Nanocontact-induced catalytic activation in palladium nanoparticles†
Changlong
Jiang
a,
Sadananda
Ranjit
a,
Zhongyu
Duan
a,
Yu Lin
Zhong
a,
Kian Ping
Loh
a,
Chun
Zhang
*ab and
Xiaogang
Liu
*a
aDepartment of Chemistrys, National University of Singapore, Singapore 117543. E-mail: chmlx@nus.edu.sg; Fax: +65-65161791
bDepartment of Physics and Centre for Computational Science and Engineering, National University of Singapore, Singapore 117543. E-mail: phyzc@nus.edu.sg
First published on 29th September 2009
Abstract
We report the synthesis and catalytic studies of novel palladium nanostructures assembled from small nanoparticles by a surfactant-templated method. These one-dimensional nanomaterials comprise high-density nanocontacts of ∼1 nm in contact length at the particle–particle interface. In contrast to dispersed Pd nanoparticles (∼5 nm), the polycrystalline palladium nanowires exhibit enhanced (∼200 times) catalytic reactivity towards carbon–carbon cross-couplings under mild conditions. Theoretical modeling studies suggest that the presence of nanocontacts triggers electron transfer and localized charge redistribution in the contact region. The charge redistribution causes the nanocontacts to become highly attractive to charged organic molecules, resulting in the facilitation of organic transformations.
An important goal in catalyst research is to establish model systems that enable the desirable activity and selectivity to be achieved via an economical process.1–3Transition metal-based homogenous catalysts show remarkable activity and selectivity during organic transformations, but suffer from a lack of recyclability and high cost of waste disposal. In contrast, metal nanoparticlecatalysts are advantageous over their homogeneous counterparts in terms of easy separation from reaction mixtures and high level of recoverability, but are generally less selective and active. In this regard, there has been considerable recent interest in the rational design and shape-controlled synthesis of metal or metal-oxidenanostructures that offer both superior catalytic efficiency and recyclability.4–11 We reason that the introduction of nanocontacts12–15 between nanoparticles should significantly impact material reactivity for organic synthesis, as metallic nanocontacts are known to exhibit different characteristics in electronic, optoelectronic, electrical and magnetic properties from individual non-interacting nanoparticles. Here, we report the synthesis of novel palladium (Pd) nanostructures composed of a network of nanoparticles with high-density point contacts between the particles. We present theoretical modeling and experimental evidence for significant nanocontact-induced catalytic activation of the nanomaterials for cross-coupling reactions in water-based solvents and at ambient conditions.
The formation of Pd nanomaterials with high-density nanocontacts was achieved by a low-temperature solution reaction using poly(N-vinylpyrrolidone) (PVP) as a stabilizing ligand. PVP has been well studied as a polymeric capping reagent for the shape-controlled formation of metal nanoparticles and nanowires.16–19 In our studies, we employed trace copper powder as a reducing agent to control the slow rate of particle growth and subsequently the formation of high density nanocontacts at the particle–particle interface. A plausible mechanism for the nanocontact formation is shown in Scheme 1. Pd ions were first reduced by the copper metal to form Pd nanoparticles, which were subsequently assembled into one-dimensional nanostructuresvia the PVP template. Upon annealing at 50 °C without stirring for 24 h, Pd nanowires comprising a network of nanocontacts were synthesized in nearly quantitative yield. It should be noted that the PVP concentration employed in the synthesis dictates the shape of the as-prepared nanomaterials. For example, only dispersed small particles (∼5 nm in average diameter) were formed in the presence of 0.0025 mmol PVP (Fig. S1 of the ESI† ). As the concentration of PVP increases, the rate of particle aggregation becomes predominant, resulting in the formation of uniformly interconnected particle nanowires (Fig. S1).
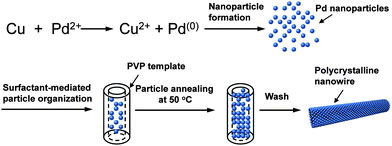 | ||
| Scheme 1 A schematic illustration of formation of Pd nanocontacts and nanowire growth. | ||
The surface morphology and crystal structure of the as-synthesized nanowires were characterized by SEM, XRD, TEM, and XPS. The results are summarized in Fig. 1. As evident from SEM, these nanowires have a uniform morphology with an average diameter of 100 nm (Fig. 1a). High-magnification SEM shows that each individual nanowire consists of corn-like elongated particle assemblies (Fig. 1b). The XRD pattern of the Pd nanowires is shown in Fig. 1c. All peaks can be indexed to face-centered cubic Pd (Joint Committee on Powder Diffraction Standards Card No. 05-0681). We attribute the peak sharpening to the particle aggregation resulting from the formation of high-density nanocontacts. In agreement with SEM studies, TEM shows densified nanoparticle arrays along the long axis direction of the nanowire (Fig. 1d). A three-dimensional web of interconnected particles (∼5 nm in diameter) can be visually traced from the outer edge of the nanowire by high-resolution TEM (Fig. 1e). Lattice fringe analysis shows high-density nanocontacts between the nanoparticles, indicating the polycrystalline nature of the nanowire. The fringe distance of 0.22 nm corresponds to the {111} planes of the Pd (Fig. 1e). The porous network structure has a pore size distribution centered at 5 nm and a surface area of 45 m2 g−1. (Fig. S3 of the ESI† ). The elemental composition of the as-prepared nanowires was determined by XPS. The Pd (3d5/2) and Pd (3d3/2) peaks were observed at 335.48 and 340.67 eV respectively, which are the characteristic values for Pd metal (Fig. 1f).
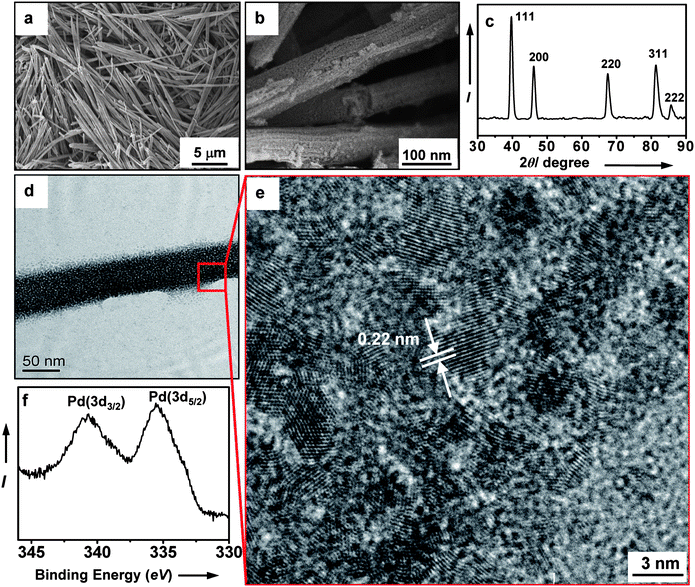 | ||
| Fig. 1 (a) SEM image of the as-synthesized Pd nanowires. (b) High-magnification SEM image showing surface morphology of the nanowires. (c) X-ray powder diffraction pattern of the nanowires. (d) TEM image of a single Pd nanowire. (e) High-magnification TEM image of the nanowire shown in Fig. 1d. (f) XPS spectrum of the nanowires. | ||
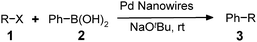
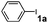
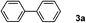
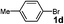
In a further set of experiments, the nanowires were examined for catalytic activity during Suzuki cross-coupling reactions under very mild conditions. Several phenyl halides were first examined for their ability to react with phenylboronic acid in the presence of the nanowires at room temperature (∼23 °C). For example, addition of phenylboronic acid (2) to a mixture of iodobenzene (1a), sodium t-butoxide and Pd nanowires in water provided biphenyl product (3a) in an almost quantitative conversion after 24 hours (Table 1, entry 1). It is worth noting that the insoluble biphenyl product formed at water–air interface can be easily separated from the catalyst precipitated to the bottom of the solution (Fig. S4a and b of the ESI† ). In contrast, the PVP-stabilized dispersed Pd nanoparticles (∼5 nm) only afforded a relatively low yield of product (50%) for numerous attempts under these conditions (Table 1, entry 4). Indeed, early reports of the Suzuki reaction using dispersed Pd nanoparticles as the catalysts were typically carried out under reflux conditions (78 ∼ 120 °C).20–27 Our experimental results also revealed that the use of ethanol as a solvent generally shortens the time (∼4 h) needed to complete the conversions (Table 1, entries 3, 5, 8, and 15). The enhanced reactivity can be largely attributed to improved solubility of the organic substrates in ethanol. Nonetheless, a binary combination of solvents in ethanol/water (2 : 3; v:v) offers comparably efficient conversions for a variety of substrates listed in Table 1 (entries 2, 9, 10, 12, 13, 16, 17, and 19). Importantly, the reaction between the phenyl chloride and phenylboronic acid can also proceed in high yields (entries 20 and 21) using polyethylene glycol as the solvent.
| Entry | R–X | Solvent | t/h | Product | % Conv.b |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), phenylboronic acid (1.2 mmol), NaOtBu (2 mmol), Pd nanowirecatalyst (6.5 mol%), total solvent volume (10 mL). b GC-MS yields. c Values in parenthesis are isolated yields. d E/W refers to a solvent mixture of ethanol and water (2 : 3; v:v). e The entry relates to an analogous reaction using Pd nanoparticles as catalysts. f The reaction is carried out at 50 °C. g PEG refers to polyethylene glycol. h The reaction is carried out at 80 °C. | |||||
| 1 |
|
H2O | 24 |
|
99 |
| 2 | 1a | E/Wd | 4 | 3a | 99 |
| 3 | 1a | EtOH | 4 | 3a | 99(93)c |
| 4e | 1a | H2O | 24 | 3a | 50(40)c |
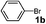
| 5 |
|
EtOH | 4 | 3a | 95 |
| 6 | 1b | H2O | 24 | 3a | 99 |
| 7 |

|
H2O | 24 |
|
44 |
| 8 | 1c | EtOH | 4 | 3b | 99 |
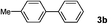
| 9 | 1c | E/Wd | 4 | 3b | 99 |
| 10 |
|
E/Wd | 4 | 3b | 99 |
| 11 |
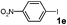
|
H2O | 24 |
|
76 |
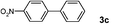
| 12 | 1e | E/Wd | 4 | 3c | 99 |
| 13 |
|
E/Wd | 4 | 3c | 99 |
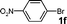
| 14 |
|
H2O | 24 |
|
72 |
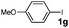
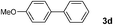
| 15 | 1g | EtOH | 4 | 3d | 99 |
| 16 | 1g | E/Wd | 4 | 3d | 99 |
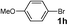
| 17 |
|
E/Wd | 4 | 3d | 99 |
| 18 |

|
H2O | 24 |
|
27 |
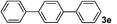
| 19 | 1i | E/Wd | 4 | 3e | 98 |
| 20f |
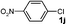
|
PEG g | 5 | 3c | 91 |
| 21h |
|
PEG g | 24 |
|
87 |
The catalytic activity of Pd nanowires are not limited to Suzuki reactions. It can be readily extended to other coupling reactions such as Heck reactions. Selected results for nanowire-catalyzed Heck reactions are summarized in Table S1 (ESI† ). For instance, the chemical reaction of an iodobenzene with a styrene in the presence of the nanowirecatalyst exhibits stereoselectivity with a high conversion (85%) for trans-coupling (Table S1, entry 1) at room temperature, albeit with the need of a polar aprotic solvent (dimethylformamide). Alternatively, the trans-coupling product can be favored in aqueous solvents at elevated temperatures (Table S1, entry 3), while its dispersed Pd nanoparticle counterpart gives rise to a low yield of product (Table S1, entry 4) under these conditions.
To benchmark the method, we further compared the activity of our Pd nanomaterials with bulk metal, dispersed nanoparticle counterparts, and palladium(II) acetate [Pd(OAc)2] as well as commercially available Pd catalyst (10% Pd/C) for coupling reactions by UV–vis spectroscopy. The formation of the biphenyl product for two different batch catalysts was monitored by quantitative absorption analysis of aliquot samples taken from the reaction at different time intervals as shown in Fig. 2a. The bulk metal (Pd foil, 1.6 mg), dispersed nanoparticles (∼5 nm; 1 mg), 10% Pd/C catalyst (10 mg), and nanowires (1 mg) exhibit markedly different kinetic profiles for the coupling reaction between the phenylboronic acid and iodobenzene substrates at room temperature. The bulk metal did not show any catalytic activity toward the coupling reaction, while the nanoparticles and the Pd/C catalyst exhibited moderate catalytic activities. In contract, the Pd nanowires exhibit a rate constant that is about 200 and 34 times greater than that of the 5-nm nanoparticles and Pd/C catalyst, respectively (Fig. 2b). It is worth noting that the Pd(OAc)2 catalyst shows a reactivity pattern similar to that of the Pd nanowires, but poses a substantial recycling challenge.
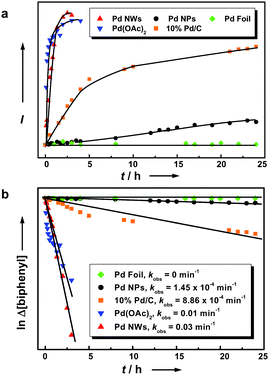 | ||
| Fig. 2 (a) Formation of biphenyl product in ethanol as a function of time at room temperature by different catalysts including Pd nanowires, Pd nanoparticles, Pd foil, commercial Pd/C particles, and Pd(OAc)2. The data points (average of two runs) were derived by acquiring the biphenyl absorption band (centered at 247 nm) for aliquot samples taken from the reaction at different time intervals. (b) Linear regression plot for the determination of the observed rate constants kobs. The values were determined from a ln plot of the change in iodobenzene concentration versus time for respective reactions. | ||
The scope of the Pd catalyst was further examined by recycling it from the reaction of iodobenzene with phenylboronic acid (Table 1, entry 1). It was observed that the catalyst exhibits consistent catalytic activity (∼99%) over six recycles (Table S2 of the ESI† ). It should be noted that the nanowirecatalyst exhibits considerable thermal stability and mechanical robustness even after repeated ultrasonic treatment at elevated temperatures (Fig. S4c and d of the ESI† ).
To provide insight into the nanocontact-induced catalytic activation, we performed first-principles density-functional theory (DFT) calculations on a contact between two Pd nanoparticles of ∼1.2 nm in diameter. The DFT calculations include the generalized gradient approximation,28 using a plane wave basis (kinetic energy cutoff Ecut = 14.6 Ry) and pseudo-scalar relativistic soft pseudo-potentials.29 Two Pd particles are contacted in either the (100) or (111) direction. The distance between two bulk layers, L, measures the length of the contact region. The bond length between two nanoparticles is denoted as d (see Fig. 3a). The electronic and atomic structures of the contact were calculated for different values of L. Two outermost layers of the contact region (see the enlarged contact region for 100 connection in Fig. 3a) are fixed in bulk structure during the structure optimization. This is consistent with a previous report that the change of L only has significant effects on a few atomic layers of the contact region.30 The difference of the total energy (δE = E − E0) upon compression of the contact (δL = L − L0, L0 = 8.25 Å for 100 connection and 11.76 Å for 111 connection, respectively) is shown in Fig. 3b, where E0 is the total energy of the two nanoparticles and L0 is the length of the contact prior to compression. For the lowest energy state, L and d are found to be 7.95 (11.56) Å and 2.66 (2.60) Å for 100 (111) connection, respectively. The charge redistribution in the contact region due to the bonding of two particles of 100 connection is shown in the inset of Fig. 3b, where the green color represents the accumulation of electrons, and the red color is the depletion of electrons. Clearly, electron transfer (ca. 1.2 e) occurs from the nanoparticles to the interfacial region to form bonds. For the (100) connection, the amount of the charge transfer is about 1.2 electrons, while for the (111) connection, it is about 1.1 electrons. This charge redistribution makes the nanocontact highly attractive to organic molecules, thus resulting in the enhanced catalytic reactivity of the nanomaterials.
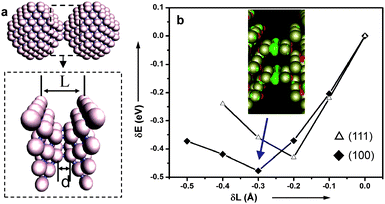 | ||
| Fig. 3 (a) Geometry of two Pd nanoparticles contacted in the (100) direction. The enlarged contact region is indicated by an arrow. L is defined as the distance between two fixed outermost layers, and d is the bond length between two particles. (b) Total energy difference (E − E0) for the (100) or (111) connection versus compression distance (L − L0) of the two Pd particles. E0 and L0 are the total energy and the length of the contact as defined as the distance between two fixed outermost layers as shown in (a) (Inset: the isosurface of the excess electronic charge for the (100) connection in green). | ||
In conclusion, we have reported the synthesis of novel Pd nanostructuresvia a combination of the PVP-templated method and a slow particle crystallization process. The as-prepared nanostructured materials show distinctive catalytic properties in sharp contrast to bulk metal and individual nanoparticles. These studies are important not only because they provide a potential platform to study the underlying principle that governs the catalytic reactivity of nanomaterials, but also because they shed insight into designing highly reactive and recyclable catalytic systems for organic transformations. Along these lines, we are currently investigating electron transport within the polycrystalline nanowires and exploring the fabrication of polycrystalline nanomaterials with various material compositions.
Acknowledgements
This work was supported by the NUS Academic Research Fund (R-143-000-317 and R-143-000-342) and a Young Investigator Award (R-143-000-318) to X. L. by the NUS. X. L. is grateful for H. C. Zeng for helpful discussions. We thank B. Liu, C. Tang, and Y. Nie for technical assistance. C. Z. acknowledges the NUS Start-up Fund (R-144-000-237). Computations were performed at the Centre for Computational Science and Engineering at NUS.References
- J. Grunes, J. Zhu and G. A. Somorjai, Chem. Commun., 2003, 2257 RSC.
- G. A. Somorjai and R. M. Rioux, Catal. Today, 2005, 100, 201 CrossRef CAS.
- Z. Duan, S. Ranjit, P. Zhang and X. Liu, Chem.–Eur. J., 2009, 15, 3666 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS.
- N. Tian, Y. Zhou, S. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- H. Song, R. M. Rioux, J. D. Hoefelmeyer, R. Komor, K. Niesz, M. Grass, P. Yang and G. A. Somorjai, J. Am. Chem. Soc., 2006, 128, 3027 CrossRef CAS.
- X. Liu, N. Wu, B. H. Wunsch, R. J. Barsotti Jr. and F. Stellacci, Small, 2006, 2, 1046 CrossRef CAS.
- X. Lu, H.-Y. Tuan, J. Chen, Z.-Y. Li, B. A. Korgel and Y. Xia, J. Am. Chem. Soc., 2007, 129, 1733 CrossRef CAS.
- X. Liu, Angew. Chem., Int. Ed., 2009, 48, 3018 CrossRef CAS.
- C. Jiang, F. Wang, N. Wu and X. Liu, Adv. Mater., 2008, 20, 4826 CrossRef CAS.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332 CrossRef CAS.
- U. Landman, R. N. Barnett, A. G. Scherbakov and P. Avouris, Phys. Rev. Lett., 2000, 85, 1958 CrossRef CAS.
- E. Tosatti, S. Prestipino, S. Kostlmeier, A. Dal Corso and F. Di Tolla, Science, 2001, 291, 288 CrossRef CAS.
- D. O. Demchenko and L.-W. Wang, Nano Lett., 2007, 7, 3219 CrossRef CAS.
- B. Pérez-García, J. Zúñiga-Pérez, V. Muñoz-Sanjosé, J. Colchero and E. Palacios-Lidón, Nano Lett., 2007, 7, 1505 CrossRef CAS.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS.
- Y. Sun, B. Gates, B. Mayers and Y. Xia, Nano Lett., 2002, 2, 165 CrossRef CAS.
- Y. Xiong, J. Chen, B. Wiley and Y. Xia, J. Am. Chem. Soc., 2005, 127, 7332 CrossRef CAS.
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- L. K. Yeung and R. M. Crooks, Nano Lett., 2001, 1, 14 CrossRef CAS.
- M. Ooe, M. Murata, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 1604 CrossRef CAS.
- E. H. Rahim, F. S. Kamounah, J. Frederiksen and J. B. Christensen, Nano Lett., 2001, 1, 499 CrossRef CAS.
- J. C. Garcia-Martinez, R. Lezutekong and R. M. Crooks, J. Am. Chem. Soc., 2005, 127, 5097 CrossRef CAS.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181 CrossRef CAS.
- L. Strimbu, J. Liu and A. E. Kaifer, Langmuir, 2003, 19, 483 CrossRef CAS.
- S.-W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2004, 108, 8572 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- N. Troullier and J. L. Martins, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 1993 CrossRef CAS.
- C. Zhang, R. N. Barnett and U. Landman, Phys. Rev. Lett., 2008, 100, 046801 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on the materials and methods used to fabricate and characterize the Pd nanocontacts and nanowires. See DOI: 10.1039/b9nr00093c |
| This journal is © The Royal Society of Chemistry 2009 |