Synthesis of LiyMnSiOx and LiMnPO4nanostructures†
Bettina
Milke
,
Peter
Strauch
,
Markus
Antonietti
and
Cristina
Giordano
*
Max-Planck-Institute of Colloids and Interfaces, Colloid Chemistry Research Campus Golm, Am Muehlenberg, D-14476, Golm, Germany
First published on 28th August 2009
Abstract
Nanosized LiyMnSiOx and LiMnPO4 have been synthesized by a hydrothermal route. Simply by changing parameters such as metal precursors and/or template, high surface area LiyMnSiOx with different morphology and sizes were prepared, in particular hollow spheres and plate-like nanoparticles, however with poorly developed crystallinity. In the case of LiMnPO4, highly crystalline nanocrystals were prepared.
Introduction
It is a trend in materials science to analyze new pathways towards nanomaterials which improve the performance of Li batteries, at the same time reducing cost and toxicity. Current cathode materials are of the type LixMO2 (M = Co, Ni), possess high performance and have been already largely used, however they are still rather expensive or toxic. At the present, the most promising candidates for substitution seems to be iron silicates and iron phosphates, followed by the more robust but more challenging corresponding Mn derivatives. Indeed, these systems exhibit a very favorable Li capacity and cell voltage, but a poor electronic conductivity. The latter negative aspect can however be compensated by reducing structural size1,2 and/or using suitable conductive layers for electron transport, such as carbon.3–5 Despite this importance, there are not many publications on the synthesis of well defined, nanostructured Mn derivatives, probably due to the difficulty in handling such materials. In this communication we analyse a simple and relatively fast route to prepare both LiyMnSiOx and LiMnPO4 with different sizes and shapes, also illustrating the textural complexity of the system.Experimental
LiyMnSiOx were synthesised by a hydrothermal process by mixing water solutions of lithium acetate (0.125 M) and manganese(II) acetate (0.125 M) and adding solid Cab-o-sil M5 (Riedel-de Haën, namely SiO2, MW 60.8, CAS number 7631-86-9) or Ludox HS-40 (namely colloidal SiO2, 40 wt% suspension in water, Sigma-Aldrich CAS number 112945-52-5) as a silica source in the desired ratio. The Cab-o-sil particle size is d < 5nm for primary particles, which however form larger chain-like secondary aggregates, while Ludox is constituted by individual spherical particles of about 15 nm in diameter. The silicon source was changed to check the influence of the potential nanostructure of the educt on the final product. After addition of the silicate precursor, the reaction mixture was stirred to get a homogeneous white dispersion. This dispersion was transferred into a Teflon-lined stainless-steal autoclave and kept in an oven at 200 °C for at least three days to complete the reaction. After cooling to room temperature, the product was washed several times with water. Thereafter, the products were dried in a vacuum oven and then milled to obtain a fine white or light beige powder in the case of Cab-o-sil derived samples and a fine light brown powder in the case of Ludox derivatives.For the LiMnPO4 samples, 1 mmol of manganese(II) acetate tetrahydrate (Mn 22% (typical), Alfa Aesar) was dissolved in 20 mL water to get a 20 M solution. Then 1 mmol of phosphoric acid (85 wt% solution in water, ACS reagent, Sigma-Aldrich) was added. After the manganese precursor and the phosphoric acid were completely mixed, 3 mmol of lithium hydroxide (98+%, Sigma-Aldrich) was added to the colourless solution, and a brown precipitate was produced immediately. The dispersion was stirred intensely to guarantee a well-mixed dispersion. The dispersion was placed into a stainless-steel autoclave. The dispersions were treated at different temperatures and different reaction times to find the best conditions. In Table 1, experimental details of the prepared samples are summarized.
| Sample name | Lithium precursor | Manganese precursor | Anionic source | Ratio |
|---|---|---|---|---|
| [LiyMnSiOx]-HS | C2H3LiO2·2H2O | Mn(CH3COO)2·4H2O | Ludox | 2:1:1 |
| [LiyMnSiOx]-HS | C2H3LiO2·2H2O | Mn(CH3COO)2·4H2O | Cab-o-sil | 2:1:1 |
| [LiyMnSiOx]-NP | LiCl | MnCl2·4H2O | Ludox | 1:1:1 |
| [LiyMnSiOx]-NP | LiCl | MnCl2·4H2O | Cab-o-sil | 1:1:1 |
| LiMnPO4 | LiOH | Mn(CH3COO)2·4H2O | H3PO4 | 3:1:1 |
Characterization was performed by elemental analysis and X-ray diffraction (by ICDD-PDF4+ database). Infrared spectra were recorded using a Biorad FTS 6000 FTIR Spectrometer under ATR (attenuated total reflection) conditions. XRD measurements were performed on a D8 Diffractometer from Bruker instruments (Cu-Kα radiation, λ = 0.154 nm) equipped with a scintillation counter.
Transmission electron microscopy (TEM) images were taken using a Zeiss EM 912Ω operated at an acceleration voltage of 120 kV. Samples were ground and then suspended in ethanol. One drop of this suspension was put on a 400 mesh carbon-coated copper grid and left in air to dry. A HRTEM Philips CM 200 LaB6, operated at an acceleration voltage of 200 kV, was used for higher magnifications.
SEM was performed on a LEO 1550-Gemini instrument. The samples were loaded on carbon coated stubs and coated by sputtering a Au/Pd alloy prior to imaging.
For determination of the surface area, all the samples were degassed at 200 °C for 20 hours before measurements. Nitrogen sorption experiments were done with a Quantachrome Autosorb-1 or Quadrasorb at liquid nitrogen temperature, and data analysis was performed with the Quantachrome software.
ICP analyses were performed using a Varian Visat-MPX CCD Simultaneous ICP-OES with radial plasma.
Results and discussion
Following the recipes and independently of using Ludox or Cab-o-sil as the silica source, the resulting LiyMnSiOx is obtained as an exciting nanostructure. When starting from Mn(CH3COO)2 (MnAc), structurally homogeneous and rather monodisperse hollow spheres are obtained (Fig. 1A). In contrast, while using MnCl2 as a manganese precursor, multiple rounded nanoplatelets are formed (Fig. 1B) | ||
| Fig. 1 TEM images of LiyMnSiOx in the form of hollow spheres (A, using MnAc and Ludox) and plate-like nanoparticles (B, using MnCl2 and Cab-o-sil). Corresponding particle size distribution patterns are reported in ESI† Fig. S2. | ||
The hollow structure seems not to be created as a consequence of the silica source (by acting as a template at the same time), as changing the silica source does not seem to have an effect on the morphology. Changing the metal precursor however has a strong influence. The exposure of certain faces by adding halogen ions in hydrothermal recipes is however not unusual and has for instance recently be used to preferentially expose the (001) face of anatase.6
The change of silica source causes a slightly different size of the structure, best seen by the increase of the BET surface area or the final particle volume (Table 2). Reducing the reaction time (2 days instead of 3) does not affect the size and morphology
| Sample name | Surface area (m2/g) | Size by BET (d = nm) | Size (nm) by TEM | Vhs/nm3 | |
|---|---|---|---|---|---|
| dint | dext | ||||
| a HS = hollow sphere morphology, NP = nanoparticles. | |||||
| [LiyMnSiOx]-HSa by Ludox | 406.9 | 8.0 | ∼5 | ∼10 | 230 |
| [LiyMnSiOx]-HS by Cab-o-sil | 531.0 | 9.6 | ∼5 | ∼8 | 303 |
| [LiyMnSiOx]-NPa by Ludox | 141.4 | 32.6 | — | 11–18 | — |
| [LiyMnSiOx]-NP by Cab-o-sil | 101.6 | 21.6 | — | 13–25 | — |
The very high homogeneity of the sample and the absence of secondary structural side products can be best ascertained by SEM investigation. The results are shown in Fig. 2. The specific surface areas of these structures are between 150–550 m2/g and are to our knowledge among the highest reported for such ternary structures.
 | ||
| Fig. 2 Representative SEM images of LiyMnSiOx samples prepared by using Ludox (A) and Cab-o-sil (B) as silicate precursors and different Mn precursors (A, B MnAc and C,D MnCl2). | ||
It is clear that for neither a small sized spherical nanobubble with nanometer-sized shell thickness nor for curved platelets with nanometer thickness are well structured XRD patterns to be expected. It is however a highly interesting question if these structures are nanocrystalline or rather amorphous. In Fig. 3 the XRD patterns of LiyMnSiOx prepared using two different silica sources are shown (the two hollow sphere samples).
 | ||
| Fig. 3 XRD patterns of LiyMnSiOx prepared using two different silica precursors: A) Cab-o-sil and B) Ludox. For comparison, patterns of the pure silicates are also shown. | ||
The direct comparison with the silica source shows that the sample is indeed not amorphous, as the typical glassy amorphous halo around 20° is practically completely suppressed (this peak is typical for all silica based glasses, even when containing larger amounts of a ternary metal). Instead, broad but still distinguished reflections can be seen, suggesting clusters in the 1 nm size region. However, it was neither possible to assign this pattern to any known crystal structure in the Li–Mn–SiOx systems or correlated structures, nor to attribute them to the expected Li2MnSiO4.
As we find no manganese salts in the supernatant, we are however quite sure that the sample contains the desired amount of manganese. On the other hand, ICP analysis revealed that Li incorporation is still rather low. This could also explain the poor capacity (∼8 mAh/g) shown by this material in a preliminary electrochemical test. A better analysis of the local structure, together with the characterization of the electrochemical performance of the system teaching us about the state of the manganese in the structure, will however be the subjects of forthcoming work.
FTIR measurements (see ESI† Fig. S1) are also indicative for the generation of a novel structure with individual vibration bands, i.e. the successful conversion of the silica can be proven. However, at the moment it remains unclear what type of local structure of the nanoclusters has been formed, and analytical tools like EXAFS, EELS, or XPS might be favourably applied.
For LiMnPO4, the situation is much more along classical lines and expectations. Differently to the silicate case, hydrothermal synthesis results in a highly crystalline material, as shown by XRD. The pattern could be attributed to orthorhombic lithiophilite (LiMnPO4 ref. ICDD-PDF4 + 04-002-3617), as show in Fig. 4. The difference in some peak intensities with respect to the expected ones could be due to the minor presence of a side product such as Li3PO4 (ref. ICDD-PDF4 + 00-025-1030), but may also be due to nanosize and shape control by the reaction medium.
 | ||
| Fig. 4 XRD pattern of LiMnPO4. For comparison, the calculated pattern is also reported (dotted line). Marked peaks are attributed to Li3PO4. | ||
The crystallite sizes estimated by the Scherrer equation are 25–50 nm in diameter. In some experiments, we also added a carbon source for conductive coatings, but this seems not to affect the primary particle formation of the LiMnPO4 structures.
Low resolution TEM images shown larger crystals of ca ∼100 nm–200 nm diameter but a closer look reveals the presence of a tectonic array, formed from clusters of smaller particles (Fig. 5). Such clustering was recently observed in a number of sol-gel recipes, such as for WO37 and SnO2,8 and was recently reviewed in a broader context.9
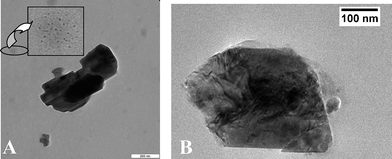 | ||
| Fig. 5 TEM images at different resolution of as-prepared LiMnPO4. In the insert, an enlargement of the nanoparticle in the background is shown. | ||
Looking more carefully to the background of the sample, we indeed find particular islands of ∼3 nm nanoparticles, as can be seen from Fig. 5A. Further experiments will have to use specific dispersion agents, like for instance the o-diphenol type successfully used for titania10 or WO311 to clarify the real primary particle size of those structures. It is clear that oriented alignment can hamper the determination of primary size by both TEM and XRD, and electrochemical performance will seriously depend on those quantities. At the moment, we can only say that these particles are, presumably due to polarization interactions, highly interactive and not protected by a stabilizing shell of an inert material. Consequently, the surface area as determined by BET was rather low, going with the surface of the secondary aggregates.
Conclusion
We present a first attempt to synthesize nanostructures of LiyMnSiOx and LiMnPO4 (both relevant Li battery cathode materials) by a simple hydrothermal route. Simply by changing parameters such as metal precursor and/or silica source, very high surface area LiyMnSiOx structures with very homogeneous morphology and sizes could be prepared, in particular hollow nanospheres and contorted plate-like nanoparticles. However, although not amorphous, these particles still exhibit a poorly developed crystallinity, which we attribute to the bond angle flexibility of the Si–O bonds.In the case of LiMnPO4, highly crystalline nanocrystals were obtained which obviously undergo aligned particle aggregation and mesocrystal formation.
A further improvement of the procedure in order to get smaller, well separated nanocrystals (in the case of phosphate) and more crystalline compounds (for silicate) is certainly advisable in order to use these materials for practical electrochemical applications. Current experiments of our group include the use of further calcination steps and employment of stabilizing ligands for particle separation which at the same time can act as a source to establish the required conductive carbon coating tightly bound to the particle surface.
References
- H. Huang, S. C. Yin and L. F. Nazar, Electrochem. Solid-State Lett., 2001, 4, A170 CrossRef CAS.
- Z. H. Chen and J. R. Dahn, J. Electrochem. Soc., 2002, 149, A1184 CrossRef CAS.
- R. Dominko, M. Bele, M. Gaberscek, M. Remskar, D. Hanzel, S. Pejovnik and J. Jamnik, J. Electrochem. Soc., 2005, 152, A607 CrossRef CAS.
- R. Dominko, J. M. Goupil, M. Bele, M. Gaberscek, M. Remskar, D. Hanzel and J. Jamnik, J. Electrochem. Soc., 2005, 152, A858 CrossRef CAS.
- Yi-Xiao Li, Zheng-Liang Gong and Yong Yang, J. Power Sources, 2007, 174, 528 CrossRef CAS.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS.
- J. Polleux, N. Pinna, M. Antonietti and M. Niederberger, J. Am. Chem. Soc., 2005, 127, 15595 CrossRef CAS.
- J. H. Ba, J. Polleux, M. Antonietti and M. Niederberger, Adv. Mater., 2005, 17, 2509 CrossRef CAS.
- M. Antonietti, M. Niederberger and B. Smarsly, Dalton Trans., 2008, 18 RSC.
- J. Polleux, N. Pinna, M. Antonietti and M. Niederberger, Adv. Mater., 2004, 16, 436 CrossRef CAS.
- J. Polleux, A. Gurlo, N. Barsan, U. Weimar, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2006, 45, 261 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR spectra of LiyMnSiOx prepared with different silica sources and different metal precursors; particles size distributions of LiyMnSiOx in the form of hollow spheres and plate-like nanoparticles; ICP technical details. See DOI: 10.1039/b9nr00149b |
| This journal is © The Royal Society of Chemistry 2009 |
