Asymmetric synthesis of trans-disubstituted cyclopropanes using phosphine oxides and phosphine boranes†
Abstract
The stereocontrolled synthesis of trans-disubstituted cyclopropylketones has been achieved from β-alkyl, γ-benzoyl phosphine oxides via a three-step cascade reaction incorporating an
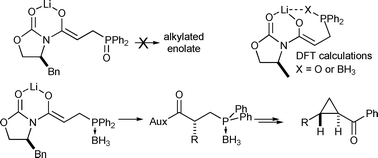
- This article is part of the themed collection: In memory of Stuart Warren

 Please wait while we load your content...
Please wait while we load your content...