Boswellic acids: a group of medicinally important compounds
Bhahwal Ali
Shah
,
Ghulam Nabi
Qazi
and
Subhash Chandra
Taneja
*
Indian Institute of Integrative Medicine (CSIR), Canal Road, Jammu Tawi, 180001, India. E-mail: sc_taneja@yahoo.co.in; Fax: +91 191 2569017/2569333; Tel: +91 191 2569011
First published on 24th October 2008
Abstract
Covering: primarily 1980 to 2008
This review, containing over 276 references, covers the progress made in the chemistry and bioactivity of this important group of triterpenoids. Though initially known for their anti-inflammatory and anti-arthritic activities through a unique 5-LO inhibition mechanism, boswellic acids have recently attained significance due to their anti-cancer properties. The phytochemistry and chemical modifications, including mechanism of action, are discussed.
 Bhahwal Ali Shah | Bhahwal Ali Shah was born in Mendhar, Jammu & Kashmir (India), in 1982. He post-graduated in organic chemistry from Ch. Charan Singh University, Meerut, in 2004. He joined Dr S. C. Taneja's research group at Indian Institute of Integrative Medicine, Jammu and submitted his Ph.D. thesis to Guru Nanak Dev University, Amritsar, on the subject “Structure modifications of some natural products for bioactive lead molecules”. Currently he is working as a scientist in the area of drug development; his research interests also include biocatalytic transformations and synthetic methodology development. |
 Ghulam Nabi Qazi | Ghulam Nabi Qazi was born in 1946 at Srinagar in Jammu & Kashmir (India). He had his early education in the Kashmir valley. He obtained his post graduate degree in Biochemistry and Ph.D. in Microbiology form M S University, Baroda (India). He did his post-doctoral research at the University of Dortmund (Germany). He has had a research career lasting over 40 years in the area of Biochemistry & Microbial Biotechnology and bioprospecting of natural molecules, and was the director of the Indian Institute of Integrative Medicine (IIIM) at Jammu under the Council of Scientific and Industrial Research up to August 2008. Dr Qazi has over 170 publications to his credit and has filed 69 patents. Dr Qazi is a recognized Ph.D. guide for 12 Indian universities and has supervised 20 Ph.D. students. |
 Subhash Chandra Taneja | Born in 1950, Subhash Chandra Taneja obtained his masters degree in Organic Chemistry in 1971 and thereafter completed his Ph.D. at the Birla Institute of Technology and Science, Pilani (Rajasthan), in 1975 under the guidance of Prof. H. P. Tiwari. His early natural product related work published in 1974 included the first report and identification of iridoid glycosides in the plant family Acanthaceae. In 1975 he moved to IIIM Jammu (erstwhile RRL), and was appointed as senior scientific assistant in 1977; he was thereafter promoted at regular intervals to various scientific positions, including the present position of Scientist ‘G’ in 2005, and is currently acting director. During this time he visited the Institute of Organic Chemistry, Warsaw, Poland. Dr Taneja has about 100 publications in international journals, and 38 patents, of which 14 are US/PCT patents. His research interests also include biocatalysis, kinetic resolutions, drug development, organic synthesis, methodology development, terpenes and glyco-chemistry. |
1 Introduction
Boswellic acids (BAs) are pentacyclic triterpenoids belonging to ursane group, which are the major constituents of the gum derived from the plant Boswellia serrata Roxb. ex Colebr. (family Burseraceae, Syn. B. glabra), commonly known by the names Salai guggal, white guggal, Indian olibanum or dhup.1 Historically, the gum exudate or the resin obtained from the bark of the tree has been widely used by the practitioners of the Indian systems of medicine for various medical conditions such as arthritis, asthma, ulcers, and skin diseases; currently it is extensively used in various formulations for the treatment of inflammation related disorders.2–4
B. serrata is a deciduous middle-sized tree widely distributed in the Indian subcontinent and Africa, and is documented to be of high medicinal as well as economic importance.5–7 In India it occurs in the dry hilly forests of Rajasthan, Madhya Pradesh, Gujarat, Bihar etc. The gum is tapped from incisions made on the trunk of the tree, which is then stored in specially made bamboo baskets and converted into different grades of material according to the flavour, colour, shape and size. The fresh gum from the tree has a hot, bitter taste, with a good flavour. Its depletion due to excessive harvesting is creating much concern.8 A multitude of phytochemical and pharmacological properties of the gum resin have been documented.9 It is the frankincense of the ancient Egyptians, Greeks and Romans, who used it as a prized incense and fumigant, as well as a multipurpose aroma.10 The frankincense used is generally that derived from the second or third harvest in the year.11 Around 1200 B.C., approximately 304![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 093 bushels of fragrant resin were used in the Temple of Amon in Thebes in one year.12 Earlier it was used as an ingredient of plaster and also in pastilles used for fumigating.13 Quincy portrayed it as very glutinous and thus used it in some compound strengthening plasters.14 It is also used for the treatment of gout and believed to be good for teeth and gums.11 Dioscorides suggested the use of frankincense mixed with leek juice to stop bleeding, especially epistaxis.15 Culpeper recommended frankincense for the stopping up of old ulcers and flesh wounds, and to stop bleeding.16
093 bushels of fragrant resin were used in the Temple of Amon in Thebes in one year.12 Earlier it was used as an ingredient of plaster and also in pastilles used for fumigating.13 Quincy portrayed it as very glutinous and thus used it in some compound strengthening plasters.14 It is also used for the treatment of gout and believed to be good for teeth and gums.11 Dioscorides suggested the use of frankincense mixed with leek juice to stop bleeding, especially epistaxis.15 Culpeper recommended frankincense for the stopping up of old ulcers and flesh wounds, and to stop bleeding.16
The other species of genus Boswellia include B. ovalifoliolata Bal. & Henry (India), B. carterii Birdw. (Somalia), B. sacra Fluckiger (Oman and Yemen), B. pirottae Chiov. (Ethopia), B. dalzielii Hutch. (West Africa), B. frereana Birdw. (Somalia), B. neglecta S. Moore (Kenya), B. papyrifera (Del.) Hochst. (Ethopia), B. rivae Engl. (Ethopia), B. hildebrandtii Engl. (Somalia), B. ogadensis Vollesen (Ethopia), B. popoviana Hepper (Yemen), B. nana Hepper (Yemen), B. bullata Thul. & Gifri (Yemen), B. dioscorides Thul. & Gifri (Yemen), B. elongata Balf. f. (Yemen), B. ameero Balf. f. (Yemen) and B. socotrana Balf. f. (Yemen).17,18B. serrata is the most investigated of all the species, while some phytochemical studies, as well as bioactivity-related investigations, have been reported for B. carterii, B. ovalifoliolata and B. papyrifera. The presence of BAs in almost all the species of Boswellia is a characteristic of this genus.
Fatty acids and triterpenoids are well known to be a part of the cuticular films or deposits which form the outermost extracellular matrix in the epidermis of the primary tissues in terrestrial plants. This waxy film may be involved at various stages of development of a plant, besides being involved in forming a protective shield. The triterpenoids are synthesized through isopentenyl pyrophosphate route (IPP) from a squalene intermediate, and their role is yet to be fully understood, though they are certainly involved in defence mechanisms, as many of them have been reported to possess diverse biological activities19 that include immunostimulation,20 antimicrobial,21 anti-inflammatory,22 anti-cancer23 and antiviral properties.24 Betulinic acid, BAs, oleanolic acid and glycyrrhetic acid are some of the examples which led to development of renewed interest in polycyclic triterpenoids as a pharmacophore for new drugs.
The work done on various aspects of the bioactivity of the gum resin of B. serrata has been reviewed previously.25,26 The present review is more comprehensive, covering not only the chemistry of the genus Boswellia but also bioactivity-related studies, carried out primarily on B. serrata extract (BSE) and its major constituents (BAs), with emphasis on anti-inflammatory and anti-cancer properties.
2 The chemical constituents of Boswellia species
2.1 The constituents of B. serrata
B. serrata gum resin is a complex mixture of terpenoids and sugars comprising more than 200 different substances27 including polysaccharides, essential oils, proteins and inorganic compounds.28B. serrata gum resin contains 8–12% essential oils, 45–60% polysaccharides and 25–35% higher terpenoids.29,30 The chemistry and pharmacology has been a subject of an earlier review.31 The major chemical components of gum resin can be divided into three groups: volatile oils or lower terpenoids, higher terpenoids, and carbohydrates, which are discussed below. | ||
| Fig. 1 The structures of boswellic acids. | ||
A large volume of original research has accumulated on the chemistry of higher terpenoids ever since the first isolation of BA was reported in 1898 by Tschirch et al.34 Since then number of chemists have worked on its structure elucidation.35 By disclosing the stereo-identity of functional groups, Allen36 provided chemical evidence that the hydroxyl and carboxyl functions in BA were axial, as well as assigning configurations at C-5, C-8, C-10, C-13 and C-17. BA occurs in the form of an isomeric mixture, i.e. (α + β)-BA; similarly, ABA is also a mixture of (α + β) isomers (5, 6); moreover, both the isomers can be easily resolved by HPLC.37 BA is generally accompanied with a diene derivative, namely 3-O-acetyl-9,11-dehydro-β-boswellic acid (7),38 which is believed to originate from 3-O-11-hydroxy-β-boswellic acid (9) via dehydration.39 The diene derivative may be isolated by repeated crystallization of the methyl ester of BA.40 The structures of all the major pentacyclic triterpenes, which include BAs and the diene derivative (7), have been established by NMR spectroscopy.41 The structures of ABA (3)42 and the methyl ester of BA37 have also been confirmed by X-ray crystallographic studies. In addition, α-amyrin (10) and 3-hydroxy-urs-9,11-dien-24-oic acid (8) have also been isolated from the gum resin. Several tetracyclic terpenoic acids have also been reported by Pardhy et al.,43 and these include 3α-hydroxy-tirucall-8,24-dien-21-oic acid (11), 3α-acetoxy-tirucall-8,24-dien-21-oic acid (12), 3β-hydroxy-tirucall-8,24-dien-21-oic acid (13) and 3-keto-tirucall-8,24-dien-21-oic acid (14). Isolation of a new diterpene alcohol, serratol (15), believed to be responsible for the typical odour of the gum, has also been reported.43 Mahajan et al. described the structure elucidation of two new triterpenoids from acidic and neutral fractions of the gum extract,44 2,3-dihydroxy-urs-12-ene-24-oic acid (16) and urs-12-ene-3α,24-diol (17).45 The presence of another isomeric diol, viz. urs-12-ene-3β,24-diol (18), has also been confirmed in the neutral fraction.46 Of late, lupane triterpene, 3α-hydroxy-lup-20(29)-en-24-oic acid (19)47 and 3α-acetoxy-lup-20(29)-ene-24-oic acid (20)48 have also been isolated from B. serrata. β-Sitosterol (21) has also been isolated from the bark of B. serrata by Beri et al.,49 and a diterpene alcohol, incensole (22), has also been reported from this species (Fig. 2).50
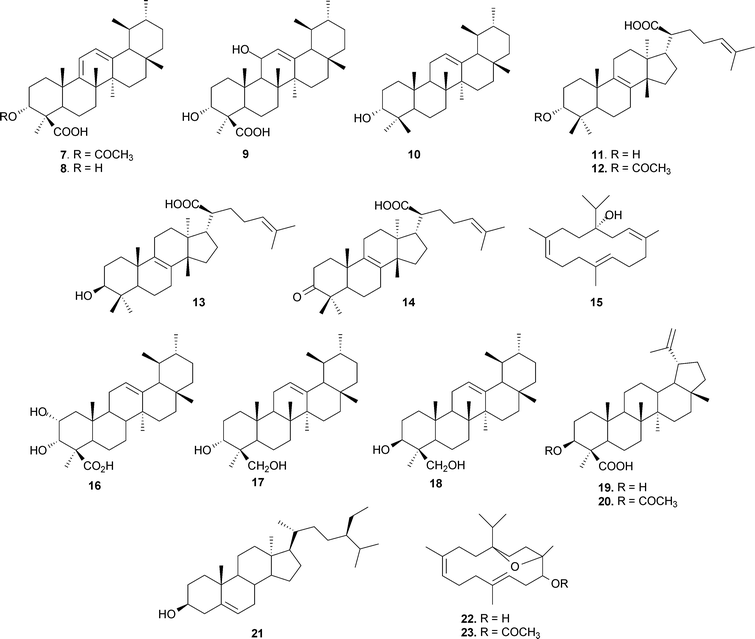 | ||
| Fig. 2 The structures of various terpenoids from B. serrata. | ||
2.2 The chemistry of other Boswellia species
Not many chemical investigations have been reported for other species of the genus Boswellia, though there have been sporadic preliminary investigations of the volatile and other constituents. n-Octyl acetate and n-octanol are the principal volatile components of B. carterii53 and B. papyrifera,54 and α-pinene is the major component of the oil of B. frereana.55B. neglecta has been found to contain α-thujene, α-pinene, β-pinene and p-cymene as the major components.56 The oil of B. rivae is characterized by the presence of limonene and α-pinene, and trans-verbenol as the minor component; whereas in B. pirottae, high contents of trans-verbenol and terpinen-4-ol were reported.57All the species of the genus Boswellia consist of various quantities of BAs and other di- and triterpenoids. B. carterii has been found to contain 3α-acetoxy-lup-20(29)-ene-24-oic acid (20),58 incensole acetate (23)59,60 along with incensole (22)50 and BAs. Some other diterpenes have also been isolated from B. carterii, including cembrene A (24),61,62 isocembrene (25),53 cembrene C (26),63 incensole oxide (27),32 isoincensole oxide (28),64 verticillia-4(20),7,11-triene (29), verticillol (30) and ent-verticillene (31), along with four hydroxylated verticillane derivatives (32–35).65 The isolation of sitost-4-en-3-one (36)66 along with α-amyrin (4)67,68 was reported from B. ovalifoliolata. In addition, there are two species in the genus containing phenolics. The macrocyclic diaryl ether heptanoids ovalifoliolatins A (37) and B (38)69 and acetogenin C (39)70,71 have been reported from B. ovalifoliolata, and trans-4,5-dihydroxy-3-methoxystilbene-5-O-{R-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-α-D-glucopyranoside (40), trans-4,5-dihydroxy-3-methoxystilbene-5-O-[α-L-rhamnopyranosyl-(1→6)]-α-D-glucopyranoside (41) are present in B. papyrifera, which also contains BAs and 3α,27-dihydroxylup-20(29)-en-24-oic acid (42), 3α-acetoxy-27-hydroxylup-20(29)-en-24-oic acid (43), 3-keto-tirucall-8,24-dien-21-oic acid (14) and α-sitosterol (44).72 The presence of lupeol (45) and its epimer (46) has been reported from B. frereana (Fig. 3).73
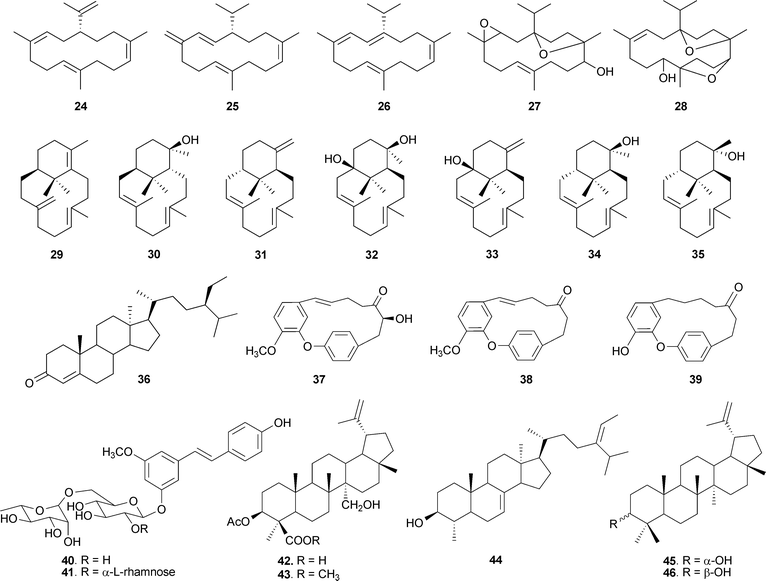 | ||
| Fig. 3 The structures of various compounds from other Boswellia sp. | ||
2.3 Method of isolation of boswellic acids
Historically, there has been a good demand of the gum resin for its use in medicines and incenses, and as a fumigant.74 BAs, the major pentacyclic triterpenes, may be separated by a modified method developed earlier by Winterstein and Stein.75 A flowchart for the isolation of BAs from the gum resin as optimized in the authors’ laboratory is given in Scheme 1. The yield of the higher triterpenoids (BAs), which are reported to be bioactive and responsible for the most of the medicinal attributes of the gum resin, may vary due to factors such as the age of the tree, the time of collection and the climate. Methods using alkali–acid treatment of the concentrated alcoholic extract provide BAs of higher purity. | ||
| Scheme 1 Flowchart for isolation of various terpenoids. | ||
Chromatographic methods may also be used for the separation of the individual acids and other constituents. However, an earlier report claimed that AKBA (4) could not be separated from a mixture of ABA (3) and AKBA by any of the chemical methods, such as ketal formation76 or semi-carbazide formation.77 The commercial formulations and most of the market preparations generally utilize extracts of B. serrata comprising ∼60–70% total acids/BAs. In addition, enrichment of AKBA by ∼100% is achievable by oxidation of ABA with chromic acid78,79 or photo-oxidation with NBS–water–dioxane.80
3 Semi-synthetic modifications of BAs
The structures and bioactivities of BAs have been well documented, and OTC formulations and nutraceutical preparations in diverse forms comprising Boswellia serrata extract (BSE) in combination with other plant extracts or molecules have been in use for many years. Surprisingly, not much has been reported regarding chemical modifications or preparation of structural analogues of the key constituents.However, the authors’ group have prepared 3-epi analogues of BAs, which do not occur naturally.81 The synthesis initiated with the conversion of BAs 1 and 2 to corresponding methyl esters 47 and 48 with diazomethane (CH2N2)40 followed by oxidation with PCC82 or sodium dichromate over silica,77 yielding the 3-keto derivatives (49 and 50). These were quantitatively reduced by NaBH438 to 51 and 52, with (S) or β-configuration at C-3; the preferred epimer formation being achieved due to the steric hindrance caused by the substituent groups in the A/B rings (e.g. 25-Me, 24-COOMe). The methyl esters thus obtained were hydrolyzed using KOH/MeOH in a high pressure reaction vessel at 98–100 °C to obtain β-epimeric BAs 53 and 54 respectively in 88% yield, which were then converted to corresponding acetates (55 and 56) (Scheme 2).
 | ||
| Scheme 2 Synthesis of 3-epi BAs. | ||
Methyl β-boswellic acid ester on treatment with POCl3 in dry pyridine83 gave methyl urs-2,12-dien-24-oate (57). Similarly, methyl 11-oxo-urs-2,12-dien-24-oate (58) was also synthesized (Scheme 3).
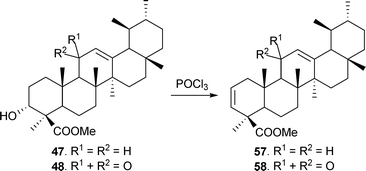 | ||
| Scheme 3 Synthesis of diene analogues of BAs. | ||
The carboxylic group in 1 can be easily removed and nor-β-boswellenone (59) may be obtained from BAs by treating with chromic anhydride in glacial acetic acid.84 Subsequent treatment with chromic acid gave nor-β-boswellendione (60) (Scheme 4).79
 | ||
| Scheme 4 Synthesis of a nor-analogue of BA. | ||
Methyl-3α-acetoxy-12-oxo-urs-24-oate (62) was prepared from methyl-3α-acetoxy-urs-24-oate (61) by reacting it with either hydrogen peroxide in glacial acetic acid85 or with potassium permanganate in glacial acetic acid,84 the product being further hydrolysed to methyl-3α-hydroxy-12-oxo-urs-24-oate (63) (Scheme 5).
 | ||
| Scheme 5 Synthesis of a 12-keto analogue of BA. | ||
Heterocyclic analogues from BA and KBA were also synthesized by treating 3-keto esters 49 and 50 with ethyl formate in dry benzene86 in the presence of sodium hydride to afford the 2-hydroxy methylene analogues (64 and 65), which were transformed to the corresponding pyrazoles (66 and 67) using hydrazine hydrate in ethanol (Scheme 6).87 Likewise, pyrazoles 68–70 (obtained from 59, 60 and 63) have also been prepared (Fig. 4).37
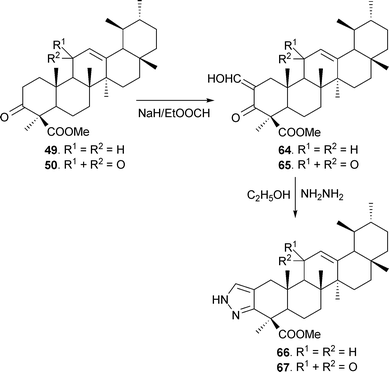 | ||
| Scheme 6 Synthesis of pyrazole derivatives of BAs. | ||
 | ||
| Fig. 4 Other pyrazoles synthesized from BAs. | ||
A BA analogue (71) having a carboxyl group at C-17 with structural similarity to a triterpene (3α-hydroxy-urs-12-ene-23,28-dioic acid, isolated from Schefflera octophylla) has also been synthesized from 3-epi-ursolic acid (72).88 The basic idea was to synthesize a molecule based on the structures of both BA and 3-epi-ursolic acid (Scheme 7).
 | ||
| Scheme 7 Synthesis of a BA analogue having a carboxyl group at C-17. | ||
Recently our group have prepared 4-amino analogues of BA (73), KBA (74) and their epimers via isocyanate intermediates (75 and 76).81 The objective was to replace the carboxyl with an amino function to study its cytotoxic behaviour against various human cancer cell lines (Scheme 8). We also reported the preparation of a triterpene diol derivative (77) by reducing BA with LiAlH4;89 the semi-synthetic molecule thus prepared has also been isolated naturally from B. serrata,37 and displays potent anti-apoptotic activity (Scheme 9).
 | ||
| Scheme 8 Synthesis of 4-amino analogues of BAs. | ||
 | ||
| Scheme 9 Synthesis of a diol analogue of BA. | ||
As well as the above-mentioned derivatives, 3-acyl (formyl, acetyl, propyl, butyl etc.) analogues of BAs and their epimers have also been prepared,90 in order to study the effect of acylation with respect to anti-cancer as well as anti-inflammatory activities.
4 Chromatography
Evidence for the presence of BAs and their acetates in archaeological frankincense has been provided by analysing the samples of resin-like materials recovered from excavations at Qasr Ibrahim, Egypt.91 Frankincense investigated by a GC–MS method was found to contain 15 triterpenoids, with BAs and their acetates constituting the major part.92 Four frankincense samples unearthed from Yemen have also been analysed for their constituents using GC–MS analysis.93 Analysis of the lower terpenes from frankincense was established through a headspace solid-phase micro-extraction method (SPME).94B. carterii and B. sacra olibanum have been found to have quite similar chemical compositions as analysed by headspace SPME–GC/MS in a study of six different olibanum samples of certified botanical origin.95 The constituents of B. serrata, B. papyrifera and B. frereana were also identified by the same method.Several HPLC methods have been reported for the detection and quantification of BAs. A highly sensitive reverse-phase HPLC method for the detection and analysis of BAs in B. serrata was developed using an acidic mobile phase at 60 °C.96 Buchele et al.97 reported an HPLC method for simultaneous determination of 12 different triterpenic acids from Indian and African frankincense as well as related phytopharmaceuticals. Another reverse-phase HPLC method was claimed to be able to determine 15 different triterpenes in frankincense at once.98 An HPLC method for the separation and individual identification of different acids in B. serrata resin as well as in multi-component herbal preparations has also been described.99 In another method involving isolation and separation of BAs by HPTLC, methanol was found to be the most appropriate solvent for extraction, and used for simultaneous quantitative estimation of major BAs from B. serrata.100 Later, another method was established to analyze 12 different triterpenic acids of the BA family in human plasma by HPLC, by combining serial extraction on diatomaceous earth and graphitized carbon black followed by reverse-phase HPLC using photodiode array detection.101
An HPTLC method was used for the separation of four major BAs on silica gel by automated multiple development (AMD) using solvent gradients, and later applied to check significant variation in the content of these pharmacologically active compounds in commercial samples.102 Very recently, another rapid and sensitive HPTLC method was established for quantitative estimation of BAs in the formulations containing BSE and KBA in human plasma.103
The effects of concomitant food intake on the bio-availability of distinct BAs from the test preparation of a dry extract from B. serrata gum resin have been investigated.104 The detailed kinetics of BAs after oral dosing and profound effect of food intake on the pharmaco-kinetic profile of BAs provided important evidence, which may be useful for their therapeutic use, if ever considered. Similarly, the pharmaco-kinetics of KBA in humans has been reported105 and a GC–MS method was applied for their quantitative estimation in plasma.106 The recommended interval for oral administration of BSE was 6 hours based on the blood profile of 12 volunteers after the administration of BSE for one week. A highly sensitive LC/MS method for the simultaneous determination of KBA and AKBA in the plasma and brain of wistar rats 3 h after a single administration has also been published, and involved matrix-assisted liquid–liquid extraction on Extrelut NT followed by separation by reverse-phase HPLC.107 The extracts of the gum resin of B. serrata, B. carterii, B. frereana and B. sacra along with a BA fraction derived from it have been found to be equipotent non-selective inhibitors of the major drug-metabolizing CYP 450 enzymes, as analyzed by LC–LC–ESI-MS.108
The development of chromatographic methods has assisted the pharmaco-kinetics and ADME studies of BAs, and has provided an insight into the bioavailability and distribution of different BAs in blood, plasma, brain etc., which may be useful in determining the safety and effectiveness of various formulations comprising them.
5 Boswellia species and the management of inflammation
5.1 Boswellia species and BSE
In the past few years an exciting array of drugs for treatment of a wide category of painful conditions have entered the market. Group of drugs like NSAIDs have heralded a new era of improved efficiency and low side effects. The gum resin of B. serrata has been widely used in Ayurvedic preparations as an anti-inflammatory agent since antiquity,109,110 and current research trends in the area of anti-inflammatory drug development highlight the contributions from B. serrata.111 Exhaustive work carried out on B. serrata extract (BSE) for anti-inflammatory and anti-arthritic activity in the authors’ institute has established the inhibition of carrageenan-induced rat hind paw oedema by oral and intraperitoneal administration.112 The gum resin has been claimed to have marked analgesic activity in addition to sedative effects,113 and a US patent has utilized the extract of the gum resin of B. serrata in a phytonutrient formulation for the relief of chronic pain resulting from inflammation.114 Compositions enriched in BAs compared to commercial extracts displayed enhanced biological activity toward a variety of proliferative and/or inflammatory afflictions in mammalian hosts, and demonstrated synergism with other phytochemicals. B. carterii resin and its constituents (including BAs and other components) have also been reported to display anti-inflammatory activity.115 An anti-inflammatory effect was equally well marked in adrenolactomized rats. Boswellin®, a commercial product based on the methanolic extract of the gum resin exudates of B. serrata, and comprising naturally occurring triterpenoids, displayed marked inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced increase in skin inflammation, epidermal proliferation, the number of epidermal cell layers, and tumour promotion in 7,12-dimethylbenzo[a]anthracene-initiated mice.116The anti-arthritic activity of BSE is also well recorded,117 and it is frequently added to various herbal compositions for the management of osteoarthritis.118 Early biochemical investigations of formaldehyde-induced arthritis when compared with normal animals and that of phenylbutazone-treated animals showed significant alterations in several biochemical markers.119 Through oral administration, it was established that BSE strongly inhibited the infiltration of polymorphonuclear leucocytes (PMNL) and reduced the volume of pleural fluid in carrageenan-induced pleurisy in rats.120 During pharmacological studies into carrageenan-induced oedema in rats and mice and dextran oedema in rats, BSE displayed marked anti-inflammatory activity and was equally effective in adrenolactomized rats.121 However, no significant effect was observed in the cotton pellet-induced granuloma test, but in formaldehyde and adjuvant arthritis, BSE displayed prominent anti-arthritic activity. A study reported leucocyte migration into the inflammatory fluid caused by carrageenan administration, establishing a marked inhibitory effect on both volume and leucocyte population of pleural fluids.122
Many formulations comprising B. serrata have been evaluated for their possible anti-inflammatory effect for the management of arthritis. For instance, BHUx (Sandhika®), a polyherbal formulation containing B. serrata, was studied for its artherosclerosis-preventative effect.123 Sandhika® has also been revealed to cause a significant enhancement in calcium nodule formation in a dose-dependent manner, indicating a potential anabolic effect of this polyherbal formulation on osteoblast-like cells under inflammatory conditions.124 Another herbomineral formulation, evaluated in a randomized, double-blind, placebo-controlled cross-over study, resulted in significant improvement in patients with osteoarthritis.125 Clinical evaluations on 358 patients illustrated the potential efficacy and safety of a formulation comprising B. serrata for the symptomatic treatment of osteoarthritis of the knees.126 The acetone extract of B. carterii has shown a significant anti-arthritic, anti-inflammatory effect,127 and is a potent agent against adjuvant arthritis.128In vivo effects of BSE on glycosaminoglycan metabolism were compared with that of ketoprofen in male albino rats.129 Glycosaminoglycan content was found to be decreased in the ketoprofen-treated group, whereas it remained unaltered in the BAs- or BSE-treated groups. Gupta et al.130 evaluated the anti-arthritic activity of a number of known clinically effective drugs in papaya latex induced rat paw inflammation. All the drugs were tested in three graded doses and showed dose-related anti-inflammatory activity.
In a double-blind placebo-controlled study on 30 patients with osteoarthritis of the knee, BSE resulted in significant improvement.131 Chronic toxicity studies of BSE132 have also been performed on 16 normal healthy monkeys divided into four groups, each group containing two male and two female monkeys. Biochemical, haematological, histopathological and other observations of the animals, which were kept under uniform husbandry conditions throughout the experiment, revealed no toxicity. A herbal dietary supplement providing symptomatic support in canine osteoarthritic disease was recommended in a veterinary clinical trial of BSE on dogs with manifestations of chronic joint and spinal disease.133 Pachnanda et al.134 conducted clinical trials of this drug on 175 volunteers suffering from musculoskeletal rheumatism inducing rheumatoid arthritis and ankylosing spondylitis of the moderate to severe type. Of the 175 patients, 122 were either bed-ridden or unable to carry out normal work, and showed abatement of symptoms within 2–4 weeks of initiation of treatment. Seventeen of these patients, when put on placebo treatment, showed recurrence of the symptoms within 10 days. Of the remaining 53 patients, 35 showed good results and the other 18 had no appreciable improvement within a week after starting treatment. None of these patients complained of any undesirable side-effects. However, Sander et al.135 questioned the use of BSE as drug in chronic polyarthritis. In their multi-centric but limited clinical trial on 78 patients in four groups, they found that only one patient in each group showed a good response in all parameters (which included efficacy parameters, Ritchie's Index for swelling and pain, erythrocyte sedimentation rate, C-reactive protein etc.), whereas four patients in each group worsened, and the others showed no alteration in their disease indices.
BSE has also been used in the treatment of colitis.136,137 It has proven its efficacy in reducing diarrhoea in patients with inflammatory bowel disease.138 In a clinical trial on patients suffering from Crohn's disease, the efficacy of BSE was also compared with mesalazine.139 Considering safety and efficacy parameters, BSE appeared to be superior to mesalazine in terms of a benefit–risk evaluation. The effect of BSE in patients suffering from ulcerative colitis grade II and III with patients receiving sulfasalazine as control have also been studied.140 The various parameters in the study (which included stool properties, histopathology and scan microscopy of rectal biopsies, and blood parameters including Hb, serum iron, calcium, phosphorus, proteins, total leukocytes and eosinophils) showed results that were comparable with controls, namely 82% of treated patients went into remission, whereas 75% of sulfasalazine-treated patients went into remission. In a similar study on 30 patients (17 males and 13 females) with chronic colitis, 20 patients were given a preparation of BSE (900 mg daily divided into three doses for 6 weeks) and 10 patients were given sulfasalazine (3 g daily divided into three doses for 6 weeks) as a controls.141 Of the 20 patients treated with BSE, 18 showed an improvement in one or more of the parameters (including stool properties and histopathology, in addition to haemoglobin, serum iron, calcium, phosphorus, proteins, total leukocytes and eosinophils), whereas similar results for the same parameters were obtained for 6 out of 10 patients in the control group. Moreover, out of 20 patients treated with BSE, 14 went into remission, compared to 4 out of 10 for sulfasalazine, corroborating that the gum resin preparation from B. serrata was effective for the treatment of chronic colitis with minimal side effects. BSE's claims to ameliorate the symptoms of ulcerative colitis and Crohn's disease was considered to be superior over its counterparts in terms of safety, but found to be ineffective in controlling dextran sulfate or trinitrobenzene sulfonic acid-induced colitis in mice, though individual BAs were demonstrated to increase the basal and IL-1β-stimulated NF-κB activity in intestinal epithelial cells in vitro as well as reverse proliferative effects of IL-1β.142 BSE has also been shown to be highly effective in clinical trials for the treatment of collagenous colitis.143 BSE and its constituent AKBA have also been shown to result in improvement in tissue injury in indomethacin-induced ileitis in rats.144 A recent report has also claimed that combined therapy of Boswellia and antifibrotic Scutellaria extracts have a protective effect on the development of colonic fibrosis in rats.145 In addition, the immunostimulatory effect of a bioactive fraction from B. serrata has also been demonstrated.146
5.2 Boswellic acids
Although most of the anti-inflammatory activity studies in this area have been on BSE, the bioactivities of BAs have also been reported. For example, a composition of four BAs has been used for the treatment of lymphoproliferative and inflammatory disorders including rheumatoid arthritis and osteoarthritis, and also in colon, prostate and breast cancers and a broad range of neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease, as disclosed in three patents.147 The authors’ institute has developed a process for extraction, isolation and standardization of BAs exhibiting anti-inflammatory, anti-arthritic and anti-ulcerogenic activities.148 The biological activity of BAs has been found to be enhanced by acetylation or mild oxidation, which improved the ratio of AKBA to BA, ABA and KBA.149 Singh et al.150 have studied the effects of a mixture of five BAs (with β-BA as the major component) for its effectiveness in both adjuvant-induced arthritis and established arthritis; they showed antipyretic effects with no ulcerogenic effect. In another study, orally administered BAs significantly reduced the population of leucocytes in a knee injected with bovine serum albumin (BSA), and changed the electrophoretic pattern of the synovial fluid proteins, showing that the leucocyte-inhibitory activity of BAs is not due to its cytotoxic effect.151Detailed investigations of the therapeutic effect of BAs and BSE in adjuvant-induced arthritic rats in relation to urinary excretion of connective tissue metabolites disclosed that the arthritic animals showed an increase in the excretion of these metabolites in urine, and that both BAs and BSE could offer partial protective action against the changes induced by adjuvant-induced arthritis.152 The synergistic effect of BAs and glucosamine against inflammation and arthritis in rats has also been recently reported.153 The activities of BAs against ulcerative colitis154,155 and Crohn's disease156 are also documented. A semi-synthetic form of AKBA significantly reduced the disease activity in experimental murine colitis induced by dextran sodium sulfate (DSS).157 The treatment of established colitis with semi-synthetic AKBA largely prevented the P-selectin upregulation normally associated with DSS colitis. A recent report has also demonstrated that BAs are also effective against inflammatory disorders through topical application.158 The group has also demonstrated that BAs inhibit ulcer formation, possibly by increasing the gastric mucosal resistance and local synthesis of cytoprotective prostaglandins.159
5.3 Mechanisms of anti-inflammatory action of BSE and BAs
A number of studies have been reported in the literature aiming to understand the mechanism of action of both BSE and BAs. As BAs are the main constituents of BSE, the two forms of their preparation should be construed as interchangeable in the context of their mechanisms of action.BAs are reported to be non-redox, non-competitive inhibitors of 5-lipoxygenase (5-LO).160–162 5-LO is mainly expressed in hematopoietic cells163 and is a key enzyme in the biosynthesis of leukotrienes from arachidonic acid (AA).164 Leukotrienes are naturally produced eicosanoid lipid mediators, which may be responsible for the effects related to asthma, allergies and inflammation, and BAs are accepted inhibitors of leukotriene biosynthesis.165,166 Schweizer et al.39 observed that 3-O-acetyl-9,11-dehydro-β-boswellic acid almost totally inhibited 5-LO activity in PMNLs. Their data indicated that the conditions chosen for the workup of BSE could significantly influence the potency of biological actions and their potential therapeutic effectiveness. Of the individual BAs characterized, AKBA proved to be the most potent inhibitor of 5-LO. BAs have also shown to exhibit a synergistic inhibitory effect on both COX-2 and LO when mixed with Carthamus tinctorious extracts.167
The molecular basis of the anti-inflammatory effects of BSE in a system of TNFα-induced gene expression in human microvascular endothelial cells (HMEC) has been tested.168 Out of 522 genes induced by TNFα in HMEC, 113 were clearly sensitive to BSE treatment. The family of these genes are directly related to inflammation, cell adhesion, and proteolysis. In order to evaluate the significance of AKBA in the anti-inflammatory properties of BSE, effects of BSE containing 3% AKBA and 30% AKBA (5-loxin) were compared.169 Pre-treatment of HMECs for 2 days with BSE potently prevented TNFα-induced expression and activity of MMP-3 (matrix metalloproteinase), MMP-10, and MMP-12. In vivo, BSE protected against experimental arthritis and in all experiments, both in vitro and in vivo, BSE with 30% AKBA was more effective than that with 3% AKBA, thus supporting earlier findings that BSE has potent anti-inflammatory properties both in vitro as well as in vivo.168
BAs, though inhibiting leukotriene synthesis via 5-LO, did not affect 12-LO and COX activities. Thus they are specific, non-redox inhibitors of leukotriene synthesis, which either interact directly with 5-LO or block its translocation.170 Additionally, BAs did not impair the peroxidation of AA by iron and ascorbate. They also decreased the formation of leukotrienes, with AKBA exerting the most pronounced inhibition of LO.171 In contrast to redox-type 5-LO inhibitor nordihydroguaiaretic acid, BA in concentrations up to 400 µM did not impair the COX and 12-LO in isolated human platelets or the peroxidation of AA by Fe-ascorbate. However, a recent study has shown that BAs, particularly AKBA, inhibit COX-1 product formation in intact human platelets as well as the activity of isolated COX-1 enzyme in cell-free assays; in contrast, COX-2 was less efficiently inhibited by BAs.172
The effects of natural pentacyclic triterpenes such as BA, α-amyrin, 11-keto-α-amyrin and AKBA on 5-LO activity were compared, and it was concluded that the pentacyclic triterpene ring system is crucial for binding to the highly selective effectors site, whereas functional groups (especially the 11-keto function in addition to a hydrophilic group on C-4 of the ring A) are essential for 5-LO inhibitory activity.173 AKBA is the only leukotriene synthesis inhibitor so far identified that inhibits 5-LO activity as an allosteric regulator and not by a reducing or competitive mechanism.174 AKBA's effectors site was characterized by a photoaffinity analogue, which inhibited 5-LO activity as efficiently as the lead compound and specifically labelled human 5-LO protein, showing that AKBA binds in the presence of calcium to a site which is distinct from the substrate binding site of 5-LO, and that the binding site is likely to be identical to a regulatory, second arachidonate binding site of the enzyme.
BAs with 5-LO inhibitory activity block human leukocyte elastase (HLE) activity.175 HLE is a serine protease and may play a role in several diseases, such as pulmonary emphysema, cystic fibrosis, chronic bronchitis, acute respiratory distress syndrome, glomerulonephritis and rheumatic arthritis.176 HLE inhibition is established for many lipophilic compounds, but a dual HLE and 5-LO inhibitory property is unique to pentacyclic triterpenes from the BA series. Leukotriene levels and HLE release are increased in parallel in many inflammatory diseases and hypersensitivity-based reactions,177 and BA derivatives such as AKBA might provide a tool to help in coping with such patho-physiological processes. Although BAs are inhibitors of 5-LO, studies have also shown that 3-oxo-tirucallic acid (a tetracyclic triterpenoid constituent of BSE) enhanced 5-LO formation in ionophore-challenged polymorphonuclear cells (PMNLs) in intact cells,178 whereas in free cells they failed to do so. More information is required concerning the bioactivity of 3-oxo-tirucallic acid and related molecules in the extracts, in order to help understand their collective role with regard to BAs.
Although leukotrienes generated via 5-LO pathways are believed to act as important lipid mediators of inflammatory diseases, a number of potent leukotriene antagonists and inhibitors failed in clinical studies, casting doubts on the significance of these mediators. A general idea in chronic inflammation is the stimulation of pro-inflammatory transcription factors AP-1 and NF-κB.179 The activation of NF-κB is a multistep process that involves activation of the IKK complex. Studies have demonstrated that both ABA and AKBA are novel selective inhibitors of IKK, and that they represent a potential therapeutic target for the suppression of NF-κB-dependent cytokine expression.180 AKBA reportedly exerts its anti-inflammatory effect by inhibiting NF-κB.181 BSE also acts against inflammatory bowel diseases through an NF-κB pathway.182 Other than BAs, IA has also been shown to exert anti-inflammatory effects by inhibiting NF-κB.59 BSE as well as pure isolated compounds (BAs) have shown down-regulation of Th1 cytokines IFN-γ and IL-12, while the Th2 cytokines IL-4 and IL-10 were up-regulated upon treatment.183
Inhibitory effects of BAs on NO production – the expression of pro-inflammatory cytokines and mediators via inhibition of phosphorylation of the mitogen-activated protein kinases (MAPKs), JNK, and p38 – have been observed, while no inhibition was seen in ERK phosphorylation in LPS stimulated human PBMCs. The activation of cellular 5-LO may occur by at least two different pathways (which may act in conjunction): either by elevation of intracellular Ca2+or by Ca2+-independent pathways involving ERK- and/or p38 MAPK-mediated 5-LO phosphorylation.184,185 Since MAPKs respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation and cell survival/apoptosis,186 BSE and its constituent BAs activate the MAPK, p42MAPK and p38 in isolated PMNL and mobilize intracellular Ca2+. Removal of Ca2+ by chelation partially inhibited the activation of MAPK by AKBA.187 The efficacy of nonredox-type 5-LO inhibitors in isolated human PMNL depends on the 5-LO activation pathway, and AKBA operates through cell stress-induced 5-LO product formation involving 5-LO kinase pathways. Thus, compared with Ca2+ mediated 5-LO product formation, the enzyme activation involving 5-LO phosphorylation events specifically and strongly alters the susceptibility of 5-LO toward nonredox-type inhibitors in intact cells.188
BAs are also considered to act via G(i/0) protein(s) stimulating signalling pathways that control functional leukocyte responses as they activate pertussis toxin, which inactivates G(i/0) protein subunits, preventing MAPK activation and Ca2+ mobilization induced by KBAs.189 Moreover, KBA-induced ROS formation is Ca2+-dependent and is mediated by NADPH oxidase involving PI3-K and p42/44(MAPK) signalling pathways. Also, the release of AA depends on Ca2+ and p42/44(MAPK), whereas the pathways stimulating 5-LO are not readily apparent. Poeckel et al. dealt with effects of BAs on central signalling pathways in human platelets and on various platelet functions. They found that the 11-methylene analogue of KBA caused a pronounced mobilization of Ca2+ from internal stores and induced the phosphorylation of p38 MAPK, extracellular signal-regulated kinase (ERK2), and Akt.190 In contrast to PMNLs, AKBA concentration-dependently decreased the basal Ca2+, not only in resting Mono Mac (MM6) cells but also in cells where Ca2+ had been elevated by stimulation with platelet-activating factor (PAF), suggesting that AKBA interferes with pivotal signalling events in monocytic cells that are usually required for monocyte activation by pro-inflammatory stimuli and may represent a possible mechanism underlying the reported anti-inflammatory properties of AKBA.191 BAs can also enhance the liberation of AA in the human leukocytes and platelets.192 Both BA and AKBA markedly enhanced the release of AA via cytosolic phospholipase A2 (cPLA2), whereas for generation of 12-hydroperoxyeicosatetraenoic acid (12-HPETE), BA was more potent than AKBA. In addition, BA-induced release of AA and formation of 12-HPETE was more rapid and occurred in the absence of Ca2+.
6 Boswellia extracts and compounds as anticancer agents
6.1 Anticancer activity of Boswellia extracts
Cancer is clearly a disease directly associated with the genes, and risk of cancer rises in the final decades of life.193 Since the dawn of molecular therapeutics, there has been an associated revolution in the development of anti-cancer drugs.194BSE is reported to moderate the breast cancer and the brain tumour metastases.195 It is a known inducer of apoptosis196 and the ethanolic extract tested for cytotoxic, cytostatic and apoptotic activity against leukaemia and brain tumour cells has shown to induce apoptosis and to act as a potent anti-proliferative agent.197 BSE containing 60% BAs have reportedly inhibited tumours and inflammation in mice.198 BSE has also been reported for anti-carcinogenicity in mice with ehrlic ascites carcinoma and S-180 tumour by inhibiting the cell proliferation and growth inhibition due to the interference with biosynthesis of DNA, RNA and proteins.199 It reduced the tumour cell proliferation and induced apoptosis in several in vitro experiments with animals.200 The efficacy of BSE against peri-tumoural oedema can be increased by enhancing the bioavailability of AKBA.201 A composition of B. carterii has been shown to induce the cell differentiation in HL-60 cells at significantly low concentrations.202,203B. carterii extract has also been reported for pro-apoptotic effects in HL-60 cells.204
In separate clinical studies, a reduction in perifocal oedema was observed when a herbal medication of BSE was administered to seven patients for treatment of glioblastoma and five patients for leukencephalopathy. Although no tumour response was seen in the patients, leukencephalopathy patients showed a clinical benefit.205 A clinical study with brain tumour patients has also been conducted by Heldt et al.206 and Boker et al.,207 in which BSE was administered to 29 patients having gliomas in three groups with different doses (3 × 1200 mg/d, 3 × 800 mg/d, 3 × 400 mg/d) prior to surgical intervention. After seven days of treatment, the reduction in size of perifocal oedema was found to be the largest in case of a group having highest intake of extract, to a lesser extent in the group receiving 3 × 800 mg/d, with no effect being seen in the group receiving the smallest dose. Though both these studies suggest a possible beneficial effect of BSE on brain oedema, their applicability in treatment due to the participant selection and study design has been questioned. Moreover, the radiologic method (CT scan) to evaluate the oedema size was claimed as unreliable.208 BSE has also been used as a coating material in the drug delivery of 5-fluorouracil for the treatment of colorectal cancer.209
6.2 Anticancer activity of BAs
BAs have attracted considerable attention as anticancer agents in the recent past,210 especially from the time when 5-LO inhibitors were also recognized as cancer chemo-preventive agents.211 The study on natural BAs indicated potent inhibitory activity against human neuroblastoma cells212 and human leukaemia cells.213 One of the constituents, AKBA, inhibited the growth in meningioma cells, phosphorylation of Erk-1/2 and impaired the motility of meningioma cells stimulated with platelet-derived growth factor BB at 2–8 µM concentrations.214,215 AKBA was also found to inhibit basic fibroblast growth factor (bFGF) induced angiogenesis using an in vivo Matrigel Plug assay. Recent studies also demonstrated BAs can act as anti-angiogenic agents.216ABA has been demonstrated as a specific myeloid leukaemia differentiation inducer, attributing the effects to inhibition of 5-LO without giving any insight into the mechanism.217 ABA induced apoptosis in human myeloid leukaemia cells (HMLCs) through activation of caspase-8 by induced expression of DR4 and DR5.218 Caspase-8 either directly activates caspase-3 by cleavage or indirectly by cleaving Bid, which subsequently decreased mitochondria membrane potential, where as the levels of Bcl-2, Bax and Bcl-XL were not modulated at all. A general caspase inhibitor and a specific caspase-8 inhibitor blocked ABA-induced apoptosis, whereas a caspase-9 inhibitor had minimal effect on apoptosis, thereby suggesting that a mitochondria-mediated pathway does not play an important role in ABA-mediated apoptosis. Both KBA and AKBA have also shown apoptotic and anti-proliferative effects on colon cancer (HT-29) cells.219 Here also, caspase-3 and caspase-8 inhibitors completely blocked the apoptotic pathway, whereas caspase-9 inhibitors partially blocked the same, implying that the caspase-8 pathway was involved in apoptosis in HT-29 cells, leading to the activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase (PARP). The anti-cancer activities of BAs were in the order of AKBA as the most active, then KBA, ABA and BA, the least active.
BA has also been used in vivo against malignant glioma.220 The treatment of rats with the drug in four groups with different dosages after 14 days of inoculation of C6 tumour cells, revealed that the survival time was twice as long in high-dosage groups than in the control group. In another experiment, the animals were sacrificed after 14 days’ treatment following implantation of C6 tumour cells, and their brains examined microscopically. The proportion of apoptotic tumour was high in the high-dose group compared to the low dose group. BAs have also been explored for the treatment of tumours221 and as anti-oedema agents in patients with brain tumours.222 Glaser et al.223 reported the cytotoxicity of BAs against human malignant glioma. Though steroids are important for the control of oedema in malignant glioma patients, BAs are an alternative drug: the levels of anti-apoptotic proteins such as Bax and Bcl-2 remain unaltered, thus demonstrating that Boswellia and its constituents might represent a new therapeutic option for glioma growth in human beings. In contrast to steroids, BAs synergize the cytotoxic cytokine CD95 ligand, in inducing glioma cell apoptosis. The effect is probably mediated by the inhibition of RNA synthesis and is not associated with the changes of CD95 expression at the cell surface. BAs can even be considered as alternative drugs to corticosteroids, as they have been shown to reduce cerebral peri-tumoural oedema by modulating P-glycoprotein (Pgp) function.224 Pgp has gained importance as the transporter, mainly for drug disposition and the resulting clinical response; BAs as well as BSE inhibited the transport activity of Pgp in the micro-molecular range.
Palliative treatment of 19 children and adolescents with intracranial tumours with H15® (a herbal preparation of Boswellia) showed an improvement of their general health status, transient improvement of neurological symptoms such as pareses and ataxia, and increased muscular strength and weight gain.225 During investigations of apoptotic and anti-proliferative activity of two major constituents, KBA and AKBA, on liver cancer, Hep G2 cells caspase-3 and caspase-8 inhibitors were found to completely block the activity, whereas caspase-9 only partially blocked it.226 A recent study also showed that AKBA induces apoptosis through caspase-3 and caspase-8 in human prostate cancer cells.227 In flow cytometeric analysis, the cells were arrested in the G1 phase, and the percentage of S phase cells decreased, thereby proving that BAs inhibited the proliferation of Hep G2 cells. The mechanism of BA-induced apoptosis and inhibition of the cell viability was different, as caspase inhibitors did not show any effect on the cell viability. The apoptotic and anti-proliferative effects of AKBA were stronger than KBA. AKBA also inhibited growth in colon cancer cells by a p-21-mediated pathway, and may have further implications in the use as anti-cancer drug.228
ABA gave excellent DNA fragmentation in melanoma and fibrosarcoma, whereas in the normal cells it didn't accelerate apoptosis.229 ABA is a cytostatic rather than a cytotoxic agent, as it induces differentiation, apoptosis and cytostasis in various cell lines, and can be used in chemo-preventive intervention strategies, either to interrupt the occurrence of a primary tumour or to decrease the likelihood of metastasis. BAs have been shown to induce cell differentiation and inhibit topoisomerase I and II,230 and AKBA has been described as a catalytic inhibitor of topoisomerase I and IIα.231 AKBA inhibited this enzyme in a cell-free DNA relaxation assay, at concentrations of ≥10 µM, whereas amyrin (having a very similar chemical structure) did not show any effect up to 50 µM. The inhibition of topoisomerase I by AKBA may explain the observed apoptotic cell death. These observations were further reinforced by the expression of the mRNA of topoisomerase I, topoisomerase IIα and topoisomerase IIβ in HL-60 and CCRF-CEM cells, though it was slightly lower in CCRF-CEM as compared with HL-60 cells, and the difference was most prominent for topoisomerase I.232 ABA inhibited topoisomerase by directly binding to enzyme and not by binding to DNA or by complex formation with enzyme and DNA. The same mechanism operates for both topoisomerases I and IIα, i.e. by competing with DNA for topoisomerase binding, as they did not effect the activity of bovine DNase I. Other pentacyclic triterpenes such as oleanolic acid, ursolic acid and betulinic acid are also known to possess antitumour activity; they probably operate through the same mechanism as KBAs.
As mentioned earlier, both ABA and AKBA inhibit NF-κB signalling,179 which has a probable role in the mechanism of action against cancer cells.233 In prostate cancer cells such as PC-3 and DU-145, BAs triggered apoptosis in vitro; these cell lines are otherwise resistant to induction of apoptosis even by highly potent agents such as staurosporine and camptothecin.234 The induction of apoptosis was through mitochondrial cytochrome c release and DNA fragmentation. The constitutive activation of NF-κB has been shown to be responsible for both the chemo-resistance and a highly malignant phenotype of prostate cancer.235 These compounds reportedly inhibited constitutively activated NF-κB signalling at a molecular level by intercepting the IκB kinase (IKK) activity; they have specificity for IKK inhibition.
The topical application of water-soluble AKBA–γ-cyclodextrin complex on PC-3 tumours xenografted onto chick chorioallantoic membranes induced concentration-dependent inhibition of proliferation as well as apoptosis. Similarly, in nude mice carrying PC-3 tumours, the systemic application of AKBA–γ-cyclodextrin inhibited the tumour growth and triggered apoptosis with no detectable systemic toxicity. 3α-Acetyl-11-keto-α-boswellic acid, synthesized by a radical-type reaction using bromine, had also been stated to induce apoptosis in PC-3 prostate cancer cells xenotransplanted onto the chick chorioallantoic membrane.236 AKBA potentiated the apoptosis induced by TNF and chemotherapeutic agents, and inhibited the receptor activator of NF-κB ligand-induced osteoclastogenesis, all of which are known to require NF-κB activation.237 AKBA also inhibited NF-κB-regulated gene transcription and NF-κB-regulated gene products involved in cell proliferation (e.g., cyclin D1 and COX-2), anti-apoptosis (e.g., survivin, IAP1, XIAP, Bcl-2, Bcl-xL, Bfl-1/A1, and FLIP), and the invasion (MMP-9 and vascular endothelial growth factor). Moreover, AKBA did not directly affect the binding of NF-κB to the DNA, but inhibited sequentially the TNF-induced activation of IκBα kinase (IKK), IκBα phosphorylation, IκBα ubiquitination, IκBα degradation, p65 phosphorylation and p65 nuclear translocation. Furthermore, AKBA inhibited the NF-κB-dependent reporter gene expression activated by TNFR type 1, TNFR-associated death domain protein, TNFR-associated factor 2, NF-κB-inducing kinase, and IKK, but not that activated by the p65 subunit of NF-κB. These results indicate the ability of AKBA to enhance apoptosis, and suppress the invasion and inhibition of osteoclastogenesis, and thus provide novel targets for the cancer therapy. AKBA also caused a decrease of androgen receptor expression, crucial for the development and progression of the prostate cancer, at mRNA and protein levels.238
In addition to natural isolates, semi-synthetic acyl analogues of BAs have displayed significant cytotoxicity against various human cancer cell lines in vitro, and markedly induced apoptosis in HL-60 cells.90 Most of the acyl analogues displayed improved cytotoxicity compared to their natural counterparts. A natural diol derivative of BA (also synthesized semi-synthetically) (77) has also shown anti-cancer activity in in vivo models and also induced apoptosis.89 In an attempt to establish the mechanism of apoptosis by diol of BA,239 it was observed that the effect was mediated through an extrinsic pathway via activation of TNF family of proteins (TNF-R1, DR4), with the generation of NO and ROS leading to caspase-8 activation. Likewise, semi-synthetic amino alcohol analogues have displayed improved cytotoxicity in vitro compared to the parent BAs against various human cancer cell lines.81 The active molecules also induced apoptosis in HL-60 cells.
7 Miscellaneous bioactivities of BSE and BAs
Besides exhibiting anti-inflammatory and anticancer activities, the constituents of B. serrata in different forms have been used for various disorders. B. serrata extract (BSE) is used as an ingredient of certain ointments for rheumatism and nervous disorders,240 and is reported to exhibit antibacterial241 analgesic and sedative effects.242 It has also shown its potential as a hepatoprotective243 and reno-protective agent.244 Also described is the effect of BSE as an anti-insect agent;245 the oil of B. serrata is reported to cause malformation of both larval and pupal stages of Culex pipiens.246 Frankincense oil demonstrated immunostimulant activity when assessed by a lymphocyte proliferation assay.247 Moreover, the essential oil of the gum resin has also shown a stimulatory effect on skeletal muscle and spasmogenic effects on smooth muscle of guinea pig.248BSE displayed immunomodulatory property with promising anti-anaphylactic and mast-cell-stabilizing activity.249 Recently, immune responses of the water-soluble biopolymeric fraction from B. serrata were studied,250 and it was found to be a potent enhancer of antigen-specific Th1 and Th2 immune responses in comparison to alum with Th2 limitation, thus indicating the potential of the biopolymeric fraction of B. serrata as an adjuvant for vaccine applications. Similarly, the lymphocyte transformation of BAs and other purified compounds have shown more activity than the parent extract of B. carterii.251 A purified mixture of BAs from B. carterii has displayed in vitro carrier-dependent immunomodulatory activities,252 and the methanolic extract of B. carterii has been shown to have significant activity against the hepatitis C virus.253 The molluscicidal activity of the methanolic extract of B. dalzielii has been reported.254,255 The essential oils of B. serrata, B. rivae, B. carterii and B. papyrifera have also been reported to exhibit antimicrobial activity at fairly low concentrations.256 The methanolic extracts of B. ameero, B. elongata reportedly exhibited antibacterial257 and anti-viral activity.258 The aqueous extract of B. glabra has demonstrated anti-diabetic activity in rats,259 and the methanolic extract of B. elongata has shown inhibitory potency against neutral endopeptidase and aminopeptidase N.260 The medicinal properties of B. ovalifoliolata are well known,261 and a protocol for in vitro micro-propagation of B. ovalifoliolata has been developed.262 The neuroprotective action of IA has also been recently reported.263 In a more recent report it has been claimed that diterpene alcohol incensole acetate (23) elicits psychoactivity by activating transient receptor potential vanilloid-3 (TRPV-3, an ion channel implicated in the perception of warmth in the skin) causing anxiolytic-like and anti-depressive-like behavioural effects in wild-type mice.264
BSE's clastogenic effects are also documented.265 The activity of BSE against asthma has been illustrated by conducting a double-blind placebo-controlled six-week clinical study on forty patients.266 The data signified a definite role of BSE in the treatment of bronchial asthma, as 70% of patients showed improvement, including disappearance of physical symptoms and signs such as dyspnoea, bronchi, number of attacks, increase in FEV subset1, FVC and PEFR as well as decrease in eosinophilic count and ESR. BAs have also been used as a monotherapy in an allogeneic mouse heart transplantation model to evaluate the effect on the severity and the time course of rejection.267 Although ultimate rejection could not be inhibited, there was a significant delay in the development of the morphological signs of rejection. These findings are comparable to the results previously reported after monotherapy with high doses of steroids or toxic doses of other anti-inflammatory drugs such as acetylsalicylic acid. The clear advantage of B. serrata-derived agents is the absence of known side-effects, even when used in high doses as in the described model. Further studies are needed to evaluate its effect as part of a combination therapy to potentially allow a reduction in steroid dose in transplant patients.
BAs and their derivatives have been found to inhibit normal and increased leucocytic elastase or plasmin activity, and can be subsequently used in the treatment of diseases such as pulmonary emphysema, acute respiratory distress syndrome, shock lung, cystic fibrosis (mucoviscidosis), glomerulonephritis and rheumatoid arthritis caused by the increased activity of leucocytic elastase or plasmin.268 An earlier study also reported that BSE exhibits anti-diabetic activity in rats against Streptozocin-induced diabetes.269 BSE also reduces cholesterol level, as demonstrated in a study conducted on wistar rats.270 AKBA has been used to soften or to relax skin by its topical application.271 A composition comprising BAs has also been used in the treatment of hair and skin.272
8 Safety aspects
As well as phytochemical analysis, animal experiments on the anti-inflammatory/anti-arthritic and anti-ulcer effects, and acute and chronic toxicity studies have been carried out,81 providing proof of the efficacy of BSE in Phase II studies for anti-inflammatory/anti-arthritic effects. In rats and mice, BA was revealed to be safe up to 3000 mg/kg. The chronic toxicity studies (six months) was carried out in rodents (rats) and primates (monkeys) in doses of 250, 500 and 1000 mg. In vitro toxicity investigations were performed on polymorph leucocytes, and showed no toxicity up to 1 mg/ml.273 Recent studies have also confirmed that B. serrata and AKBA exert quite low toxicity on human skin cells.274Various NSAID drugs currently being used for the treatment of arthritis can raise the risk of heart attacks. Currently, COX-2 inhibitors, a new generation of NSAIDs, are used for the treatment of arthritis due to lower level of damage they cause to the stomach lining compared to traditional NSAIDs containing Ibuprofen. However, a COX-2 inhibitor drug, Celebrex, was found to double the risk of heart attacks.275 Yet another arthritis drug, Vioxx, has been withdrawn from the market due to the high heart attack risk associated with it.276 In light of these findings, NSAID drugs derived from Nature, such as BSE, might prove to be more beneficial. As already mentioned, BAs are 5-LO inhibitors having a pathway entirely different from COX-2 inhibitors. Moreover, BSE preparations sold in the market as OTC anti-inflammatory formulations are considered to be quite safe. Ayurvedic practitioners also claim B. serrata to be a safe and effective dietary supplement against joint disorders. Other than B. serrata, the formulations may contain certain other ingredients such as cellulose, silicon dioxide, stearic acid, croscarmellose sodium, magnesium stearate and vegetable glaze. BSE is also sold by Source Naturals as an anti-inflammatory drug under the trade-name Boswellia Extract®, and contains 70% BAs together with dibasic calcium phosphate, stearic acid, modified cellulose gum, magnesium stearate and colloidal silicon dioxide. Shallaki®, sold by Morpheme, is claimed to be useful as an adjuvant for arthritis, and as an anti-inflammatory agent with cholesterol- and triglyceride-lowering properties, helping weight reduction. Another drug, Boswellin®, is marketed by Nature's Herbs as a dietary supplement for inflammatory diseases. Nature's Herbs also market Boswellin® cream for pains and aches due to rheumatism and arthritis. In addition, there are several other formulations comprising BSE available on the market all over the globe.
9 Conclusions
Boswellia sp. gum extracts and their triterpenic constituents, especially boswellic acids, have drawn the attention of medicinal chemists, biochemists and pharmacologists since the first report of their anti-inflammatory activity. Their effectiveness in the treatment of rheumatoid arthritis and chronic inflammation without many side effects (such as the ulceration and heart failures generally associated with NSAIDs), combined with almost negligible toxicity and distinctive mode of action via inhibition of the 5-lipoxygenase (5-LO) pathway, should make it a preferred therapeutic agent in its category. The cytotoxicity and anticancer studies carried out in the last few years have provided an additional dimension to its bioactivity. While several other structurally related semi-synthetic molecules, such as those derived from oleanolic acid and betulinic acid, are at various stages of clinical assessment, the potential of BAs has not been fully exploited despite its proven biological effects. Therefore, more effort in building diverse libraries based on its chemical scaffold and the generation of biological data, including target-based studies, are necessary.10 Acknowledgements
The authors thankfully acknowledge CSIR, New Delhi, for support and Mr Ajay Kumar, IIIM Jammu, for useful input regarding the mechanism of action.11 References
- P. G. Buvari, Med. Diss., Universitat Heidelberg, 2001 Search PubMed
.
-
K. R. Kirtikar and B. D. Basu, in Indian Medicinal Plants, I, 2nd edn, M/s Periodical Experts, Delhi, India, 1935, p. 521 Search PubMed
.
- G. K. Chatterjee and S. D. Pal, Indian Drugs, 1984, 21, 431 Search PubMed
.
- G. Yuan, M. L. Wahlqvist, G. He, M. Yang and D. Li, Asia Pacific J. Clin. Nutr., 2006, 15, 143 CAS
.
- D. Ennet, F. Poetsch and D. Schopka, Deutsche Apotheker Zeitung, 2000, 140, 1887 Search PubMed
.
-
The wealth of India: Raw materials, Council of Scientific & Industrial Research, New Delhi, 1948, p. 196 Search PubMed
.
- S. K. Chuo, S. H. Wong and P. C. Leong, Singapore J. Pharmacy Ind., 1982, 10, 19 Search PubMed
.
- P. D. Moore, Nature, 2006, 444, 829 CrossRef CAS
.
- S. Mukherjee, A. K. Banerjee and B. N. Mitra, Ind. J. Pharma., 1970, 32, 48 Search PubMed
.
- R. M. Hillson, Roy. Soc. Med., 1988, 81, 542 Search PubMed
.
- T. J. Abercrombie, National Geographic, 1985, 168, 474 Search PubMed
.
-
J. Thorwald, in Science and secrets of early medicine, Thames & Hudson, London, 1962, p. 69 Search PubMed
.
-
H. G. Greenish, in A textbook of materia medica, Churchill, London, 2nd edn, 1909, p. 552 Search PubMed
.
-
J. Quincy, in Pharmacopoeia officinalis and extemporanea or a compleat English dispensatory, A. Bell, W. Taylor and J. Osborn, London, 2nd edn, 1719, p. 97 Search PubMed
.
-
F. H. Garrison, in An introduction to the history of medicine, W. B. Saunders, Philadelphia, 4th edn, 1929, p. 136 Search PubMed
.
-
N. Culpeper, in Pharmacopoeia Londinensis or the London dispensatory, ed. P. Cole, London, 6th edn, 1656, p. 44 Search PubMed
.
- A. O. Tucker, Econ. Bot., 1986, 40, 425 CAS
.
-
J. J. W. Coppen, in Non-wood Forest Products 1: Flavours and Fragrances of Plant Origin, FAO, Rome, 1995, p. 81 Search PubMed
.
- J. D. Conolly and R. A. Hill, Nat. Prod. Rep., 2002, 19, 494 RSC
.
- J. B. Press, R. C. Reynolds, R. D. May and D. stJ. Marciani, Stud. Nat. Prod. Chem., 2000, 24, 131 Search PubMed
.
- G. Bringmann, W. Saeb, L. A. Assi, G. Francois, A. S. S. Narayanan, K. Peters and E. M. Peters, Planta Med., 1997, 63, 255 CrossRef CAS
.
- J. L. Rios, M. C. Recio, S. Manez and R. M. Giner, Stud. Nat. Prod. Chem., 2000, 24, 93 Search PubMed
.
- T. Konoshima and M. Takasaki, Stud. Nat. Prod. Chem., 2000, 24, 607 Search PubMed
.
- F. Li, R. Goila-Gaur, K. Salzwedel, N. R. Kilgore, M. Reddick, C. Matallana, A. Castillo, D. Zoumplis, D. E. Martin, J. M. Orenstein, G. P. Allaway, E. O. Freed and C. T. Wild, Proc. Natl. Acad. Sci. USA, 2003, 100, 13555 CrossRef CAS
.
- D. Poeckel and O. Werz, Curr. Med. Chem., 2006, 13, 3359 CrossRef CAS
.
- A. Sharma, A. S. Mann, V. Gajbhiye and M. D. Kharya, Phcog. Rev., 2007, 1, 137 Search PubMed
.
- C. Kreck and R. Saller, Versicherungsmedizin, 1999, 51, 122 Search PubMed
.
- H. P. Ammon, Wien. Med. Wochenschr., 2002, 152, 373 CrossRef CAS
.
- G. G. Bhargava, J. J. Negi and H. R. D. Ghua, Indian Forestry, 1978, 104, 174 Search PubMed
.
- A. Kumar and V. K. Saxena, Indian Drugs, 1979, 16, 80 Search PubMed
.
- V. N. Gupta, D. S. Yadav, M. P. Jain and C. K. Atal, Indian Drugs, 1987, 24, 1 Search PubMed
.
- R. Nicoletti and M. L. Forcellese, Tetrahedron, 1968, 24, 6519 CrossRef CAS
.
-
(a) R. S. Pearson and P. Singh, Indian Forest Records, 1918, 6, 321 Search PubMed
; (b) J. L. Simonson and L. N. Owen, in The Terpenes, University Press, Cambridge, vol. 2, 1949, p. 10 Search PubMed
; (c) E. S. Guenther, Am. Perfumer, 1943, 45, 41 Search PubMed
; (d) P. P. Bhargawa, Perf. Ess. Oil Rec., 1963, 54, 740 Search PubMed
; (e) J. B. Girgune and B. D. Garg, J. Sci. Res. (Bhopal), 1979, 1, 119 Search PubMed
; (f) T. J. Dennis, Bull. Med. Ethno. Bot. Res., 1980, 1, 353 Search PubMed
.
- A. Tschirch and O. Halbey, Arch. Pharma., 1898, 236, 487 Search PubMed
.
-
(a) C. Ferdinando, Ann. Chim. App., 1937, 27, 178 Search PubMed
; (b) B. Bischof, O. Jerger and L. Ruzicka, Helv. Chim. Acta, 1949, 32, 1911 CrossRef CAS
; (c) L. Ruzicka, O. Jerger and W. Ingold, Helv. Chim. Acta, 1944, 27, 1859 CrossRef CAS
; (d) L. Ruzicka and W. Wirz, Helv. Chim. Acta, 1940, 23, 132 CrossRef CAS
; (e) L. Ruzicka and W. Wirz, Helv. Chim. Acta, 1941, 24, 248 CrossRef CAS
; (f) P. Bilham, G. A. R. Kon and W. C. J. Ross, J. Chem. Soc., 1942, 35 RSC
.
-
(a) G. G. Allan, Chem. Ind., 1965, 1497 Search PubMed
; (b) G. G. Allan, Phytochemistry, 1968, 7, 963 CrossRef CAS
.
-
D. S. Yadav, Ph.D. Thesis, Jiwaji University, Gwalior 1989
.
- J. L. Beton, T. G. Halsall and E. R. H. Jones, J. Chem. Soc., 1956, 2904 RSC
.
- S. Schweizer, A. F. W. Von Brocke, S. E. Boden, E. Bayer, H. P. T. Ammon and H. Safayhi, J. Nat. Prod., 2000, 63, 1058 CrossRef CAS
.
- H. Elkhadem, Z. M. El-Shafei, M. A. S. Elsekely and M. M. A. A. Rahman, Planta Medica, 1972, 22, 157 CrossRef CAS
.
- K. Belsner, B. Buchele, U. Werz, T. Syrovets and T. Simmet, Magn. Reson. Chem., 2003, 41, 115 CrossRef CAS
.
- V. K. Rajnikant, V. D. Gupta, S. R. Rangari, R. B. Bapat and R. Agarwal Gupta, Cryst. Res. Technol., 2001, 36, 93 CrossRef CAS
.
-
(a) R. S. Pardhy and S. C. Bhattacharya, Ind. J. Chem., Sect. B, 1978, 16, 174
; (b) R. S. Pardhy and S. C. Bhattacharya, Ind. J. Chem., Sect. B, 1978, 16, 176
.
- B. Mahajan, V. K. Sethi, S. C. Taneja and K. L. Dhar, Phytochemistry, 1995, 39, 453 CrossRef CAS
.
- R. S. Pardhy and S. C. Bhattacharya, Ind. J. Chem., Sect. B, 1978, 16, 171
.
-
B. Mahajan, in Ph.D. Thesis, Jammu University, Jammu 1993
.
- G. Culioli, C. Mathe, P. Archier and C. Vieillescazes, Phytochemistry, 2003, 62, 537 CrossRef CAS
.
- K. Belsner, B. Buchele, U. Werz and T. Simmet, Magn. Reson. Chem., 2003, 41, 629 CrossRef CAS
.
-
(a) R. M. Beri and M. G. Karnick, Curr. Sci., 1963, 32, 324 CAS
; (b) R. M. Beri and M. G. Karnick, Ind. Forest Leaflet, 1964, 175, 7 Search PubMed
.
- S. Corsano and R. Nicoletti, Tetrahedron, 1967, 23, 1977 CrossRef CAS
.
- M. A. J. Malandkar, Ind. Inst. Sci., 1925, 8, 240 Search PubMed
.
- A. K. Sen, A. K. Das, N. Banerji and M. R. Vignon, Carbohyd. Res., 1995, 25, 321
.
- S. M. Wahab, E. A. Aboutabl, S. M. El-Zalabani, H. A. Fouad, H. L. De Pooter and B. El-Fallaha, Planta Med., 1987, 53, 382 CrossRef CAS
.
- A. Dekebo, M. Zewdu and E. Dagne, Bull. Chem. Soc. Ethiop., 1999, 13, 93 CAS
.
- S. Hayashi, H. Amemori, H. Kameoka, M. Hanafusa and K. Furukawa, J. Essent. Oil Res., 1998, 10, 25 CAS
.
- G. J. Provan, A. I. Gray and P. G. Waterman, Flavour Fragr. J., 1987, 2, 115 CrossRef CAS
.
- K. H. C. Baser, B. Demirci, A. Dekebo and E. Dagne, Flavour Fragr. J., 2003, 18, 153 CrossRef CAS
.
- J. Y. Zhou and R. Cui, Yao Xue Xue Bao, 2002, 37, 633 CAS
.
- A. Moussaieff, E. Shohami, Y. Kashman, E. Fride, M. L. Schmitz, F. Renner, B. L. Fiebich, E. Munoz, Y. Ben-Neriah and R. Mechoulam, Mol. Pharmacol., 2007, 72, 1657 CrossRef CAS
.
- H. Obermann, Dragoco Rep., 1977, 24, 260 Search PubMed
.
- E. N. Schimdt, N. K. Kashtanova and V. A. Pantegova, Khim. Prirod. Soedineii, 1970, 6, 694 Search PubMed
.
- V. D. Patil, U. R. Nayak and S. Dev, Tetrahedron, 1973, 29, 341 CrossRef CAS
.
- A. O. Barakat and J. Rullkoter, Organic Mass Spectrometry, 1993, 28, 157 CrossRef CAS
.
- M. L. Forcellese, R. Nicoletti and C. Santarelli, Tetrahedron Lett., 1973, 39, 3783 CrossRef
.
- S. Basar, A. Koch and W. A. Konig, Flavour Frag. J., 2001, 16, 315 CrossRef CAS
.
- K. C. Joshi, R. K. Bansal and P. Singh, Indian J. Chem., Sect. B, 1974, 12, 903 Search PubMed
.
- D. E. U. Ekong and J. I. Okogun, Phytochemistry, 1969, 8, 669 CrossRef CAS
.
-
U. V. Ahmad and Atta-ur-Rahman, in Handbook of Natural Products Data, Vol. 2, Pentacyclic Triterpenoids, Elsevier, Amsterdam, The Netherlands, 1994, 718 Search PubMed
.
- V. L. N. Reddy, K. Ravinder, M. Srinivasulu, T. V. Goud, S. M. Reddy, D. Srujankumar, T. P. Rao, U. S. Murty and Y. Venkateswarlu, Chem. Pharm. Bull. (Tokyo), 2003, 51, 1081 CrossRef CAS
.
- M. Kubo, M. Nagai and T. Inoue, Chem. Pharm. Bull., 1983, 31, 1917 CAS
.
- G. I. Gonzalez and J. Zhu, J. Org. Chem., 1997, 62, 7544 CrossRef CAS
.
- Atta-ur-Rahman, H. Naz, Fadimatou, T. Makhmoor, A. Yasin, N. Fatima, F. N. Ngounou, S. F. Kimbu, B. L. Sondengam and M. I. Choudhary, J. Nat. Prod., 2005, 68, 189 CrossRef
.
- G. Proietti, G. Strapaghetti and S. Corsano, Planta Med., 1981, 41, 417 CrossRef CAS
.
- Y. K. Sarin and C. K. Atal, Pafai J., 1982, 4, 13 Search PubMed
.
- A. Winterstein and G. Stein, Z. Physiol. Chem., 1932, 9, 208 Search PubMed
.
- J. Barney, Magerlein and R. H. Levin, J. Am. Chem. Soc., 1955, 77, 1904
.
- R. P. Singh, H. N. Subbarao and Sukhdev, Tetrahedron, 1981, 37, 843 CrossRef CAS
.
- US Pat., 20040073060
.
- J. C. E. Simpson and N. Williams, J. Chem. Soc., 1938, 1712 RSC
.
- J. Jauch and J. Bergmann, Eur. J. Org. Chem., 2003, 4752 CrossRef CAS
.
- B. A. Shah, A. Kumar, P. Gupta, M. Sharma, V. K. Sethi, A. K. Saxena, J. Singh, G. N. Qazi and S. C. Taneja, Bioorg. Med. Chem. Lett., 2007, 17, 6411 CrossRef CAS
.
- H. Singh, T. R. Bhardwaj, M. Kumar and B. Singh, Ind. J. Chem., Sect. B, 1986, 25, 1118
.
- L. H. Sarett, J. Am. Chem. Soc., 1948, 70, 1454 CrossRef CAS
; L. H. Sarett, J. Am. Chem. Soc., 1949, 71, 2443 CrossRef CAS
.
- J. C. E. Simpson and N. Williams, J. Chem. Soc., 1938, 686 RSC
.
- R. Budziarek, J. D. Johnston, W. Manson and F. S. Spring, J. Chem. Soc., 1951, 3019 RSC
.
- C. D. Xenos and P. Catsoulacos, Synthesis, 1985, 307 CrossRef CAS
.
- R. O. Clinton, R. L. Clarke, F. W. Stonner, A. J. Manson, K. F. Jennings and D. K. Phillips, J. Org. Chem., 1962, 27, 2800 CAS
.
- L. Bore, T. Honda and G. W. Gribble, J. Org. Chem., 2000, 65, 6278 CrossRef CAS
.
- G. N. Qazi, S. C. Taneja, J. Singh, A. K. Saxena, A. K. Shahi, V. K. Sethi, D. M. Mondhe, B. K. Kapahi, S. Bhushan, S. S. Andotra, B. A. Shah, S. Singh, H. C. Pal, F. Malik, A. Kumar and M. Sharma, Pat. Appl. 0570/DEL/2007.
-
G. N. Qazi, S. C. Taneja, J. Singh, A. K. Saxena, A. K. Shahi, V. K. Sethi, B. A. Shah, B. K. Kapahi, S. S. Andotra, A. Kumar, S. Bhushan, F. Malik, D. M. Mondhe, S. Muthiah, S. Singh, M. Verma and S. K. Singh, Pat Appl. 0606/DEL/2008
.
- P. F. Van Bergen, T. M. Peakman, E. C. Leigh-Firbank and R. P. Evershed, Tet. Lett., 1997, 38, 8409 Search PubMed
.
- C. Mathe, G. Culioli, P. Archier and C. Vieillescazes, J. Chromatogr. A, 2004, 1023, 277 CrossRef CAS
.
- C. Mathe, J. Connan, P. Archier, M. Mouton and C. Vieillescazes, Ann. Chim., 2007, 97, 433 CrossRef CAS
.
- S. Hamm, E. Lesellier, J. Bleton and A. Tchapla, J. Chromatogr. A, 2003, 1018, 73 CrossRef CAS
.
- S. Hamm, J. Bleton, J. Connan and A. Tchapla, Phytochemistry, 2005, 66, 1499 CrossRef CAS
.
- M. Ganzera, W. M. Stoggl, G. K. Konn, I. A. Khan and H. Stuppner, J. Sep. Sci., 2003, 26, 1383 CrossRef CAS
.
- B. Buchele, W. Zugmaier and T. Simmet, J. Chromatogr. B, 2003, 791, 21 CrossRef
.
- C. Mathe, G. Culioli, P. Archier and C. Vieillescazes, Chromatographia, 2004, 60, 493 CrossRef
.
- M. Ganzera and I. A. Khan, Planta Med., 2001, 67, 778 CAS
.
- K. Krohn, M. S. Rao, N. V. Raman and M. Khalilullah, Phytochem. Anal., 2001, 12, 374 CrossRef CAS
.
- B. Buchele and T. Simmet, J. Chromatogr. B, 2003, 795, 355 CrossRef
.
- O. N. Pozharitskaya, S. A. Ivanova, A. N. Shikov and V. G. Makarov, J. Sep. Sci., 2006, 29, 2245 CrossRef CAS
.
- S. A. Shah, I. S. Rathod, B. N. Suhagia, D. A. Patel, V. K. Parmar, B. K. Shah and V. M. Vaishnavi, J. Chromatogr. B, 2007, 848, 232 CrossRef CAS
.
- V. Sterk, B. Buchele and T. Simmet, Planta Med., 2004, 70, 1155 CrossRef CAS
.
- S. Sharma, V. Thawani, L. Hingorani, M. Shrivastava, V. R. Bhate and R. Khiyani, Phytomedicine, 2004, 11, 255 Search PubMed
.
- A. Kaunzinger, A. Baumeister, K. Cuda, N. Haring, B. Schug, H. H. Blume, K. Raddatz, G. Fischer and M. J. Schubert-Zsilavecz, Pharm. Biomed. Anal., 2002, 28, 729 CrossRef CAS
.
- K. Reising, J. Meins, B. Bastian, G. Eckert, W. E. Mueller, M. Schubert-Zsilavecz and M. Abdel-Tawab, Anal. Chem., 2005, 77, 6640 CrossRef CAS
.
- A. Frank and M. Unger, J. Chromatogr. B, 2006, 1112, 255 CAS
.
- J. E. Chrubasik, B. D. Roufogalis and S. Chrubasik, Phytother. Res., 2007, 21, 675 CrossRef CAS
.
- H. P. Ammon, Planta Med., 2006, 72, 1100 CrossRef CAS
.
-
S. C. Taneja and K. L. Dhar, in Supplement To Cultivation & Utilization Of Medicinal Plants, ed. Handa & Kaul, 1996, p. 525 Search PubMed
.
-
(a) C. K. Atal, G. B. Singh, S. Batra, S. Sharma and O. P. Gupta, Ind. J. Pharm., 1980, 12, 59 Search PubMed
; (b) C. K. Atal, O. P. Gupta and G. B. Singh, Br. J. Pharm., 1981, 74, 203
.
- M. K. Menon, Life Science, 1969, 8, 1023
.
- US Pat., 20040037903
.
- N. Banno, T. Akihisa, K. Yasukawa, H. Tokuda, K. Tabata, Y. Nakamura, R. Nishimura, Y. Kimura and T. Suzuki, J. Ethnopharmacol., 2006, 107, 249 CrossRef CAS
.
- M. T. Huang, V. Badmaev, Y. Ding, Y. Liu, J. G. Xie and C. T. Ho, Biofactors, 2000, 13, 225 Search PubMed
.
- D. Khanna, G. Sethi, K. S. Ahn, M. K. Pandey, A. B. Kunnumakkara, B. Sung, A. Aggarwal and B. B. Aggarwal, Curr. Opin. Pharmacol., 2007, 7, 344 CrossRef CAS
.
- US Pat., 6,492,429
.
- S. Gupta, A. K. Saxena, G. B. Singh and C. K. Atal, Ind. J. Pharm., 1982, 14, 74 Search PubMed
.
- M. L. Sharma, A. Khajuria, A. Kaul, S. Singh, G. B. Singh and C. K. Atal, Agents Actions, 1988, 24, 161 Search PubMed
.
- G. B. Singh and C. K. Atal, Agents Actions, 1986, 18, 407 Search PubMed
.
- C. K. Atal, M. L. Sharma, A. Koul, A. Khajuria and G. B. Singh, Ind. J. Pharm., 1983, 15, 38 Search PubMed
.
- Y. B. Tripathi, M. M. Reddy, R. S. Pandey, J. Subhashmi, O. P. Tiwari, B. K. Singh and P. Reddanna, Inflammopharmacology, 2004, 12, 131 CrossRef CAS
.
- Y. B. Tripathi, P. Tripathi, K. Korlagunta, S. C. Chai, B. J. Smith and B. H. Arjmandi, Inflammation, 2008, 31, 1 CrossRef
.
- R. R. Kulkarni, P. S. Patki, V. P. Jog, S. G. Gandage and B. Patwardhan, J. Ethnopharmacol., 1991, 33, 91 CrossRef CAS
.
- A. Chopra, P. Lavin, B. Patwardhan and D. Chitre, J. Clin. Rheumatol., 2004, 10, 236 CrossRef
.
- A. Y. Fan, L. Lao, R. X. Zhang, A. N. Zhou, L. B. Wang, K. D. Moudgil, D. Y. Lee, Z. Z. Ma, W. Y. Zhang and B. M. Berman, J. Ethnopharmacol., 2005, 101, 104 CrossRef CAS
.
- A. Y. Fan, L. Lao, R. X. Zhang, L. B. Wang, D. Y. Lee, Z. Z. Ma, W. Y. Zhang and B. M. Berman, J. Altern. Complement. Med., 2005, 11, 323 CrossRef
.
- G. K. Reddy, G. Chandrakasan and S. C. Dhar, Biochem. Pharmacol., 1989, 38, 3527 CrossRef CAS
.
- O. P. Gupta, N. Sharma and D. Chand, J. Pharmacol. Toxicol. Methods, 1994, 31, 95 CrossRef CAS
.
- N. Kimmatkar, V. Thawani, L. Hingorani and R. Khiyani, Phytomedicine, 2003, 10, 3 Search PubMed
.
- C. K. Atal, B. Singh, S. Kour, S. Singh, G. B. Singh and C. L. Gupta, IPS (Abstract), Chandigarh, 1982, 15 Search PubMed
.
- J. Reichling, H. Schmokel, J. Fitzi, S. Bucher and R. Saller, Schweiz. Arch. Tierheilkd., 2004, 146, 71 Search PubMed
.
-
(a) V. K. Pachnanda, S. Singh, G. B. Singh, O. P. Gupta and C. K. Atal, Ind. J. Pharm., 1981, 13, 63 Search PubMed
; (b) V. K. Pachnanda, RRL News Letter, Jammu, 1982, 3, 9 Search PubMed
; (c) V. K. Pachnanda, Graduates Pharmaceutica, 1982, 5, 76 Search PubMed
.
- O. Sander, G. Herborn and R. Rau, Z. Rheumatol., 1998, 57, 11 CrossRef CAS
.
- S. Joos, T. Rosemann, J. Szecsenyi, E. G. Hahn, S. N. Willich and B. Brinkhaus, BMC Complement. Altern. Med., 2006, 6, 19 Search PubMed
.
- N. Nyhlin, J. Bohr, S. Eriksson and C. Tysk, Aliment Pharmacol. Ther., 2006, 23, 1525 CrossRef CAS
.
- F. Borrelli, F. Capasso, R. Capasso, V. Ascione, G. Aviello, R. Longo and A. A. Izzo, Br. J. Pharmacol., 2006, 148, 553 CrossRef CAS
.
- H. Gerhardt, F. Seifert, P. Buvari, H. Vogelsang and R. Repges, Z. Gastroenterol., 2001, 39, 11 Search PubMed
.
- I. Gupta, A. Parihar, P. Malhotra, G. B. Singh, R. Ludtke, H. Safayhi and H. P. Ammon, Eur. J. Med. Res., 1997, 2, 37 Search PubMed
.
- I. Gupta, A. Parihar, P. Malhotra, S. Gupta, R. Ludtke, H. Safayhi and H. P. Ammon, Planta Med., 2001, 67, 391 CrossRef CAS
.
- P. R. Kiela, A. J. Midura, N. Kuscuoglu, S. D. Jolad, A. M. Solyom, D. G. Besselsen, B. N. Timmermann and F. K. Ghishan, Am. J. Physiol. Gastrointest. Liver Physiol., 2005, 288, G798 CrossRef CAS
.
- A. Madisch, S. Miehlke, O. Eichele, J. Mrwa, B. Bethke, E. Kuhlisch, E. Bastlein, G. Wilhelms, A. Morgner, B. Wigginghaus and M. Stolte, Int. J. Colorectal Dis., 2007, 22, 1445 CrossRef
.
- C. F. Krieglstein, C. Anthoni, E. J. Rijcken, M. Laukotter, H. U. Spiegel, S. E. Boden, S. Schweizer, H. Safayhi, N. Senninger and G. Schurmann, Int. J. Colorectal Dis., 2001, 16, 88 CrossRef CAS
.
- G. Latella, R. Sferra, A. Vetuschi, G. Zanninelli, A. D'Angelo, V. Catitti, R. Caprilli and E. Gaudio, Eur. J. Clin. Invest., 2008, 38, 410 CrossRef CAS
.
- A. Khajuria, A. Gupta, P. Suden, S. Singh, F. Malik, J. Singh, B. D. Gupta, K. A. Suri, V. K. Srinivas, K. Ella and G. N. Qazi, Phytother. Res., 2008, 22, 340 CrossRef CAS
.
-
(a) World Pat., 2000/066111;
(b) World Pat., 2002/066491;
(c) US Pat., 20030185907
.
- US Pat., 5,629,351
.
- US Pat., 20030199581
.
- G. B. Singh, O. P. Gupta, S. Kour, B. Singh and C. K. Atal, Ind. J. Pharm., 1984, 16, 51 Search PubMed
.
- M. L. Sharma, S. Bani and G. B. Singh, Int. J. Immunopharmacol., 1989, 11, 647 CrossRef CAS
.
- G. K. Reddy, S. C. Dhar and G. B. Singh, Agents Actions, 1987, 22, 99 Search PubMed
.
- S. Singh, A. Khajuria, S. C. Taneja, R. K. Khajuria, J. Singh and G. N. Qazi, Bioorg. Med. Chem. Lett., 2007, 17, 3706 CrossRef CAS
.
- N. Chande, J. W. McDonald and J. K. MacDonald, Cochrane Database Syst. Rev., 2006, CD003575 Search PubMed
.
- N. Chande, J. W. McDonald and J. K. MacDonald, Cochrane Database Syst. Rev., 2008, CD003575 Search PubMed
.
- L. Langmead and D. S. Rampton, Aliment Pharmacol. Ther., 2006, 23, 341 CrossRef CAS
.
- C. Anthoni, M. G. Laukoetter, E. Rijcken, T. Vowinkel, R. Mennigen, S. Muller, N. Senninger, J. Russel, J. Jauch, J. Bergmann, D. N. Granger and C. F. Krieglstein, Am. J. Physiol. Gastrointest. Liver Physiol., 2006, 290, G1131 CrossRef CAS
.
- S. Singh, A. Khajuria, S. C. Taneja, R. K. Johri, J. Singh and G. N. Qazi, Phytomedicine, 2008, 15, 400 Search PubMed
.
- S. Singh, A. Khajuria, S. C. Taneja, R. K. Khajuria, J. Singh, R. K. Johri and G. N. Qazi, Phytomedicine, 2008, 15, 408 Search PubMed
.
- I. Schneider and F. Bucar, Phytother. Res., 2005, 19, 81 CrossRef CAS
.
- H. P. Ammon, T. Mack, G. B. Singh and H. Safayhi, Planta Med., 1991, 57, 203 CrossRef CAS
.
- S. K. Shrivastava, G. K. Singh and P. K. Basniwal, Ind. J. Nat. Prod., 2003, 19, 14 Search PubMed
.
-
(a) S. H. Itzkowitz and X. Yio, Am. J. Physiol. Gastrointest. Liver Physiol., 2004, 287, G7 CrossRef CAS
; (b) D. N. Seril, J. Lia, G. Y. Yang and C. S. Yang, Carcinogenesis, 2003, 24, 353 CrossRef CAS
.
- J. M. Balcarek, T. W. Theisen, M. N. Cook, A. Varrichio, S. M. Hwang, M. W. Strohsacker and S. T. Crooke, J. Biol. Chem., 1988, 263, 13937 CAS
.
- A. Wildfeurer, I. S. Neu, H. Safayhi, G. Metzger, M. Wehrmann, U. Vogel and H. P. Ammon, Arzneimittelforschung, 1998, 48, 668
.
- H. P. Ammon, Eur. J. Med. Res., 1996, 1, 369 Search PubMed
.
- US Pat., 20040166182
.
- S. Roy, S. Khanna, H. Shah, C. Rink, C. Phillips, H. Preuss, G. V. Subbaraju, G. Trimurtulu, A. V. Krishnaraju, M. Bagchi, D. Bagchi and C. K. Sen, DNA Cell Biol., 2005, 24, 244 CrossRef CAS
.
- S. Roy, S. Khanna, A. V. Krishnaraju, G. V. Subbaraju, T. Yasmin, D. Bagchi and C. K. Sen, Antioxid Redox Signal, 2006, 8, 653 CrossRef CAS
.
- H. P. Ammon, H. Safayhi, T. Mack and J. Sabieraj, J. Ethnopharmacol., 1993, 38, 113 CrossRef CAS
.
- H. Safayhi, T. Mack, J. Sabieraj, M. I. Anazodo, L. R. Subramanium and H. P. Ammon, J. Pharmacol. Exp. Ther., 1992, 261, 1143 CAS
.
- U. Siemoneit, B. Hofmann, N. Kather, T. Lamkemeyer, J. Madlung, L. Franke, G. Schneider, J. Jauch, D. Poeckel and O. Werz, Biochem. Pharmacol., 2008, 75, 503 CrossRef CAS
.
- E. R. Sailer, L. R. Subramanium, B. Rall, R. F. Hoernlein, H. P. Ammon and H. Safayhi, Br. J. Pharmacol., 1996, 117, 615 CAS
.
- E. R. Sailer, S. Schweizer, S. E. Boden, H. P. Ammon and H. Safayhi, Eur. J. Biochem., 1998, 256, 364 CrossRef CAS
.
- H. Safayhi, B. Rall, E. R. Sailer and H. P. Ammon, J. Pharmacol. Exp. Therap., 1997, 281, 460 CAS
.
-
P. R. Bernstein, P. D. Edwards and J. C. Williams, in Progress in Medicinal Chemistry, vol. 37, ed. G. P. Ellis and D. K. Luscombe, Elsevier, Amsterdam, 1994, p. 59 Search PubMed
.
- E. Mayatepek and G. F. Hoffmann, Pediatr. Res., 1995, 37, 1 CAS
.
- S. E. Boden, S. Schweizer, T. Bertsche, M. Dufer, G. Drews and H. Safayhi, Mol. Pharmacol., 2001, 60, 267 CAS
.
-
(a) P. P. Tak and G. S. Firestein, J. Clin. Invest., 2001, 107, 7 CrossRef CAS
; (b) S. S. Makarov, Mol. Med. Today, 2000, 6, 441 CrossRef CAS
; (c) Y. Yamamoto and R. B. Gaynor, J. Clin. Invest., 2001, 107, 135 CrossRef CAS
.
- T. Syrovets, B. Buchele, C. Krauss, Y. Laumonnier and T. Simmet, J. Immun., 2005, 174, 498 Search PubMed
.
- C. Cuaz-Perolin, L. Billiet, E. Bauge, C. Copin, D. Scott-Algara, F. Genze, B. Buchele, T. Syrovets, T. Simmet and M. Rouis, Arterioscler. Thromb. Vasc. Biol., 2008, 28, 272 Search PubMed
.
- J. O. Clarke and G. E. Mullin, Nutr. Clin. Pract., 2008, 23, 49 Search PubMed
.
- B. Gayathri, N. Manjula, K. S. Vinaykumar, B. S. Lakshmi and A. Balakrishnan, Int. Immunopharmacol., 2007, 7, 473 CrossRef CAS
.
- O. Radmark, Am. J. Respir. Crit. Care Med., 2000, 161, S11 Search PubMed
.
-
(a) O. Werz, E. Buerkert, B. Samuelsson, O. Radmark and D. Steinhilber, Blood, 2002, 99, 1044 CrossRef CAS
; (b) O. Werz, J. Klemm, B. Samuelsson and O. Radmark, Proc. Natl. Acad. Sci. USA, 2000, 97, 5261 CrossRef CAS
.
- G. Pearson, F. Robinson, G. T. Beers, B. E. Xu, M. Karandikar, K. Berman and M. H. Cobb, Endocr. Rev., 2001, 22, 153 CrossRef CAS
.
- A. Altmann, L. Fischer, M. Schubert-Zsilavecz, D. Steinhilber and O. Werz, Biochem. Biophys. Res. Commun., 2002, 290, 185 CrossRef CAS
.
- L. Fischer, D. Szellas, O. Radmark, D. Steinhilber and O. Werz, FASEB J., 2003, 17, 949 CAS
.
- A. Altmann, D. Poeckel, L. Fischer, M. Schubert-Zsilavecz, D. Steinhilber and O. Werz, Br. J. Pharmacol., 2004, 141, 223 CrossRef CAS
.
- D. Poeckel, L. Tausch, A. Altmann, C. Feisst, U. Klinkhardt, J. Graff, S. Harder and O. Werz, Br. J. Pharmacol., 2005, 146, 514 CrossRef CAS
.
- D. Poeckel, L. Tausch, S. George, J. Jauch and O. Werz, J. Pharmacol. Exp. Ther., 2006, 316, 224 CAS
.
- D. Poeckel, L. Tausch, N. Kather, J. Jauch and O. Werz, Mol. Pharmacol., 2006, 70, 1071 CrossRef CAS
.
- R. A. DePinho, Nature, 2000, 408, 248 CrossRef CAS
.
- M. L. Rothenberg, D. P. Carbone and D. H. Johnson, Nat. Rev. Cancer, 2003, 3, 303 CrossRef CAS
.
- D. F. Flavin, J. Neurooncol., 2007, 82, 91 CrossRef CAS
.
- US Pat., 20050084547
.
- K. Hostanska, G. Daum and R. Saller, Anticancer Res., 2002, 22, 2853 CAS
.
- M. T. Huang, V. Badmaev, J.-G. Xie, Y.-R. Lou, Y. P. Lu and C. T. Ho, Proc. Am. Assoc. Cancer Res., 1997, 38, 368
.
- T. Tsukada, K. Nakashima and S. Shirakewa, Biochem. Biophys. Res. Commun., 1986, 140, 812
.
- L. G. Wang, X. M. Liu and X. J. Ji, Acta Pharmacol Sinica, 1991, 12, 114 Search PubMed
.
- P. Kruger, R. Daneshfar, G. P. Eckert, J. Klein, D. A. Volmer, U. Bahr, W. E. Muller, M. Karas, M. Schubert-Zsilavecz and M. Abdel-Tawab, Drug Metab. Dispos., 2008, 36, 1135 CrossRef
.
- Y. Jing, L. Xia and R. Han, Chin. Med. Sci. J., 1992, 7, 12 Search PubMed
.
- Y. K. Jing and R. Han, Yao Xue Xue Bao, 1993, 28, 11 CAS
.
- Z. Qi, G. Zhang and W. Zhu, Hunan Yi Ke Da Xue Xue Bao, 1999, 24, 23 Search PubMed
.
- J. R. Streffer, M. Bitzer, M. Schabet, J. Dichgans and M. Weller, Neurology, 2001, 56, 1219 CAS
.
- M. R. Heldt, M. Winking and T. Simmet, J. Neuro-Oncol., 1996, 2, 30
.
- D. K. Boker and M. Winking, Deutsches Arzteblatt, 1997, 94, A-1197 Search PubMed
.
- P. C. Warnke, K. Kopitzki and C. B. Ostertag, Deutsches Arzteblatt, 1998, 95, 220 Search PubMed
.
- V. R. Sinha, A. Singh, S. Singh and J. R. Bhinge, J. Pharm. Pharmacol., 2007, 59, 359 CrossRef CAS
.
- R. Han, Stem Cells, 1994, 12, 53 Search PubMed
.
- V. E. Steele, C. A. Holmes, E. T. Hawk, L. Kopelovich, R. A. Lubet, J. A. Crowell, C. C. Sigman and G. I. Kelloff, Cancer Epidem. Biomark. Prev., 1999, 8, 467 Search PubMed
.
- T. Akihisa, K. Tabata, N. Banno, H. Tokuda, R. Nishimura, Y. Nakamura, Y. Kimura, K. Yasukawa and T. Suzuki, Biol. Pharm. Bull., 2006, 29, 1976 CrossRef CAS
.
- Y. Shao, C. T. Ho, C. K. Chin, V. Badmaev, W. Ma and M. T. Huang, Planta Med., 1998, 64, 328 CrossRef CAS
.
- Y. S. Park, J. H. Lee, J. Bondar, J. A. Harwalkar, H. Safayhi and M. Golubic, Planta Med., 2002, 68, 397 CrossRef CAS
.
- Y. S. Park, J. H. Lee, J. A. Harwalkar, J. Bondar, H. Safayhi and M. Golubic, Adv. Exp. Med. Biol., 2002, 507, 387 CAS
.
- S. K. Singh, S. Bhusari, R. Singh, A. K. Saxena, D. Mondhe and G. N. Qazi, Vascul. Pharmacol., 2007, 46, 333 CrossRef CAS
.
- Y. Jing, S. Nakajo, L. Xia, K. Nakaya, Q. Fang, S. Waxman and R. Han, Leuk. Res., 1999, 23, 43 CrossRef CAS
.
- L. Xia, D. Chen, R. Han, Q. Fang, S. Waxman and Y. Jing, Mol Cancer Ther., 2005, 4, 381 CAS
.
- J. J. Liu, A. Nilsson, S. Oredsson, V. Badmaev, W. Z. Zhao and R. D. Duan, Carcinogenesis, 2002, 23, 2087 CrossRef CAS
.
- M. Winking, S. Sarikaya, A. Rahmanian, A. Jodicke and D. K. Boker, J. Neuro-Oncol., 2000, 46, 97 CrossRef CAS
.
- US Pat., 5,919,821
.
- W. Wick and W. Kuker, Onkologie, 2004, 27, 261 CrossRef CAS
.
- T. Glaser, S. Winter, P. Groscurth, H. Safayhi, E. R. Sailer, H. P. Ammon, M. Schabet and M. Weller, Br. J. Cancer, 1999, 80, 756 CrossRef CAS
.
- C. C. Weber, K. Reising, W. E. Muller, M. Schubert-Zsilavecz and M. Abdel-Tawab, Planta Med., 2006, 72, 507 CrossRef CAS
.
- G. Janssen, U. Bode, H. Breu, B. Dohrn, V. Engelbrecht and U. Gobel, Klin. Padiatr., 2000, 212, 189 CrossRef CAS
.
- J. J. Liu, A. Nilsson, S. Oredsson, V. Badmaev and R. D. Duan, Int. J. Mol. Med., 2002, 10, 501 Search PubMed
.
- M. Lu, L. Xia, H. Hua and Y. Jing, Cancer Res., 2008, 68, 1180 CrossRef CAS
.
- J. J. Liu, B. Huang and S. C. Hooi, Br. J. Pharmacol., 2006, 148, 1099 CrossRef CAS
.
- W. Zhao, F. Entschladen, H. Liu, B. Niggemann, Q. Fang, K. S. Zaenker and R. Han, Cancer Detect. Prev., 2003, 27, 67 Search PubMed
.
- US Pat., 5,064,823
.
- T. Syrovets, B. Buchele, E. Gedig, J. R. Slupsky and T. Simmet, Mol. Pharmacol., 2000, 58, 71 CAS
.
- R. F. Hoernlein, T. Orlikowsky, C. Zehrer, D. Niethammer, E. R. Sailer, T. Simmet, G. E. Dannecker and H. P. Ammon, J. Pharmacol. Exp. Therap., 1999, 288, 613 CAS
.
- T. Syrovets, J. E. Gschwend, B. Buchele, Y. Laumonnier, W. Zugmaier, F. Genze and T. Simmet, J. Biol. Chem., 2005, 280, 6170 CAS
.
- C. Y. Wang, Jr., J. C. Cusack, R. Liu and Jr. A. S. Baldwin, Nat. Med., 1999, 5, 412 CrossRef
.
- A. Richmond, Nat. Rev. Immunol., 2002, 2, 664 CrossRef CAS
.
- B. Buchele, W. Zugmaier, A. Estrada, F. Genze, T. Syrovets, C. Paetz, B. Schneider and T. Simmet, Planta Med., 2006, 72, 1285 CrossRef
.
- Y. Takada, H. Ichikawa, V. Badmaev and B. B. Aggarwal, J. Immunol., 2006, 176, 3127 CAS
.
- H. Q. Yuan, F. Kong, X. L. Wang, C. Y. Young, X. Y. Hu and H. X. Lou, Biochem. Pharmacol., 2008, 75, 2112 CrossRef CAS
.
- S. Bhushan, A. Kumar, F. Malik, S. S. Andotra, V. K. Sethi, I. Kaur, S. C. Taneja, G. N. Qazi and J. Singh, Apoptosis, 2007, 12, 1911 Search PubMed
.
- W. Dymock, C. J. H. Warden and D. Hopper, M/S Periodical Experts, Delhi, 1980, 1, 303 Search PubMed
.
- S. Weckesser, K. Engel, B. Simon-Haarhaus, A. Wittmer, K. Pelz and C. M. Schempp, Phytomedicine, 2007, 14, 508 Search PubMed
.
- M. K. Menon and A. Kar, Planta Med., 1971, 19, 333 CrossRef CAS
.
- Y. J. Kamath, J. V. Asad and M., Pak. J. Pharm. Sci., 2006, 19, 129 Search PubMed
.
- R. S. Pandey, B. K. Singh and Y. B. Tripathi, Indian J. Exp. Biol., 2005, 43, 509 Search PubMed
.
- M. L. Roonwal, P. N. Chaterjee and R. S. Thapa, Ind. Forest Records, Entomol., 1960, 9, 215 Search PubMed
.
- H. F. Khater and A. A. Shalaby, Rev. Inst. Med. Trop. Sao Paulo., 2008, 50, 107 Search PubMed
.
- B. R. Mikhaeil, G. T. Maatooq, F. A. Badria and M. M. Amer, Z. Naturforsch. C, 2003, 58, 230 CAS
.
- M. Lis-Balchin and S. Hart, J. Ethnopharmacol., 1997, 58, 183 CrossRef CAS
.
- P. Pungle, M. Banavalikar, A. Suthar, M. Biyani and S. Mengi, Indian J. Exp. Biol., 2003, 41, 1460 Search PubMed
.
- A. Khajuria, A. Gupta, F. Malik, S. Singh, J. Singh, B. D. Gupta, K. A. Suri, P. Suden, V. K. Srinivas, K. Ella and G. N. Qazi, Vaccine, 2007, 25, 4586 CrossRef CAS
.
- F. A. Badria, B. R. Mikhaeil, G. T. Maatooq and M. M. Amer, Z. Naturforsch. C, 2003, 58, 505 CAS
.
- M. R. Chevrier, A. E. Ryan, D. Y. Lee, M. Zhongze, Z. Wu-Yan and C. S. Via, Clin. Diagn. Lab. Immunol., 2005, 12, 575 CrossRef CAS
.
- G. Hussein, H. Miyashiro, N. Nakamura, M. Hattori, N. Kakiuchi and K. Shimotohno, Phytother. Res., 2000, 14, 510 CrossRef CAS
.
- S. L. Kela, R. A. Ogunsusi, V. C. Ogbogu and N. Nwude, Rev. Elev. Med. Vet. Pays. Trop., 1989, 42, 189 Search PubMed
.
- S. L. Kela, R. A. Ogunsusi, V. C. Ogbogu and N. Nwude, Rev. Elev. Med. Vet. Pays. Trop., 1989, 42, 195 Search PubMed
.
- L. Camarda, T. Dayton, V. Di Stefano, R. Pitonzo and D. Schillaci, Ann. Chim., 2007, 97, 837 CrossRef CAS
.
- R. A. Mothana and U. Lindequist, J. Ethnopharmacol., 2005, 96, 177 CrossRef
.
- R. A. Mothana, R. Mentel, C. Reiss and U. Lindequist, Phytother. Res., 2006, 20, 298 CrossRef
.
- J. V. Kavitha, J. F. Rosario, J. Chandran, P. Anbu and Bakkiyanathan, Ind. J. Physiol. Pharmacol., 2007, 51, 29 Search PubMed
.
- A. Oleski, U. Lindequist, R. A. Mothana and M. F. Melzig, Pharmazie, 2006, 61, 359 CAS
.
- S. A. Latheef, B. Prasad, M. Bavaji and G. Subramanyam, Bioinformation, 2008, 2, 260 Search PubMed
.
- T. Chandrasekhar, T. M. Hussain and B. Jayanand, Z. Naturforsch. C, 2005, 60, 505 CAS
.
- A. Moussaieff, N. A. Shein, J. Tsenter, S. Grigoriadis, C. Simeonidou, A. G. Alexandrovich, V. Trembovler, Y. Ben-Neriah, M. L. Schmitz, B. L. Fiebich, E. Munoz, R. Mechoulam and E. Shohami, J. Cereb. Blood Flow Metab., 2008, 28, 1341 Search PubMed
.
- A. Moussaieff, N. Rimmerman, T. Bregman, A. Straiker, C. C. Felder, S. Shoham, Y. Kashman, S. M. Huang, H. Lee, E. Shohami, K. Mackie, M. J. Caterina, J. M. Walker, E. Fride and R. Mechoulam, FASEB J., 2008 DOI:10.1096/fj.07-101865
.
- S. Ghoshal, M. J. Mukhopadhyay and A. Mukherjee, Indian J. Exp. Biol., 2001, 39, 1068 Search PubMed
.
- I. Gupta, V. Gupta, A. Parihar, S. Gupta, R. Ludtke, H. Safayhi and H. P. Ammon, Eur J. Med. Res., 1998, 3, 511 Search PubMed
.
- U. Dahmen, Y. L. Gu, O. Dirsch, L. M. Fan, J. Li, K. Shen and C. E. Broelsch, Transplantation Proceedings, 2001, 33, 539 Search PubMed
.
-
(a) US Pat., 20050209169
; (b) US Pat., 20020010168
.
- F. al-Awadi, H. Fatania and U. Shamte, Diabetes Res., 1991, 18, 163 Search PubMed
.
- U. Zutsi, P. G. Rao and S. Kaur, Ind. J. Pharmacol., 1986, 18, 182 Search PubMed
.
- US Pat., 20040166178
.
- US Pat., 6,589,516
.
-
H. Singh and G. N. Qazi, presented in part at the Training Workshop on Drug Standarization and Quality Assurance, New Delhi, June 2007
.
- B. Burlando, A. Parodi, A. Volante and A. M. Bassi, Toxicol. Lett., 2008, 177, 144 CrossRef CAS
.
- M. Bertagnolli, C. Eagle, A. Zauber, M. Redston, S. Solomon, K. Kim, J. Tang, R. Rosenstein, J. Wittes, D. Corle, T. Hess, G. Woloj, F. Boisserie, W. Anderson, J. Viner, D. Bagheri, J. Burn, D. Chung, T. Dewar, T. Foley, N. Hoffman, F. Macrae, R. Pruitt, J. Saltzman, B. Salzberg, T. Sylwestrowicz, G. Gordon, E. Hawk and N. Engl, J. Med., 2006, 355, 873 CAS
.
- C. Bombardier, L. Laine, A. Reicin, D. Shapiro, R. Burgos-Vargas, B. Davis, R. Day, M. B. Ferraz, C. J. Hawkey, M. C. Hochberg, T. K. Kvien and T. J. Schnitzer, N. Engl. J. Med., 2000, 343, 1520 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2009 |
