5, 10.4 | 146.1 | 5.36, t, 6.9 | 119.8 | 5.35, t, 6.0 |
| C-3′ | – | 38.9 | – | 124.8 | – |
| C-4′ | 1.54, s | 27.7 | 1.76, s | 25.6 | 1.76, s |
| C-5′ | 1.55, s | 27.7 | 1.82, s | 18.1 | 1.82, s |
Because a retention of the configuration at position C11 was expected during the prenyl transfer reaction, this compound was identified as epi-aszonalenin C, termed as (2R,3S,11S)-aszonalenin in this paper (Scheme 1). The two minor products were identified as C2–3′-DMA-(S)-benzodiazepinedione (8) and N1–1′-DMA-(S)-benzodiazepinedione (9), respectively (Fig. 3). The presence of 8 and 9 could indicate that rearrangements could be implicated in the formation of 2. It can be speculated that the prenylation was taking place firstly at the N1-position of the indole ring resulting in the formation of 9. 8 and 2 are rearrangement products of 9, as proposed for 3-alkyl-1-allylindoles.20,21 In the case of the natural substrate of AnaPT 6, the rearrangement steps would be much more rapid than the prenylation, so only 1 was detected in the incubation mixture. Incubation of 8 or 9 with AnaPT in the presence or absence of DMAPP did not result in the formation of any enzymatic products (data not shown), indicating the independent formation of these compounds. However, the possibility cannot be excluded that the conversion of 9 to 8 and then to 2 takes place only as enzyme-bound intermediates. In summary, the two known aszonalenin diastereomers 1 and 2 could be successfully prepared from 6 and 7 by using AnaPT, respectively.
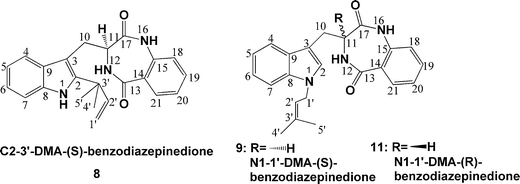 | ||
| Fig. 3 Structures of side products formed during the production of aszonalenins. | ||
Theoretically, there are two further diastereomers with a cis-configuration at positions C2 and C3, i.e. (2S,3R,11R)- (10) and (2S,3R,11S)-aszonalenin (12) (Scheme 1). To obtain these compounds, we carried out incubations with 6 and 7 in the presence of CdpNPT and DMAPP. CdpNPT from Aspergillus fumigatus was found to catalyse the reverse prenylation at position N1 of the tryptophan-containing cyclic dipeptides.22 Only the reversely N1-prenylated derivatives could be detected in the reaction mixtures of cyclo-L-Trp-L-Trp, cyclo-L-Trp-L-Tyr, cyclo-D-Trp-L-Tyr, cyclo-L-Trp-L-Phe, cyclo-L-Trp-L-Leu and cyclo-L-Trp-Gly.22 In the reaction mixtures of cyclo-L-Trp-L-Pro and cyclo-D-Trp-L-Pro, however, two additional products could be detected in each (data not shown).22 One of them was identified as the regularly prenylated derivative which was formed by rearrangement of the reversely N1-prenylated derivative.22 The structures of the second ones could not be determined in this study. However, based on the information obtained from NMR data (data not shown), we proposed that these compounds carry probably a reverse prenyl moiety at position C3 and two fused five-membered rings, similar to compounds shown in Fig. 1. Considering the structural difference between cyclic dipeptides containing proline and those containing other amino acids such as tyrosine, phenylalanine, glycine or leucine, it could be speculated that the fused ring system in the structures of cyclo-L-Trp-L-Pro and cyclo-D-Trp-L-Pro could be responsible for the formation of the C3-prenylated derivatives, which were absent in the incubation mixtures of the other tryptophan-containing cyclic dipeptides. Therefore, we expected that prenylation of 6 and 7 by CdpNPT would produce 1 and 2 as in the case of AnaPT or their isomers 10 and 12, respectively. As shown in Fig. 2C and D, both 6 and 7 were accepted well by CdpNPT. In the case of 6, a conversion rate of 84.8% could be reached and two products with a ratio of 10:1 were detected at 12.1 and 15.3 min, respectively (Fig. 2C). The minor product of 6 was identified as N1–1′-DMA-(R)-benzodiazepinedione (11) (Table 2, Fig. 3). The 1H and 13C NMR data of the predominant product 10 differed clearly from those of 1. With an exception for the signal of NH-16, the spectra of 10 were almost identical to those of 2 (Table 1). In the case of 7, the conversion rate was estimated to be 99.4%. The 1H and 13C NMR spectra of the enzymatic product 12 were almost identical to those of 1 (Table 1).This indicates that the two products 10 and 12 could be enantiomers of 2 and 1, respectively, if the stereochemistry at position C11 remained unchanged during the prenylation. To confirm the stereochemistry of 10 and 12, CD spectra of the four enzymatic products as well as those of the two substrates were taken in ethanol and compared to each other (Fig. 4). Fig. 4 shows clearly that the substance pairs 6 and 7, 1 and 12 as well as 2 and 10 have opposite Cotton effects. This proved unequivocally that they are enantiomers of each other. 10 and 12 have not been isolated from nature yet. The results provided in this study also proved that both AnaPT and CdpNPT catalyse the formation of indoline derivatives carrying fused five-membered rings with a cis-configuration. However, they introduce the ring system from opposite sides. They are therefore complementary to each other with regard to prenylation, which expands their usage in chemoenzymatic synthesis.
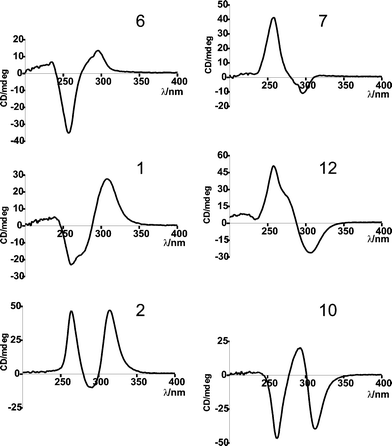 | ||
| Fig. 4 CD spectra of reported compounds. | ||
Conclusions
In conclusion, we have presented a new strategy for the synthesis of four aszonalenin stereoisomers in one-step reactions by using two soluble prenyltransferases, which could be easily produced in E. coli and purified to homogeneity in high yields. The stereoselectivity of the reactions was approximately 100% and the reaction yields were found to be between 85% to 100%. More importantly, AnaPT and CdpNPT accepted the same substrates, but introduced the five-membered ring from different sides. As a result, two compounds with different stereochemistry could be obtained from one substrate by using different enzymes. The results reported in this study provide an additional example of chemoenzymatic synthesis or enzyme-catalysed chemical transformations, which are now widely recognised as practical alternatives to traditional organic synthesis and as convenient solutions to certain intractable synthetic problems.23 The approach described in this study could also find use in the synthesis of C3-prenylated natural products, e.g. roquefortine D, amauromine, fructigenines A and B by using AnaPT and the respective cyclic dipeptides.Experimental section
Chemicals
Dimethylallyl diphosphate was prepared according to the method described for geranyl diphosphate by Woodside.24 D- and L-tryptophan were purchased from Roth (Karlsruhe, Germany). Isatoic anhydride was purchased from Fluka (Steinheim, Germany).Bacterial strains, plasmids and cultural conditions
Plasmids pWY225 and pHL525 were used for overproduction of AnaPT and CdpNPT, respectively.Escherichia coli XL1 Blue MRF′ (Stratagene, Heidelberg, Germany) was used for overexpression experiments and grown in liquid or on solid Luria-Bertani medium with 1.5% (w/v) agar at 37° C. Carbenicillin (50 µg mL−1) was used for selection of recombinant E. coli strains.
Preparation of AnaPT and CdpNPT
For preparation of AnaPT, E. coli XL1 Blue MRF′ cells harbouring pWY22 were induced by 0.5 mM of IPTG at 37 °C. His6-AnaPT was purified with Ni-NTA agarose to homogeneity as judged by SDS-PAGE and a protein yield of 25 mg of purified His6-tagged AnaPT per litre of culture was obtained.5For preparation of CdpNPT, E. coli XL1 Blue MRF′ cells harbouring pHL5 were induced by 1 mM of IPTG at 37 °C. His6-CdpNPT was purified with Ni-NTA agarose to homogeneity as judged by SDS-PAGE and a protein yield of 5 mg of purified His6-tagged CdpNPT per litre of culture was obtained.25
HPLC conditions for analysis and isolation of synthetic products
The enzymatic products of the incubation mixtures of AnaPT and CdpNPT were analysed by HPLC on an Agilent series 1200 by using a LiChrospher RP 18–5 column (125 × 4 mm, 5 µm, Agilent) at a flow rate of 1 mL min−1. 50% methanol in water (solvent A) and methanol (solvent B) were used as solvents. For analysis of enzymatic products, a linear gradient of 10–50% (v/v) solvent B in solvent A in 15 min was used. The column was then washed with 100% solvent B for 5 min and equilibrated with 10% (v/v) solvent B for 5 min. Detection was carried out by a Photo Diode Array detector and illustrated at 254 nm.For isolation, the same HPLC equipment with a Multospher 120 RP-18 column (250 × 10 mm, 5 µm, C + S Chromatographie Service, Langenfeld, Germany) was used. A linear gradient of 30–70% (v/v) solvent B in A in 12 min at a flow rate of 2.5 mL min−1 was used. The column was then washed with 100% solvent B for 8 min and equilibrated with 30% (v/v) solvent B for 5 min.
Circular dichroism spectra
The samples were dissolved in ethanol. CD spectra were recorded on a JASCO J-810 CD spectropolarimeter by using Spectra Manager Software (JASCO Inc). Spectral data were collected from 400 to 200 nm with a data pitch of 0.1 nm. A band width of 1 nm was used with a detector response time of 0.5 sec. and scanning speed of 200 nm/min. Each spectrum or data point was acquired 5 times, and mean values were used.Synthesis of benzodiazepinediones
(R)- (6) and (S)-benzodiazepinedione (7) were synthesized by using D- or L-tryptophan, triethylamine and isatoic anhydride according to the method described by Barrow and Sun,13 respectively.Enzymatic synthesis of aszonalenins
The reaction mixtures containing 6 or 7 (1 mM), DMAPP (1 mM), CaCl2 (10 mM), Tris-HCl (50 mM, pH 7.5), glycerol 1.5% (v/v), AnaPT (0.25 µM) or CdpNPT (0.38 µM) were incubated at 37 °C for 24 h and then extracted with ethyl acetate. After evaporation of the solvent, the residues were dissolved in methanol and purified by HPLC under the conditions described above. The isolated products were analysed by 1H NMR, 13C NMR spectroscopy, H-H-COSY, H-C-COSY as well as positive and negative electrospray ionization (ESI) mass spectrometry with a ThermoFinnigan TSQ Quantum. The mass spectrometer was coupled with an Agilent HPLC series 1100 equipped with a RP18-column (5 µm, 2 × 250 mm, 5 µm). For separation, the column was run with 10% (v/v) solvent B (CH3OH) in solvent A (H2O) (each containing 0.1% (v/v) HCOOH) for 5 min, followed by a gradient from 10% (v/v) to 100% B over 30 min. After washing with 100% B for 10 min, the column was equilibrated with 10% (v/v) B for 10 min. The flow rate was at 0.2 mL min−1.From the incubation mixture of AnaPT with 6.1 mg of 6, 5.5 mg of 1 was isolated. From the incubation mixture of AnaPT with 61 mg of 7, 40 mg of 2, 5 mg of 8 and 3 mg of 9 were isolated. 8 mg of 10 and 0.8 mg of 11 were obtained from the incubation mixture of CdpNPT with 12.2 mg of 6. 10 mg of 12 was obtained from the incubation mixture of CdpNPT with 12.2 mg of 7. Positive and negative ESI-MS data of the reported compounds are as following: 1: m/z: 374.3 ([M + 1]+), 372.5 ([M − 1]−); 2: m/z: 746.9 ([2M + 1]+), 374.2 ([M + 1]+), 744.8 ([2M − 1]−), 372.2 ([M − 1]−); 7: m/z: 611.1 ([2M + 1]+), 306.3 ([M + 1]+); 8: m/z: [M + 1]+, 374.2, [M − 1]−, 372.1; 9: m/z: 374.3 ([M + 1]+), 372.2 ([M − 1]−); 11: m/z: 374.1 ([M + 1]+), 396.1 ([M + Na]+);.12: m/z: 374.2 ([M + 1]+), 372.7 ([M − 1]−).
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SPP 1152: Evolution of metabolic diversity, to S.-M. Li). We thank Mark Schlegel for his help to take the CD spectra.References
- Y. Kimura, T. Hamasaki, H. Nakajima and A. Isogai, Tetrahedron Lett., 1982, 23, 225–228 CrossRef.
- A. Hayashi, S. Fujioka, M. Nukina, T. Kawano, A. Shimada and Y. Kimura, Biosci. Biotechnol. Biochem, 2007, 71, 1697–1702 CrossRef CAS.
- R. J. Capon, C. Skene, M. Stewart, J. Ford, R. A. J. O'Hair, L. Williams, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, Org. Biomol. Chem., 2003, 1, 1856–1862 RSC.
- D. Wakana, T. Hosoe, T. Itabashi, K. Nozawa, K. Okada, G. M. d. C. Takaki, T. Yaguchi, K. Fukushima and K. I. Kawai, Mycotoxins, 2006, 56, 3–6 CrossRef CAS.
- W.-B. Yin, A. Grundmann, J. Cheng and S.-M. Li, J. Biol. Chem., 2009, 284, 100–109 CAS.
- B. Bhat and D. M. Harrison, Tetrahedron, 1993, 49, 10655–10662 CrossRef CAS.
- C. Rank, R. K. Phipps, P. Harris, J. C. Frisvad, C. H. Gotfredsen and T. O. Larsen, Tetrahedron Lett., 2006, 47, 6099–6102 CrossRef CAS.
- P. M. Scott and B. P. C. Kennedy, J. Agr. Food Chem., 1976, 24, 865–868 CrossRef CAS.
- S. Takase, Y. Kawai, I. Uchida, H. Tanaka and H. Aoki, Tetrahedron, 1985, 41, 3037–3048 CrossRef CAS.
- J. E. Hochlowski, M. M. Mullally, S. G. Spanton, D. N. Whittern, P. Hill and J. B. McAlpine, J. Antibiot., 1993, 46, 380–386 CAS.
- K. Arai, K. Kimura, T. Mushiroda and Y. Yamamoto, Chem. Pharm. Bull., 1989, 37, 2937–2939 CAS.
- J. P. Karwowski, M. Jackson, R. R. Rasmussen, P. E. Humphrey, J. B. Poddig, W. L. Kohl, M. H. Scherr, S. Kadam and J. B. McAlpine, J. Antibiot. (Tokyo), 1993, 46, 374–379 CAS.
- C. J. Barrow and H. H. Sun, J. Nat. Prod., 1994, 57, 471–476 CAS.
- N. Shangguan, W. J. Hehre, W. S. Ohlinger, M. P. Beavers and M. M. Joullie, J. Amer. Chem. Soc., 2008, 130, 6281–6287 CrossRef CAS.
- B. M. Schiavi, D. J. Richard and M. M. Joullie, J. Org. Chem., 2002, 67, 620–624 CrossRef CAS.
- D. J. Richard, B. Schiavi and M. M. Joullie, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11971–11976 CrossRef CAS.
- S. Takase, Y. Itoh, I. Uchida, H. Tanaka and H. Aoki, Tetrahedron, 1986, 42, 5887–5894 CrossRef CAS.
- S. P. Marsden, K. M. Depew and S. J. Danishefsky, J. Am. Chem. Soc., 1994, 116, 11143–11144 CrossRef CAS.
- K. M. Depew, S. P. Marsden, D. Zatorska, A. Zatorski, W. G. Bornmann and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 11953–11963 CrossRef CAS.
- G. Casnati, R. Marchelli and A. Pochini, J. Chem. Soc., Perkin Trans. 1, 1974, 754–757 RSC.
- G. Casnati and A. Pochini, J. Chem. Soc., Chem. Commun., 1970, 1328–1329 RSC.
- H.-L. Ruan, W.-B. Yin, J.-Z. Wu and S.-M. Li, ChemBioChem, 2008, 9, 1044–1047 CrossRef CAS.
- K. M. Koeller and C. H. Wong, Nature, 2001, 409, 232–240 CrossRef CAS.
- A. B. Woodside, Z. Huang and C. D. Poulter, Org. Synth., 1988, 66, 211–215 CAS.
- W.-B. Yin, H.-L. Ruan, L. Westrich, A. Grundmann and S.-M. Li, ChemBioChem, 2007, 8, 1154–1161 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
