Blue flower color development by anthocyanins: from chemical structure to cell physiology
Kumi
Yoshida
*,
Mihoko
Mori†
and
Tadao
Kondo
Graduate School of Information Science, Nagoya University, Chikusa, Nagoya 464-8601, Japan. E-mail: yoshidak@is.nagoya-u.ac.jp
First published on 6th May 2009
Abstract
Covering: 1992 to 2007
Blue flower colors are primarily due to anthocyanin, a flavonoid pigment. Anthocyanin itself is purple in neutral aqueous solutions, and its color is very unstable and quickly fades. Therefore, the mechanism of blue color development in living flower petals is one of the most intriguing problems in natural product chemistry. Much progress has been made in understanding blue flower coloration since the comprehensive review by Goto and Kondo in 1991. This review focuses on the advances in the last 15 years, and cites 149 references.
 Kumi Yoshida | Kumi Yoshida was born in Nagoya (Japan) in 1958. She received her Master’s degree in 1982 and her Ph.D. in 1993 at Nagoya University (under Professor Toshio Goto). After 6 years at Amano Enzyme Inc., in 1988 she moved to Sugiyama Jogakuen University as an Assistant Professor. From 1994 to 1995 she stayed at Universität Konstanz, and in 1995 was awarded The Japan Bioscience, Biotechnology and Agrochemistry Society Award for the Encouragement of Young Scientists. In 2000 she became an Associate Professor of Nagoya University. Her research interests are the mechanisms of flower color development, structural studies and functions of plant polyphenols. |
 Mihoko Mori | Mihoko Mori received her B.Sc. in 1995 and Master’s degree in 1997 under the guidance of Professor Minoru Isobe. After 2 years at Banyu Pharmaceutical Co. Ltd., she started doctoral work at Nagoya University under the direction of Dr. Kumi Yoshida, obtaining her Ph.D. in 2005. From 2005 to 2008 she was a post-doc in the research group of Hiroyuki Osada, the Director of Riken, and in May 2008 she became an Assistant Professor at Kitasato University. Her research interests are natural product chemistry and antibiotics. |
 Tadao Kondo | Tadao Kondo was born in Nagoya (Japan) in 1941. He received his B.Sc. (1965) from Gifu Pharmaceutical University, MS (1967) and then PhD (1973) from the Department of Agricultural Chemistry, Nagoya University (under Prof. Toshio Goto). He then carried out research as a post-doc in the group of Professor Lemieux at University of Alberta, Canada (1973–1975). He became a Research Associate (1979) and an Associate Professor (1993) at the Chemical Instrument Center, and then a Professor (2000) at the Graduate School of Bioagricultural Sciences at Nagoya University. He received The Chemical Society of Japan Award for Creative Work (1998). His research field is natural product chemistry and organic synthesis. |
1 Introduction
Anthocyanin is the common name of a flavonoid pigment that possesses a hydroxylated 2-phenylbenzopyrilium chromophore (Fig. 1).1,2 Anthocyanins exist in various tissues of higher plants, including flowers, fruits, seed coats, leaves, stems, tubers and roots, and they exhibit a wide variety of colors, ranging from red to purple and from blue to black.1,3,4 Cyanin means “blue”, and most blue flower colors are due to anthocyanins. Nearly one thousand such pigments have been reported, and this progress is due to advances in structural determination (such as in HPLC, NMR and MS techniques), as well to improvements in purification methods.2,3,5 Structures of newly reported anthocyanins and their functions were reviewed periodically in a series of books entitled ‘The Flavonoids’ edited by Harborne, and in other reviews written by Harborne and co-workers.2,6–11 Grayer inherited this work and has authored two reviews in Natural Product Reports,12,13 the most recent appearing in 2008. In 2006 a comprehensive book entitled ‘Flavonoids – Chemistry, Biochemistry and Applications’, edited by Andersen and Markham, was published.5 Progress in the structural studies of anthocyanins has already been summarized in those reports. However, one of the most interesting topics in anthocyanin research, the mechanism of blue flower color development, remains to be clarified, and this will form the subject of this review.In its monomeric state, the anthocyanin molecule never exhibits a blue color in weakly acidic or neutral media; instead, a purple color is observed, and under such conditions the molecule rapidly loses its color.3,7,8,14–16 Thus, some sophisticated mechanism must exist in petal cells to permit blue coloration.
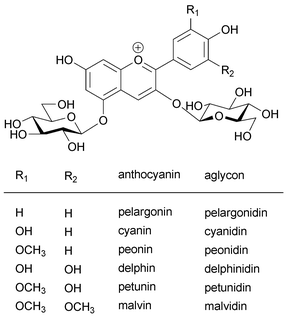 | ||
| Fig. 1 Structure of common anthocyanins and their aglycons. | ||
Goto and Kondo summarized the research on flower color variation in a review in 1991.3 Since then, 15 years have passed, and much progress has been made and reported. One of the breakthroughs in this area has been the structural determination of commelinin, a self-assembled blue metal complex pigment found in petals of blue dayflowers.17 A few years later, evidence that alkalization in colored petal cells leads to blue color was observed in morning glory petals.18 These two results provide experimental evidence for two historical hypotheses, the metal-complex theory and pH theory, about which the first controversy arose in the early 20th century.
Newly developed methods have opened the door to studies on blue color in living petal cells. Using a micro-manipulation technique, combined with microscale HPLC, spectrophotometry, electrodes, and elemental analyses, direct chemical information concerning the colored vacuoles of petals can be obtained.19,20 Put simply, the vacuole can be regarded as an extremely tiny test tube, in which all the ‘actors’ demonstrate their flower-coloring magic. Progress in instrumental analysis has contributed to the structural determination of many complex pigments, and research into the biosynthetic genes of anthocyanins has opened a new world in horticultural sciences, such as molecular breeding in pursuit of a blue rose.21
In this review, we summarize the mechanisms of blue flower color development by anthocyanins described between 1992 and 2007, and therefore supplement the review by Goto and Kondo in 1991. Following an introduction to the history of chemical research on blue flowers, we discuss metalloanthocyanin, a supramolecular self-assembled metal-complex pigment composed of anthocyanins, flavones, and metal ions in a stoichiometric ratio. Next, we describe fuzzy metal-complexes, which are also composed of anthocyanins, co-pigments, and metal ions, but not in a stoichiometric ratio. The third main part of this review is a discussion of polyacylated anthocyanins with two or more aromatic acyl residues and their blue color development by intramolecular stacking. In addition, anthocyanins covalently linked to colorless flavonoids are described. In the last section, we discuss trials for the molecular breeding of blue flowers.
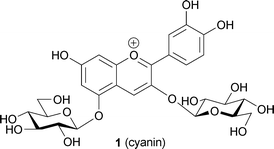
2 History and mystery of blue color development
A brief history of blue color development in plants before 1991 is presented in Table 1. In 1913 Willstätter and Everest proposed the pH theory, the first chemical hypothesis concerning blue flower color development.22 The hypothesis was based on the observation that cyanin, a pigment from blue cornflowers (1), is also found in red rose petals.23 Willstätter et al. tested the color change of 1 in aqueous solutions of various pH values, and found that in acidic media, the pigment shows a red color, while in alkaline solutions, it turns blue.24 Thus, they thought that the differences in the pH of petal cells of blue cornflowers and red roses cause the differences in petal colors. In opposition to the pH theory, a Japanese botanist and his brother, named Shibata, argued that blue color development in alkaline conditions is not relevant, because the existence of alkaline cell sap is not physiologically possible in living plants. One Shibata brother was a botanist and the other (fortunately) was an inorganic chemist, and together they studied blue coloration using metal-complexed anthocyanin. They found that when a flavonol was reduced with magnesium metal, a blue–green solution was obtained.25 A chemical analysis of the coloring matter indicated that the blue color was due to metal complexation of Mg2+ by anthocyanin. It was as a result of this that in 1919 Shibata and co-workers reported the metal-complex theory for blue color development.25| 1913 | Willstätter and Everest | pH theory |
| 1919 | Shibata and Shibata | Metal complex theory |
| 1927 | Robertson and Robinson | Synthesis of anthocyanins |
| 1931 | Robinson and Robinson | Co-pigmentation theory |
| 1958 | Hayashi et al. | Purification of a metalloanthocyanin (commelinin) |
| 1971 | Saito et al. | Isolation of a polyacylated anthocyanin (platyconin) |
| 1972 | Asen et al. | Self-association theory |
| 1982 | Goto and Kondo | Structural determination of polyacylated anthocyanin (gentiodelphin) |
| 1986 | Goto et al. | Molecular stacking theory by hydrophobic interaction |
Today, pH-dependent structural changes in the anthocyanidin chromophore and the resulting color changes are generally investigated using kinetic studies (Fig. 2).7,14,26 Furthermore, decoloration of simple anthocyanins under neutral and weakly acidic conditions is known to be due to hydration at the 2- or 4-positions of the anthocyanidin chromophore.3,7,14–16,26 Thus, the validity of the pH theory for blue color development of cyanin (1) under alkaline conditions is now considered to be doubtful. However, during the time of the Shibata brothers in the early part of the 20th century, the metal complex theory was ignored by European chemists.
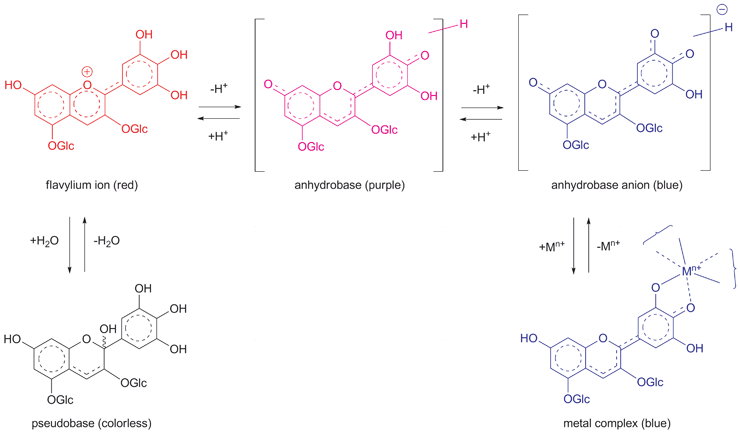 | ||
| Fig. 2 Structural changes in the anthocyanindin chromophore and their pH-dependent color changes in aqueous solution. | ||
During the next decade, Robinson and his group reported pioneering work on synthetic studies of anthocyanins.27–29 Most simple anthocyanins, anthocyanidin 3-monoglucosides and 3,5-diglucosides, were synthesized using Robinson's aldol condensation reaction.27 Simultaneously, his group studied the blue color development and the mechanism by which colorless compounds, such as amino acids, sugars and flavonoids, stabilized anthocyanins.30 To describe the effects of these co-existing compounds on anthocyanins, they used the term ‘co-pigmentation effect’, this term referring, for example, to the bathochromic shift in the absorption spectrum and to the stabilization of the chromophore. However, Robinson's group also failed to take the metal-complex theory into account.
A successor to Shibata, Hayashi, began studying various blue flowers to find evidence of metal-coordinated anthocyanins.1 Hayashi and his group tried to isolate genuine blue pigments from blue flower petals. In 1958 they grew a needle crystal from the blue dayflower Commelina communis and named it commelinin.31,32 Hayashi et al. reported that commelinin (2) is composed of not only anthocyanin but also flavone and Mg2+ ions. In the same year, Bayer isolated a blue powder from petals of the blue cornflower Centaurea cyanus and reported that Fe3+ and Al3+, but not Mg2+, may affect blue coloration.33 However, there was another argument between Hayashi and Bayer; Hayashi claimed that Mg2+ causes blue coloration,32 but Bayer denied that a divalent metal ion was involved and instead expounded his theory that trivalent ions, Fe3+ and/or Al3+, are essential.34 Their argument failed to lead to the true answer, and these problems remained until studies by Kondo et al. in 1994.35
In the 1970s Asen proposed a new concept, self-association, to describe the stabilization of anthocyanins.36 A concentrated solution of a simple anthocyanin is more stable than a dilute solution, with a non-linear increase in the molar absorption coefficient in the visible absorption spectrum;36 furthermore, the peak in the spectrum broadens. The stabilization effect due to molecular interactions seemed to be similar to the co-pigmentation effect. Goto then proposed a unified mechanism for co-pigmentation and self-association, which he called the molecular stacking theory.3,15,16 He suggested that both co-pigmentation and self-association occur by hydrophobic interactions between aromatic rings in co-pigments and anthocyanins, or between anthocyanins, not between anthocyanins interacting via hydrogen bonds.3,15,16,37,38 Therefore, the chemical driving forces for co-pigmentation and self-association are the same – the hydrophobic aromatic residues in the molecules assemble in aqueous solutions. He expanded this idea into the intramolecular stacking theory, which addressed the stability of polyacylated anthocyanins.3,15,16 In polyacylated anthocyanins, two or more aromatic acyl residues exist and the residues engage in intramolecular stacking with the anthocyanidin chromophore through the same hydrophobic interaction. Since two chromophores with different λmax stack closely in co-pigmentation, charge-transfer occurs between them, leading to a bathochromic shift. In the case of polyacylated anthocyanins, the same charge-transfer phenomenon should occur between the intramolecular aromatic residues and the anthocyanidin chromophore, thereby leading to a bathochromic shift. In contrast to these examples, self-associated anthocyanins do not show a bathochromic shift in λmax, but they do show peak-broadening of the spectrum. Interaction between the same chromophores splits the excitation energy into higher and lower levels, as occurs between neighboring bases in nucleic acids,14 and they exhibit an exiton-type Cotton effect in CD spectra, depending on whether the stacking is clockwise (a positive Cotton effect) or anti-clockwise (a negative Cotton effect).15,16
In the 1980s, the research group of Goto and Kondo reported many structures of complex anthocyanins and provided experimental evidence in support of the molecular stacking theory.3,15,16 They also reviewed mechanisms of blue-color development; however, the mystery of blue flower coloration remained. With regard to the pH theory, there were still no direct pH measurements, except for Asen's work using glass capillaries containing pH indicator.39 Concerning the metal complex theory, the entire atomic structure of commelinin was still unsolved. The intramolecular stacking conformation of polyacylated anthocyanins was supported only by long-range NOEs in NMR analysis carried out in acidic media. Briefly, scientists knew that any anthocyanin could develop a blue color when it was maintained in the anhydrobase anion form in petal cell vacuoles. The problem was how to maintain such a structure, because plant vacuoles are filled with neutral or weakly acidic aqueous media. Since that time, research into blue flower coloration has aimed at clarifying this ‘magic’.
3 Metalloanthocyanins
The term metalloanthocyanin refers to a self-assembled, supramolecular metal complex pigment; this complex is composed of stoichiometric amounts of anthocyanins, flavones, and metal ions.3,15,40 In any metalloanthocyanin, the composition is fixed at 6 : 6 : 2, respectively (Fig. 3). Three major mechanisms for blue flower coloration exist: self-association, co-pigmentation, and metal complexation. Because all the proven mechanisms for blue flower coloration (except pH) include metalloanthocyanins, they may be, in a sense, the ‘topmost pegs’ in blue flower pigments. This section discusses the structure and mechanism of blue color development of five metalloanthocyanins obtained from five different flower petals (Fig. 4). Since 1992 two X-ray crystallographic structures have been solved: commelinin (2)17 from C. communis and protocyanin (3)41 from C. cyanus. These molecular structures, as well as blue color development, are discussed in detail. In addition to these two metalloanthocyanins, three more metalloanthocyanins have been found, protodelphin (4),42,43 cyanosalvianin (5),44 and nemophilin (6, K. Yoshida, unpublished). Compounds 4 and 5 come from blue salvia petals, Salvia patens and Salvia uliginosa, while 6 comes from blue nemophila petals, Nemophila menziesii (Fig. 5). We discuss below the effects that structure and metal ion species have on blue coloration.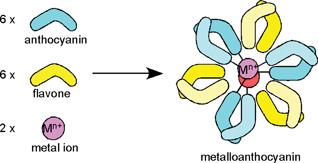 | ||
| Fig. 3 Formation of a metalloanthocyanin by self-assembly from six anthocyanin molecules, six flavone molecules and two metal ions. | ||
 | ||
| Fig. 4 Five blue flowers whose blue coloration is caused by metalloanthocyanins. | ||
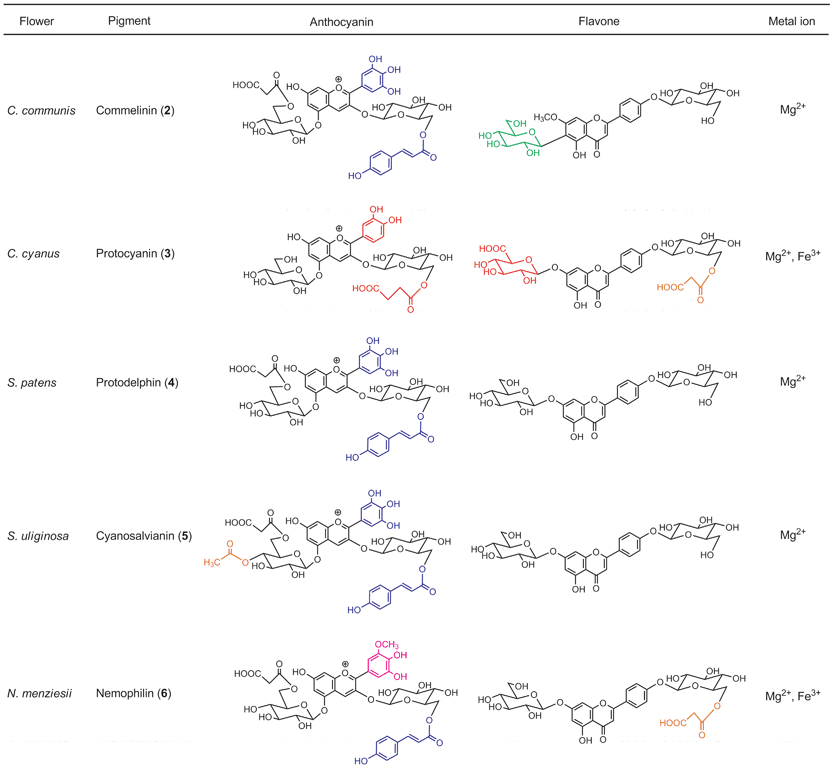 | ||
| Fig. 5 Five metalloanthocyanins and an overview of the similarities and differences in their components. | ||
3.1 Commelinin
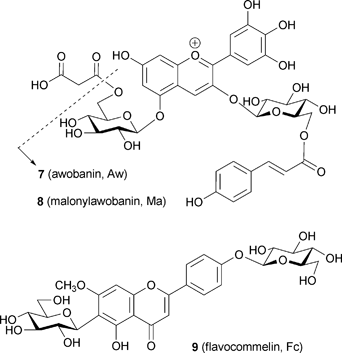
Commelinin (2) was first isolated by Hayashi et al. in 1958 as a blue crystalline pigment from petals of Commelina communis.31 For chemical studies petals of its cultivar, C. communis, cv. Ohboshibana, were chosen. This cultivar has for a long time been used to draw rough designs for Yuzen printing, because the blue color is extremely stable in concentrated solutions, but the pigment easily dissociates and becomes colorless when washed with water. In 1983 the anthocyanin component was determined to be not awobanin (Aw, 7), but rather malonylawobanin (Ma, 8),45 and 2 was found through reconstruction experiments to contain 8, flavocommelin (Fc, 9), and Mg2+ in a 6 : 6 : 2 ratio.46 Saito et al. collected X-ray reflection data of Cd-commelinin (10), but the data could not be solved completely because of the crystal's high degree of symmetry (Y. Saito et al., unpublished). In 1987 a partial structure of 10 clarified the stacking manner of anthocyanidin chromophores.16 The data revealed chiral self-association of anthocyanidin chromophores in an anti-clockwise manner and a metal-complexation structure in the B-ring;15,16 these results were consistent with the structure proposed by CD and with other experimental evidence (Fig. 6).15,16,46,47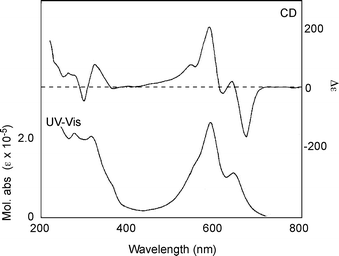 | ||
| Fig. 6 UV-Vis absorption and CD spectra of commelinin (2) in an aqueous solution at a pH of 5.0 (2: 50 µM, 25 °C). | ||
In 1992 another set of reflection data of 10 was collected using a synchrotron radiation beam, and the entire atomic structure was determined using direct methods (Fig. 7).17 Immediately after the successful X-ray crystallographic analysis of Cd-commelinin, the same group reported the structure of Mg-commelinin (2) (Table 2).48 Both crystal structures (2, 10), are similar, though several differences in the distances between metal ions and coordinated atoms exist, due to the differences in the atomic radii of the Mg and Cd ions (Fig. 8). In both crystals, a 3-fold axis exists, and two metal ions are located in the center of the molecule along this axis, approximately 0.5 nm apart from each other. A metal ion is coordinated to the 3′ and 4′ oxygen atoms in the B-ring of anthocyanin. The observation that the distance between the 3′-C and 3′-O is longer than that between the 4′-C and 4′-O indicates that Ma (8) in commelinin exists in the 4′-keto-anhydrobase anion form.17,48 In the crystal, three molecules of Ma (8) coordinate to one metal ion, and two molecules of Ma (8), which are coordinated to the different metal ions, stack on top of each other in an anticlockwise manner (Fig. 9).17,48 The distance between the two aromatic rings of 8 is approximately 0.4 nm. Similarly, two molecules of Fc (9) stack on top of each other in an anticlockwise manner. The distance between the aromatic rings of 9 is approximately 0.35 nm. In the crystal structure, self-associated MaMa and FcFc alternate in surrounding the x-axis of the molecule.17,48 In 2 and 10, co-pigmentation in a clockwise manner was observed between Ma (8) and Fc (9), and the distance between the aromatic rings of neighboring 8 and 9 was approximately 0.45 nm.
| Commelinin (2) | Cd-commelinin (10) | |
|---|---|---|
| Crystal system | Trigonal | Trigonal |
| Space group | P321 | P321 |
| Cell dimensions | a = b = 3.1191(4) nm | a = b = 3.1329(8) nm |
| c = 3.3623(8) nm | c = 3.3590(14) nm | |
| Cell volume | V = 2.833 × 10−7 nm3 | V = 2.856 × 10−7 nm3 |
| Number of molecules in a unit cell | Z = 2 | Z = 2 |
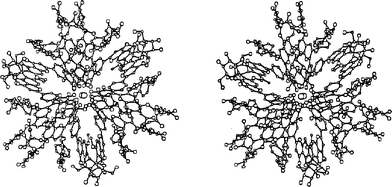 | ||
| Fig. 7 X-ray crystallographic structure of commelinin (2). The stereo-view image depicts a view along a three-fold axis. | ||
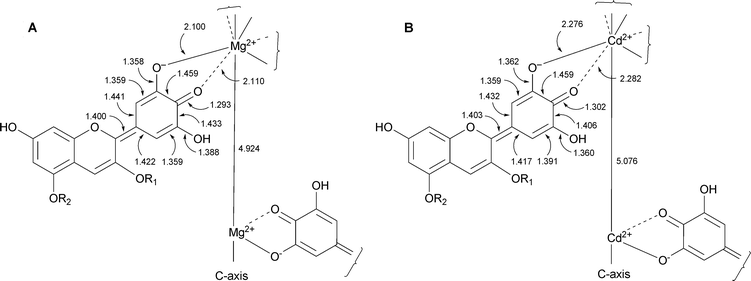 | ||
| Fig. 8 Bond lengths around the coordination center of commelinin. A: commelinin (2), B: Cd-commelinin (10). | ||
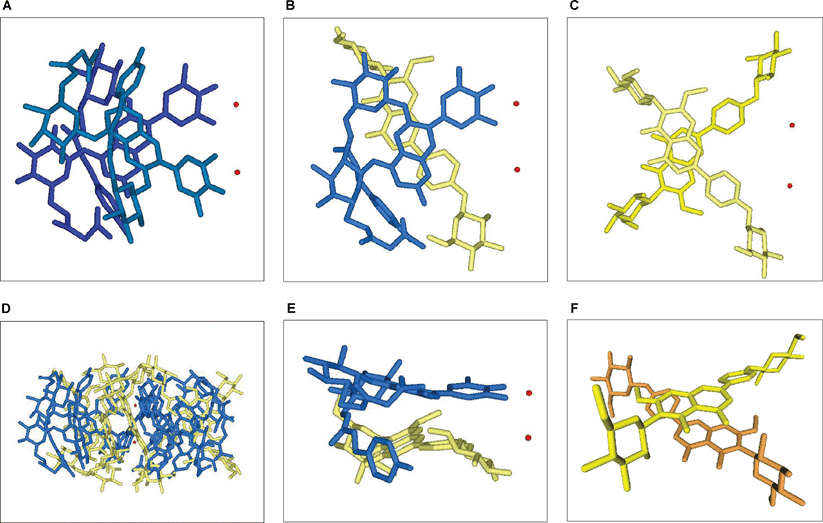 | ||
| Fig. 9 Stacking structure of commelinin (2). Blue: Ma (8), yellow and orange: Fc (9), red: Mg2+. A: A side view of a left-handed stacking of two Ma units (8) that coordinate to different Mg ions, B: A side view of a co-pigmentation of Ma (8) and Fc (9) in a right-handed stacking arrangement, C: A side view of a left-handed stacking of two Fc units (9), D: A side view of commelinin (2), E: A skew view of a co-pigmentation between Ma and Fc, F: A skew view of the self-association of two Fc units. | ||
Both structures 2 and 10 indicate that common key points are essential for blue color development. The first is generation of the 4′-keto-anhydrobase anion form of the delphinidin chromophore by coordination of the metal to the 3′,4′-oxygen atoms in the B-ring. The second is stabilization of the 4′-keto-anhydrobase anion form by molecular stacking. The crystals of commelinin from EtOH–H2O contain approximately 30% water, similar to protein crystals; however, the inside of the molecule is highly hydrophobic. This indicates that the hydrophobic interaction of benzene rings in each component is an important driving force in the assembly of the commelinin supramolecule.
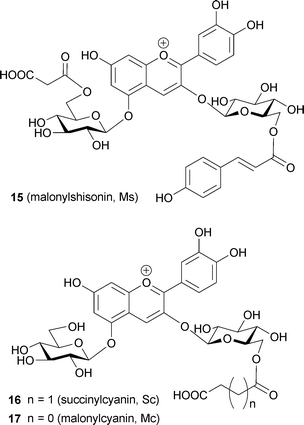
Commelinin-like supramolecules, in which one or more components are replaced, can be reconstituted.17 Indeed, metal-replaced commelinin-like pigments (10–14) have been obtained with Cd2+, Zn2+, Co2+, Ni2+, and Mn2+ (Table 3).17,47 Anthocyanin-replaced commelinin-like pigments have been obtained using awobanin (Aw, 7), malonylshisonin (Ms, 15), succinylcyanin (Sc, 16), and malonylcyanin (Mc, 17).3,15,17 Only Aw-commelinin (18) shows blue color, the others are purple (Table 3). Progress in mass spectrometry has allowed determination of the molecular weight of intact metalloanthocyanins. Previously, all of the available ionization methods, EI, CI, FAB and MALDI, destroyed the supramolecules and yielded only the mass of the monomeric molecular ions. Kondo et al. applied electrospray ionization mass spectrometry (ESI-MS) to commelinin and obtained its exact molecular weight (Fig. 10).49 Commelinin and other metal-replaced commelinins dissolved in EtOH–H2O are multivalent anions due to the presence of malonyl residues in the molecules. Deconvolution treatments have yielded the exact molecular weight of each compound (Table 4). This provides direct evidence for the composition of various metal- or anthocyanin-replaced commelinin-like pigments.49
| Compound | UV-Vis λmax/nm (log ε) | Cotton effect in CD/nm (Δε) |
|---|---|---|
| Commelinin (2) | 646 (5.00), 590 (5.34) | 675 (−160), 588 (+200) |
| Cd-commelinin (10) | 648 (4.99), 592 (5.32) | 680 (−130), 594 (+194) |
| Zn-commelinin (11) | 649 (4.98), 594 (5.31) | 680 (−167), 594 (+209) |
| Co-commelinin (12) | 658 (4.98), 602 (5.29) | 692 (−161), 604 (+191) |
| Ni-commelinin (13) | 654(5.03), 598 (5.39) | 688 (−261), 597 (+264) |
| Mn-commelinin (14) | 652 (4.98), 595 (5.32) | 680 (−133), 594 (+185) |
| Aw-commelinin (18) | 645 (4.98), 591 (5.22) | 670 (−130), 580 (+136) |
| Ms-commelinin (19) | 620 (sh), 569 (5.09) | 640 (−97), 560 (+118) |
| Sc-commelinin (20) | 620 (sh), 565(5.05) | 640 (−54), 560 (+90) |
| Mc-commelinin (21) | 620 (sh), 565 (5.09) | 640 (−68), 560 (+109) |
| Compound | Composition | Molecular weight | |
|---|---|---|---|
| Calcd. | Obsd. | ||
| Commelinin (2) | C402H414O222Mg2 | 8846 | 8849 |
| Cd-commelinin (10) | C402H414O222Cd2 | 9022 | 9023 |
| Zn-commelinin (11) | C402H414O222Zn2 | 8928 | 8931 |
| Co-commelinin (12) | C402H414O222Co2 | 8915 | 8914 |
| Ni-commelinin (13) | C402H414O222Ni2 | 8915 | 8916 |
| Mn-commelinin (14) | C402H414O222Mn2 | 8908 | 8909 |
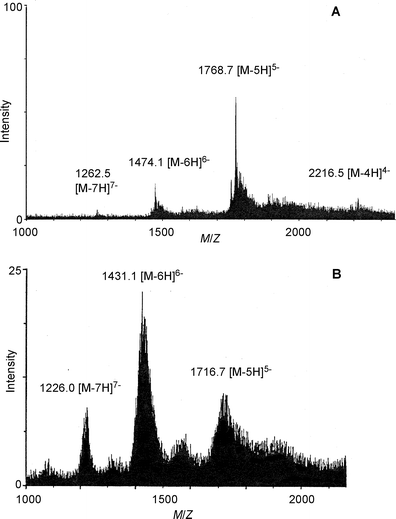 | ||
| Fig. 10 Negative mode ESI-MS spectra of metalloanthocyanins. A: commelinin (2), B: protocyanin (3). | ||
1H NMR analysis of commelinin has been reported by the same group.17 Comparison of the spectra for commelinin (2) and Ms-commelinin (19) using various 2D experiments has allowed the assignment of aromatic protons and other characteristic protons, such as anomeric protons of sugars. The protons in the B-ring of Ma (8) and Fc (9) have been observed individually, and these observations indicate that the C2–C1′ bond of both 8 and 9 in commelinin is fixed and unable to rotate freely. Close molecular stacking causes high-field shifts of aromatic ring signals. Because commelinin is a highly symmetrical molecule, only one set of signals is observed. NOE measurements indicate that the p-coumaroyl residue is folded.
The higher-order structure of commelinin suggested by NMR and CD was proven by the atomic structure obtained by X-ray analysis.3,17 Thus, the metal-complex theory and molecular association mechanism of flower-color development were simultaneously proven.
3.2 Protocyanin
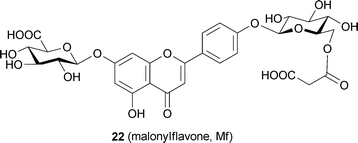
Protocyanin (3), the blue pigment of cornflower petals, has a long history of controversial chemical studies. Its coloration was first studied in 1913,22 and in 1958 Bayer isolated blue pigment from the petals and named it “protocyanin”.33 In the 1960s Hayashi50 and Asen51 also tried to purify the pigment from the petals. All three reported different compositions and metal ions that actually reflected impurities.34,50,51 In 1983 Goto and his group reported the true anthocyanin and flavone components to be succinylcyanin (Sc, 16) and malonylflavone (Mf, 22), respectively; both molecules contained labile acyl moieties.52 They also predicted the gross molecular weight to be ca. 8500 based on ultracentrifugation analysis, and they reported the stoichiometry of 16:22:Fe:Mg to be 6–8 : 6–8 : 1 : 1.3,15,16In 1994 Kondo et al. reconstituted protocyanin (3) by mixing Sc (16), Mf (22), Fe ion, and Mg ion.35 The spectroscopic features of the reconstituted 3 were identical to those of a natural one purified by repeated precipitation and gel-filtration chromatography (Fig. 11).35 They reported that both the Fe ion and Mg ion are essential for reconstituting the protocyanin.35 In the reconstitution experiment, an excess of Fe ion of more than 1/6 eq. gave a completely different blue–black pigment (Fe2-protocyanin, 23), and this pigment showed a single broad peak in the Vis absorption spectrum and a positive Cotton effect by CD (Table 5). The exact molecular weight of 3 was measured as 8511 Da by negative mode ESI-MS analysis,35 which was the same method used with commelinin (Table 6). The same protocyanin (3) was obtained by adding both Fe3+ and Fe2+, which showed a sharp signal at g = 4.2591 at 77 K in the ESR spectrum.53 The Mössbauer spectrum of 57Fe-protocyanin at 82 K revealed split signals typical of high-spin state Fe3+ (s = 2/5).53 These results indicate that Fe in 3 assumes a paramagnetic high-spin Fe3+ state in protocyanin.53
| Compound | UV-Vis λmax/nm (log ε) | Cotton effect in CD/nm (Δε) |
|---|---|---|
| Protocyanin (3) | 676 (4.63), 598 (5.14), 569 (5.21) | 640 (−85.5), 597 (+133) |
| Al,Mg-protocyanin (24) | 604 (5.11), 565 (5.36) | 628 (−150), 597 (+54.4) |
| Ga,Mg-protocyanin (25) | 606 (5.04), 566 (5.29) | 629 (−127), 597 (+42.3) |
| In,Mg-protocyanin (26) | 606 (5.08), 566 (5.32) | 630 (−137), 598 (+45.7) |
| Co,Mg-protocyanin (27) | 675 (4.48), 573 (5.20) | 668 (−106), 584 (+112) |
| Fe,Mn-protocyanin (28) | 678 (4.65), 601 (5.13), 573 (5.20) | 644 (−69.5), 597 (+109) |
| Fe,Zn-protocyanin (29) | 679 (4.56), 601 (5.00), 572 (5.06) | 643 (−85.5), 596 (+129) |
| Fe,Cd-protocyanin (30) | 679 (4.56), 601 (5.00), 572 (5.06) | 642 (−63.1), 598 (+79.8) |
| Fe2-protocyanin (23) | 588 (5.02) | 608 (+73.9), 563 (−101) |
| Compound | Composition | Molecular weight | |
|---|---|---|---|
| Calcd. | Obsd. | ||
| Protocyanin (3) | C366H384O228FeMg | 8511 | 8505 |
| Al,Mg-protocyanin (24) | C366H384O228AlMg | 8482 | 8486 |
| Ga,Mg-protocyanin (25) | C366H384O228GaMg | 8525 | 8523 |
| In,Mg-protocyanin (26) | C366H384O228InMg | 8570 | 8568 |
| Co,Mg-protocyanin (27) | C366H384O228CoMg | 8514 | 8504 |
| Fe,Mn-protocyanin (28) | C366H384O228FeMn | 8542 | 8536 |
| Fe,Zn-protocyanin (29) | C366H384O228FeZn | 8552 | 8548 |
| Fe,Cd-protocyanin (30) | C366H384O228FeCd | 8599 | 8603 |
| Fe2-protocyanin (23) | C366H384O228Fe2 | 8543 | 8539 |
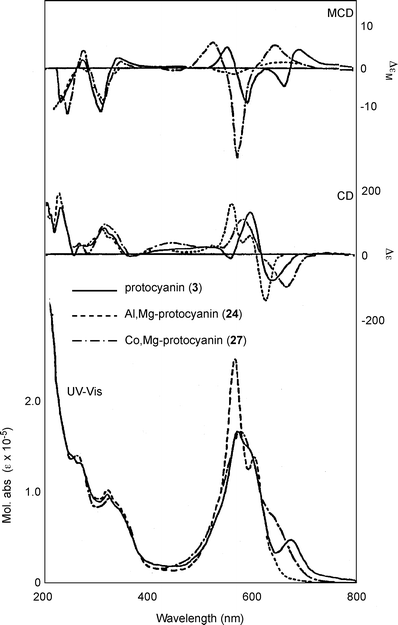 | ||
| Fig. 11 UV-Vis absorption, CD and MCD spectra of protocyanin (3), Al,Mg-protocyanin (24), and Co,Mg-protocyanin (27) in an aqueous solution at a pH of 5.0 (3: 50 µM at 25 °C). | ||
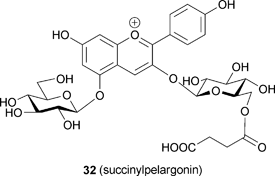
The same research group used an approach similar to that applied to commelinin to reconstitute metal-replaced protocyanin-like pigments (Tables 5 and 6). In the presence of Mg2+, Fe3+ can be replaced with Al3+, Ga3+, In3+ and Co3+ (24–27). In the presence of Fe3+, Mg2+ can be replaced with Mn2+, Zn2+ and Cd2+ (28–30).53 All the metal-replaced pigments give the expected molecular weights by ESI-MS measurements, suggesting that they assume the same self-assembled supramolecular structure (Table 6). However, not all the protocyanin-like pigments show blue color, and only Mg2+-replaced pigments – namely, Fe,Mn-protocyanin (28), Fe,Zn-protocyanin (29) and Fe,Cd-protocyanin (30) – show the same Vis and CD spectra as those of 3 (Table 5). In contrast, Fe3+-replaced protocyanins – namely, Al,Mg-protocyanin (24), Ga,Mg-protocyanin (25) and In,Mg-protocyanin (26) – show a violet color, and their spectra are similar to that of commelinin. Co,Mg-protocyanin (27) shows a blue color similar to that of protocyanin, but its spectra are different. Reconstitution experiments using malonylcyanin (Mc, 17) as the anthocyanin component yielded a protocyanin-like supramolecule (Mc-protocyanin, 31), but the same experiments using succinylpelargonin (Sp, 32) did not. These results demonstrate that the metal ions may coordinate to the 3′,4′-dihydroxyl group of the B-ring of anthocyanin, just as in commelinin (2).
The stacking of each component in protocyanin (3) was determined through sophisticated NMR measurements of Al,Mg-protocyanin (24). Kondo et al. assigned all the aromatic proton signals of 24 and examined the NOE signals between them.53 In commelinin (2), all six Ma residues (8) are equivalent, but in 3 the signals due to the Sc (16) residue, which chelates Al3+ and Mg2+, are different. The NMR and CD data provide several insights: a similar stacking mode for each Sc (16) and Mf (22) component in 3, an anticlockwise self-association of two molecules of 16 and two molecules of 22, and clockwise stacking in co-pigmentation of 16 and 22.53
The metal-replacement protocyanin-like pigment provides reasonable evidence that a ligand-to-metal charge transfer (LMCT) interaction between Sc (16) and the Fe3+ ion (Fig. 12) is involved in the mechanism of blue color development in protocyanin (3).53 The typical difference between commelinin (2) and protocyanin (3) in their Vis absorption spectra is the peak at 675 nm, which disappears in the violet-colored Al,Mg-protocyanin (24). In contrast, Co,Mg-protocyanin (27) shows a peak at 675 nm and displays a blue color; therefore, the peak of λmax around 675 nm causes the blue coloration in 3. MCD measurements of 3 and 27, which respectively contain the paramagnetic ions Fe3+ and Co3+, show two peaks at 676 and 599 nm, while these peaks are missing from the corresponding spectra of 24–26 reconstituted, respectively, with the dimagnetic ions Al, Ga, and In. The blue coloration of protocyanin (3) with the cyanidin chromophore anthocyanin (16) is due to LMCT interactions from anthocyanidin to the Fe3+ ion.
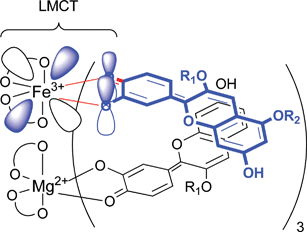 | ||
| Fig. 12 Blue coloration resulting from the LMCT interaction between chromophore of succinylcyanin (16) and Fe3+ in protocyanin (3). | ||
X-ray crystallographic analysis of protocyanin was performed in 2005 (Fig. 13).41 Shiono et al. determined the atomic structure by comparing Fe,Mg,Ca-, Fe,Mg,Ba-, and Fe,Mn,Ba-protocyanin (33–35). In the X-ray structure, protocyanin contained two more metal ions: two Ca2+ ions were found in Fe,Mg,Ca-protocyanin on the C3-axis. Shiono et al. described Ca2+ coordinated to the hydroxyl residue of 4′-glucoside in Mf (22).41 Takeda et al. suggested that those two metal ions stabilize the gross structure of protocyanin.54 In contrast, Kondo et al. reported that Ca2+ ions are not essential for reconstituting Fe,Mg-protocyanin (3); indeed, they did not detect Ca2+ in natural or reconstituted 3.35 Therefore, Ca2+ may stabilize the crystal structure but it appears not to be required for blue color development in protocyanin.
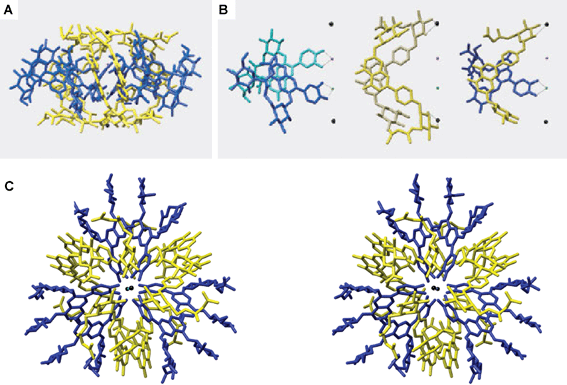 | ||
| Fig. 13 X-ray crystallographic structure of protocyanin (3). Blue, anthocyanin; yellow, flavone glycoside; red spheres, Fe3+; green spheres, Mg2+; black spheres, Ca2+. A: A side view along the pseudo-three-fold axis. B: A side view of the stacking pairs in anthocyanins (left), flavones (middle), and anthocyanin and flavone. C: A stereo-view along the pseudo-three-fold axis. (From M. Shiono et al., Nature, 2005, 436, 791, with permission). | ||
3.3 Other metalloanthocyanins
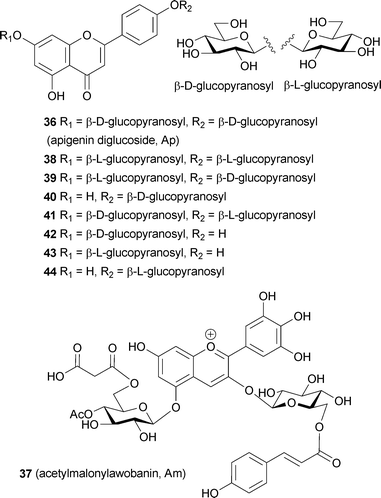
In addition to commelinin (2) and protocyanin (3), several other blue pigments have been determined to be so-called metalloanthocyanins, which can be recognized by their characteristic Vis and CD spectra. In 1994 Takeda et al. purified protodelphin (4) from Salvia patens, which has properties similar to those of commelinin.42 They identified the components of 4 as Ma (8), 7,4′-O-diglucosylapigenin (Ap, 36), and Mg2+. In 2001, Kondo et al. determined the composition to be 6 : 6 : 2 using ESI-MS analysis; a multiply charged molecular ion of 1751.8 m/z ([M − 5H]5−) corresponded to C396H408O222Mg2 (average molecular weight, 8767.95; calculated, 1751.37).43 Mori et al. working with different salvia petals, this time those of Salvia uliginosa, obtained another metalloanthocyanin, cyanosalvianin (5).44 This metalloanthocyanin is composed of acetylmalonylawobanin (Am, 37),55 Ap (36), and Mg2+. The ratio is also the same, 6 : 6 : 2; the molecular weight of reconstituted 5 was determined to be 9014.0 by ESI-MS analysis, which is consistent with the calculated average molecular weight (9008.0) of [C408H414O228Mg2] − 6H.44In metalloanthocyanins, self-association between the anthocyanin and flavone components is chiral, with the stacking occurring in a left-handed manner. In both molecules only the glucosyl residues are chiral; therefore, the chiral molecular stacking should be caused by the D-glucosyl moiety. To confirm this chiral molecular recognition in the formation of metalloanthocyanin, Kondo et al. synthesized L-glucose-substituted flavone analogs of 36 and carried out reconstitution experiments (Fig. 14).43 The flavone component in protodelphin (4) contains two glucosyl moieties; therefore, they synthesized all possible combinations of D- and L-glucosylapigenins, including one lacking a glucosyl residue at either the 7-O- or 4′-O-position (38–44). The enantiomer, apigenin 7,4′-O-di-L-glucoside (38), was found not to yield blue metalloanthocyanin; instead, it displays an unstable purple color. Interestingly, apigenin 7-O-L-glucoside-4′-O-D-glucoside (39) and apigenin 4′-O-D-glucoside (40) produce blue metalloanthocyanins, though their stability is low.43 Using conformational studies, Kondo et al. reported that three molecules of 36 bind at a pivot point through a strong hydrogen bond network involving hydroxyl groups at C-2 and C-3 of 36; these three molecules associate to form an M (minus) helical structure, similar to a propeller with three blades (Fig. 15).43
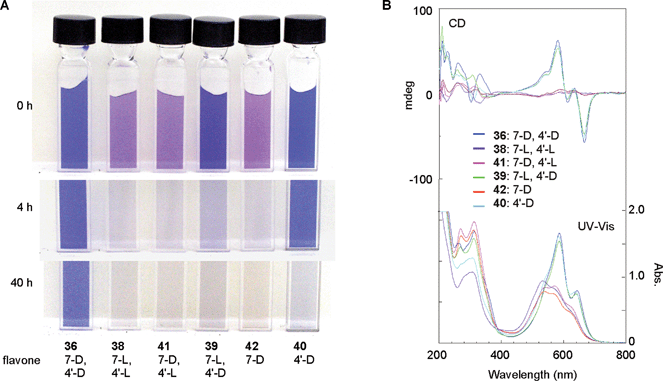 | ||
| Fig. 14 Chiral molecular recognition in the formation of protodelphin-like metalloanthocyanins. (8: 0.5 mM, glucosylapigenin: 0.5 mM, Mg2+: 2.5 mM, pH 6.0, path length 1.0 mm, 25 °C). A: The color stabilities of the various solutions. B: UV-Vis and CD spectra of the solutions. (From T. Kondo et al., Angew. Chem. Int. Ed., 2001, 40, 894–897, with permission). | ||
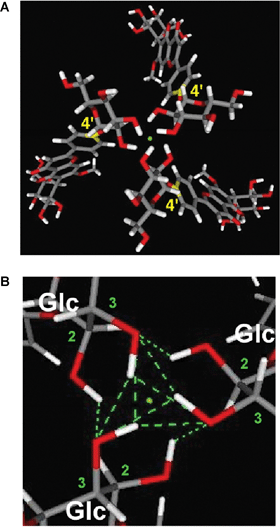 | ||
| Fig. 15 The M-helical chiral stacking arrangement of the three flavone components (apigenin 7,4′-diglucoside, 36) in protodelphin (4) stabilized by a hydrogen-bond network. A: The optimized M-helical conformer containing three molecules of 36 in 4. B: The pivot point found at the upper and the lower center of 4 formed by a hydrogen bond network of the 4′-glucosyl residues of 36. | ||
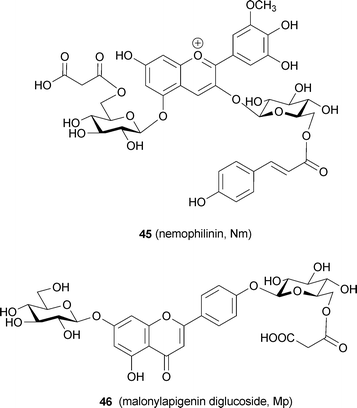
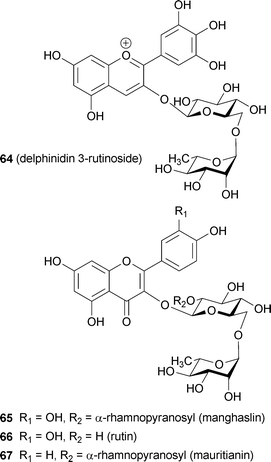
Recently, a blue petal pigment from Nemophila menziesii has been predicted to be a metalloanthocyanin and has been named nemophilin (6, K. Yoshida et al., unpublished). The anthocyanin component is petunidin 3-(6-p-coumaroylglucoside)-5-(6-malonylglucoside) (Nm, 45), the same as in 3′-methylated malonylawobanin. Two flavone components are observed in Nemophila petals; the major one is apigenin 7-glucoside 4′-(6-malonylglucoside) (Mp, 46), and the other is Ap (36). Reconstructing 6 by mixing 45 and 46 with Mg2+ and (separately) with Fe3+ and Mg2+ yields the supramolecules Mg,Mg-nemophilin (47) and Fe,Mg-nemophilin (48), respectively (Fig. 16). Mg,Mg-nemophilin (47) has a Vis spectrum similar to that of commelinin (2), but its color is purplish-blue. Fe,Mg-nemophilin (48) exhibits a blue color, with a λmax at 712 nm, which strongly indicates that the LMCT band is the same as that of protocyanin (3). The Vis and CD spectra of the Nemophila petals show certain characteristics also present in the spectra of both 47 and 48. Therefore, these two metalloanthocyanins may co-exist in the petals (K. Yoshida et al., unpublished).
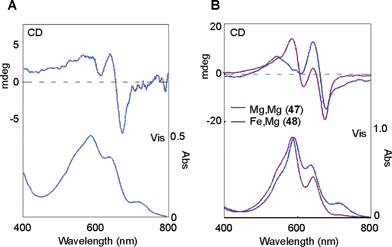 | ||
| Fig. 16 UV-Vis and CD spectra of metalloanthocyanins in Nemophila petals. A: Vis absorption and CD spectra of a blue Nemophila petal. B: Vis absorption and CD spectra of the reconstituted nemophilins (Mg,Mg-nemophilin, 47 and Fe,Mg-nemophilin, 48). | ||
So far, five metalloanthocyanins have been reported (Fig. 5). Each pigment is composed of a structurally slightly different anthocyanin and flavone. In the case of the delphinidin chromophore, Mg2+ seems sufficient for development of the blue color, while in the case of anthocyanins with cyanidin or petunidin chromophores, a 1/6 equivalent of Fe3+ is essential for blue coloration. Anthocyanin with the pelargonidin chromophore never produces a blue metal-complex, and the malvidin chromophore does not do so either (K. Yoshida, Thesis, Nagoya University, 1992). The same experiments with different combinations of anthocyanin and flavone components indicate that there are acceptable and unacceptable combinations (Table 7). Mp (46) seems to be rather tolerant in the formation of metalloanthocyanin. In contrast to this, Mf (22) produces only metalloanthocyanin with Sc (16). Interestingly, 9 cannot accept Fe3+ as a coordinating ion, but 22 needs Fe ion for formation of metalloanthocyanin in the presence of Mg2+. This may be due to some special allocation in the 3-dimensional stacking of the components. In nature, blue coloration caused by metalloanthocyanins is common, and we expect that more of these pigments will be found in the future.
| Anthocyanin | Metal ion | Flavone | ||
|---|---|---|---|---|
| Fc (9) | Mf (22) | Mp (46) | ||
| a Stable blue: the combination gave metalloanthocyanin. Unstable blue: the combination gave metalloanthocyanin but the stability was low. X: the combination did not give metalloanthocyanin. | ||||
| Ma (8) | Mg2+ | Commelinin (2) | X | Stable blue |
| Fe3+, Mg2+ | Unstable blue | X | Stable blue | |
| Sc (16) | Mg2+ | Stable blue | X | X |
| Fe3+, Mg2+ | X | Protocyanin (3) | Stable blue | |
| Nm (45) | Mg2+ | Unstable blue | X | Mg,Mg-nemophilin (47) |
| Fe3+, Mg2+ | Unstable blue | Unstable blue | Fe,Mg-nemophilin (48) | |
4 Fuzzy metal complex pigments
In blue petals, metalloanthocyanins are relatively unusual, nearly all blue flowers resulting from non-stoichiometric metal-complex pigments stabilized by co-pigmentation. These blue pigments are less stable than those of metalloanthocyanins – they are blue only in aqueous solutions, and the color disappears during isolation or crystallization. Therefore, clarification of the mechanism of blue color development by such ‘fuzzy’ metal complex pigments may be more difficult than for metalloanthocyanins. For this purpose, Yoshida et al. have proposed a new methodology, “in vivo natural product chemistry” (Fig. 17).19,20 This is combined with an intact analysis of colored vacuoles and reproduction of the blue color through reconstruction experiments. The analysis involves cell color measurement, vacuolar pH measurement, and quantitative analysis of organic and inorganic components in the cells. Color reproduction means achieving the same color by mixing components under conditions based on the analytical results. Micro-spectrophotometry is employed for color analysis, proton-sensitive microelectrodes are inserted directly into the colored vacuoles for vacuolar pH measurements, and a micro-HPLC system is used for quantitative analysis.19,20 The similarity of the reproduced color to the color of the natural petals is investigated using several spectroscopic methods: Vis absorption, CD, and NMR. In the following discussion, we describe the blue coloration of such fuzzy metal complex pigments (Fig. 18).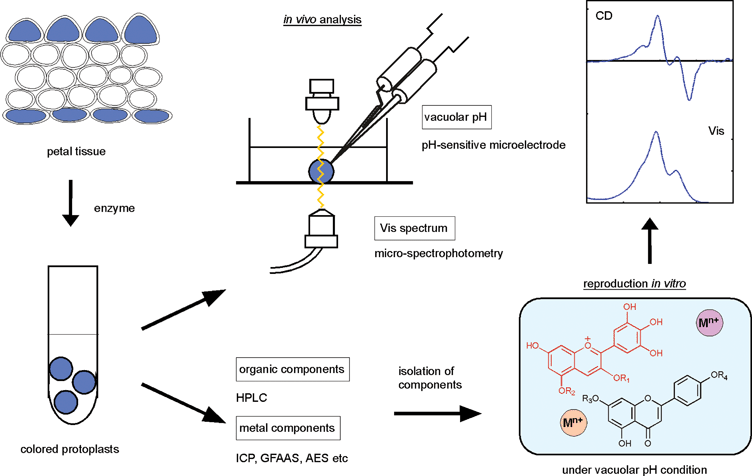 | ||
| Fig. 17 The concept and methodologies of “in vitro natural product chemistry” used to clarify the mechanism of flower color development. | ||
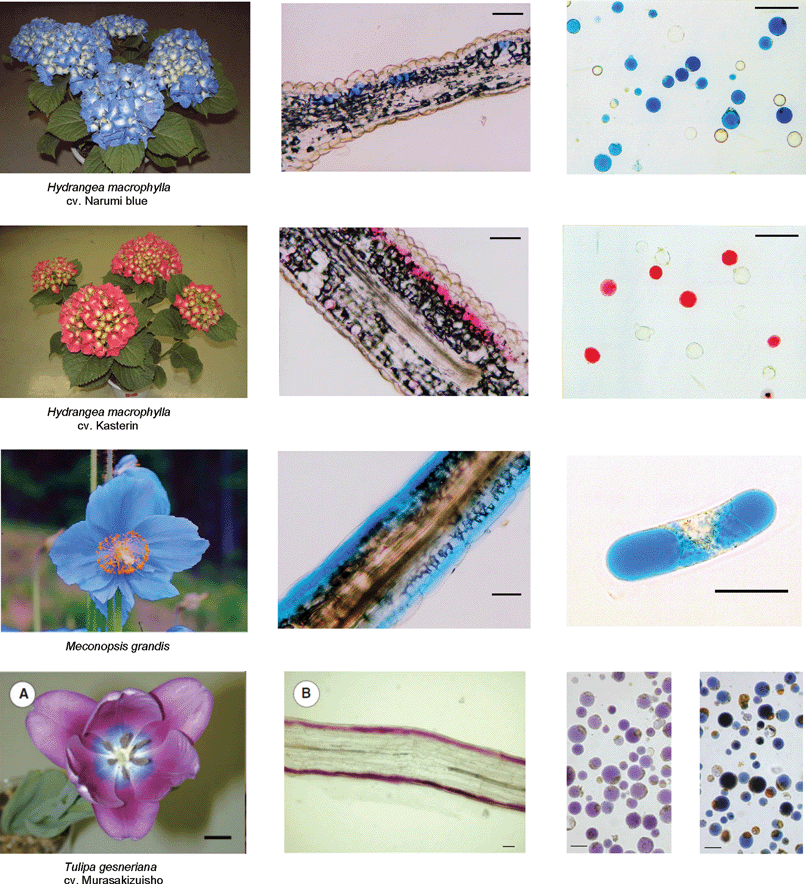 | ||
| Fig. 18 Various blue flowers (left), their transverse sections (middle), and protoplasts obtained by treatment with cellulase and pectinase (right). Scale bars for the transverse sections and protoplasts: 50 µm. (Modified from K. Yoshida et al., Plant Cell Physiol., 2003, 44, 262–268; K. Yoshida et al., Phytochemistry, 2006, 67, 992–998; and Shoji et al., Plant Cell Physiol., 2007, 48, 243–251, with permission). | ||
4.1 Blue color in hydrangea sepals
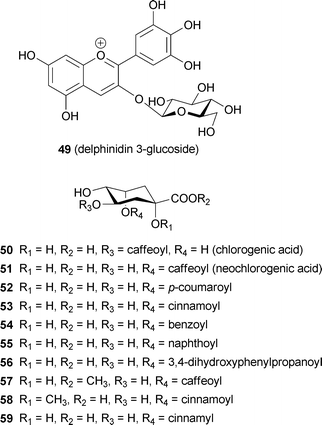
The flowers, or more specifically the petal-like sepals, of Hydrangea macrophylla show various colors, such as red, mauve, purple, violet, and blue.56 The most interesting phenomenon in hydrangea coloration is that all the colors are developed from one simple anthocyanin, delphinidin 3-glucoside (49);57 in general, blue and red flowers contain different anthocyanin chromophores.58 In blue petals, delphinidin derivatives usually exist, while in red petals, the pigment contains either pelargonidin or cyanidin chromophores. The studies on color development in hydrangea sepals have a long history, spanning more than a hundred years. The effect of acidic soils on blue coloration due to Al3+ was reported as early as the first part of the 20th century,59,60 and the existence of acylquinic acids (50–52) as co-pigments was discovered as early as the 1950s.61 From 1985 to 1990, Takeda et al. reported that a stable, blue-colored solution could be obtained by mixing 49, 5-O-acylquinic acid (51, 52), and Al3+.62–64 However, the mystery of how the same components could develop different colors in hydrangea sepals and why the color changed so easily remained unsolved.In hydrangea sepals, the colored cells are located in the second layer; therefore, Yoshida et al. prepared protoplast mixtures, from which they collected and analyzed only colored cells.19,65 Vacuolar pH (pHv) measurements of colored cells illustrated the difference between blue and red cultivars. The pH of blue cells in the blue cultivar was approximately 4.1, significantly higher than that in red cells (pH = 3.3).65 Ito et al. analyzed the composition of anthocyanin (49) and three co-pigments (50–52) by collecting approximately 150 colored cells (Table 8, D. Ito and K. Yoshida, unpublished). The results indicated that the molar ratio of 5-O-acylquinic acids (51, 52) to 49 was much higher in the blue cells than that in the red cells. The amount of Al3+ was the same; in blue cells, the molar equivalent of Al3+ to 49 was greater than 1 eq., while the amount in red cells was lower than 0.1 eq. These results were significantly different from data obtained from whole sepal tissue.66 This discrepancy emphasizes the importance of isolating and analyzing only colored cells in flower color studies. To measure the composition in colored cells with greater sensitivity, Yoshida et al. developed a single-cell analysis method. Monitoring the cell color by micro-spectrophotometry, a single cell was collected, and then the organic or inorganic components were quantified. These results showed an obvious correlation between cell color ‘blueing’ and increase in the levels of 5-O-acylquinic acid (51, 52) and Al3+.20
| Cultivar | Blue (cv. Narumi blue) | Red (cv. Kasterin) |
|---|---|---|
| a Cell color and flavonoid content were measured simultaneously using ca. 50 cells by HPLC. The vacuolar pH (pHv) was recorded using one colored cell by microelectrode, and Al3+ content was measured using ca. 20 cells by graphite furnace atomic absorption spectroscopy. pHv and Al3+ measurements were carried out in different experimental runs. b Content of co-pigment was calculated as molar equivalents relative to the anthocyanin (49). | ||
| λmax | 586 ± 3.6 nm (n = 21) | 539 ± 4.1 nm (n = 10) |
| Anthocyanin | ||
| 49 | 12.9 ± 9.1 mM | 15.4 ± 16 mM |
| Co-pigmentb | ||
| 50 | 10.4 equiv. | 18.1 equiv. |
| 51 | 8.3 equiv. | 2.4 equiv. |
| 52 | 4.5 equiv. | 1.2 equiv. |
| Al3+ | 14.8 ± 8.6 nM (n = 11) | 0.4 ± 0.4 nM (n = 8) |
| pHv | 3.6 ± 0.3 (n = 16) | 3.3 ± 0.2 (n = 21) |
Kondo et al. synthesized various co-pigment analogs and carried out reconstruction studies of hydrangea colors.67,68 To obtain a stable blue color, addition of 3 eq. of 5-O-acylquinic acid (51 or 52) to 49 turned out to be essential in the presence of 1 eq. of Al3+ at pH 4.0. The regioisomer 3-O-caffeoylquinic acid (50) did not give a stable blue solution, but instead produced a blue precipitate, which was composed of only 49 and Al3+. Kondo et al. described that Al3+ coordinated to 49 is blue but water-insoluble; the co-pigments, i.e. the 5-O-acylquinic acid derivatives, may solubilize the blue metal complex to give a stable blue solution.67 Reconstruction experiments using unnatural synthetic co-pigments (53–59) revealed the essential structure of the co-pigment for blue coloration in hydrangea (Table 9). The 5-O-ester, 1-OH, and 1-carboxyl groups in the quinic acid parts are essential for the co-pigmentation effect and formation of the water-soluble blue metal-complex pigment.67,68 Integrating all of these results, Kondo et al. proposed a schematic complex model in hydrangea, in which Al3+ coordinates to 49 through the ortho-dihydroxyl group of the B-ring and, simultaneously, the Al3+ may coordinate the oxygen atoms in the co-pigments (Fig. 19). In addition, the aromatic ring of the co-pigment moiety may stack on top of the chromophore of 49 through hydrophobic interactions. This supramolecule is labile and can easily fall apart depending on the concentrations of the components and the pH conditions; thus, the blue color of hydrangea is unstable.
| R1 | R2 | R3 | R4 | Colorb | |
|---|---|---|---|---|---|
| a Co-pigmentation effect was examined by the color and the stability of the obtained solution by mixing 1 mM 49, 3 mM co-pigment and 1 mM Al3+ in buffer at pH 4.0. b VS: very stable blue solution. S: stable blue solution. Un: a blue solution was obtained initially, but after 1 day at rt a small amount of precipitate appeared. X: a blue–black precipitate was quickly produced. | |||||
| 50 | H | H | Caffeoyl | H | X |
| 51 | H | H | H | Caffeoyl | S |
| 52 | H | H | H | p-Coumaroyl | S |
| 53 | H | H | H | Cinnamoyl | S |
| 54 | H | H | H | Benzoyl | Un |
| 55 | H | H | H | Naphthoyl | VS |
| 56 | H | H | H | 3,4-Dihydroxyl phenylpropanoyl | Un |
| 57 | H | CH3 | H | Caffeoyl | X |
| 58 | CH3 | H | H | Cinnamoyl | X |
| 59 | H | H | H | Cinnamyl | X |
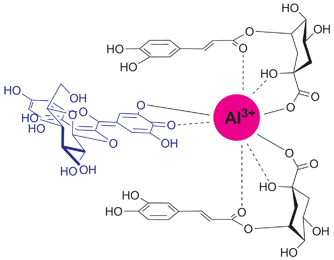 | ||
| Fig. 19 Proposed gross structure of the blue hydrangea pigment. | ||
4.2 Other blue flowers
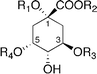
Non-stoichiometric metal-complex pigments similar to those of hydrangea have been reported. The sky-blue petals of Meconopsis grandis contain anthocyanins (60, 61) with a cyanidin chromophore,69,70 as well as flavonol glycosides (62, 63).70 Yoshida et al. reported that the blue petals have λmax values at 606 and 646 nm, and its vacuolar pH is 4.8.71 The concentrations of anthocyanins (60 and 61), flavonols (62 and 63) and Fe in the colored protoplast are 4.7 mM, 15 mM and 3.6 mM, respectively. Mixing 61, 63, and the Fe ion and Mg ion at pH 5.0 gives the same blue color, and the Vis and CD spectra of the resulting compounds are identical to those of the petals (Fig. 20). Yoshida et al. concluded that the blue petal color of M. grandis is due to a new type of metal-complex anthocyanin composed of an anthocyanin with a cyanidin chromophore (60 and 61), two or more equivalents of kaempferol derivatives (62 and 63), 1/6 equivalent of Fe ion, and excess Mg2+.71 They also stated that an excess of Fe ions with respect to anthocyanin yields a different blue–black-colored complex that is identical to that in the protocyanin.71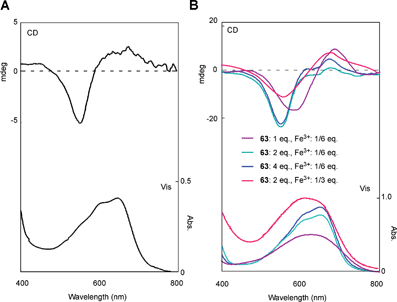 | ||
| Fig. 20 Analysis of the blue pigment in petals of the blue poppy Meconopsis grandis. A: Vis and CD spectra of M. grandis. B: Vis and CD spectra of the reconstructed pigments by mixing 61 (5 mM) and Mg2+ (25 mM) with 63 and Fe3+ (pH 5.0, path length 0.1 mm, and 25 °C). (From K. Yoshida et al., Phytochemistry, 2006, 67, 992–998, with permission). | ||
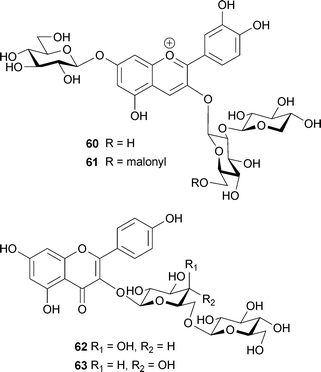
The petals of Tulipa gesneriana cv. Murasakizuisho are mostly purple, but blue near the centre of the flower. Shoji et al. reported that the blue coloration may be caused by a ‘fuzzy’ metal complex pigment.72 The only chemical difference between the purple cells and the blue cells is in the content of Fe3+; other factors, such as vacuolar pH, structure, and amount of anthocyanin (64) and glycosylated flavonols (65–67), are the same (Table 10).72 They reported that mixing anthocyanin and flavonol at pH 5.6 yields a purple color that is the same as that of the distal part of the petal, and that addition of 1 eq. of Fe3+ to the solution changes it to a blue color identical to that of the proximal part.72 In blue coloration of the tulip, the Fe3+ ion is essential, though the chromophore of the anthocyanin is delphinidin.72 Momonoi et al. recently cloned a vacuolar iron transporter gene from tulip petals and showed that the transporter plays a critical role in petal blueing by accumulating iron.73
| Protoplast | Blue (proximal part of petal) | Purple (distal part of petal) |
|---|---|---|
| a Flavonoid content and metal content were measured using a protoplast suspension (1.67 × 104 cells/mL) by HPLC and ICP, respectively. The vacuolar pH (pHv) was recorded using one colored cell by microelectrode. b Content of co-pigment was calculated as molar equivalents relative to the anthocyanin (64). | ||
| λmax | 615 nm | 544 nm |
| Anthocyanin | ||
| 64 | 14.3 ± 0.8 mM | 8.8 ± 1.3 mM |
| Co-pigmentb | ||
| 65 | 0.78 equiv. | 0.91 equiv. |
| 66 | 0.62 equiv. | 0.60 equiv. |
| 67 | 0.62 equiv. | 0.65 equiv. |
| Fe3+ | 9.5 ± 0.8 mM | 0.4 ± 0.1 mM |
| pHv | 5.6 ± 0.3 (n = 8) | 5.5 ± 0.1 (n = 9) |
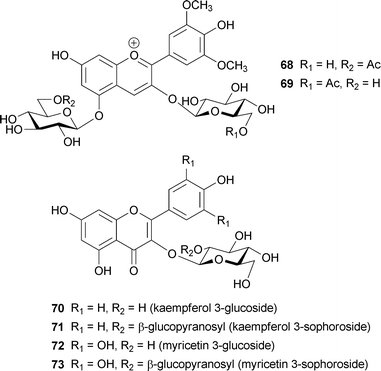
Some researchers have reported blueing mechanisms without any contributions from metal ions, but instead resulting from co-pigmentation by flavonols and flavones. Markham et al. reported that three geranium petals, the bluish-purple petals of Geranium “Johnson's Blue”, the purplish-blue petals of G. pratense, and the bluish-magenta petals of G. sanguineum, contain the same anthocyanins: malvidin 3-O-glucoside-5-O-(6-O-acetylglucoside) (68), with a small amount of malvidin 3-O-(6-O-acetylglucoside)-5-O-glucoside (69).74 The difference between the three colored petals is in the molar ratios of the flavonols, 3-O-glucosyl- and 3-O-sophorosyl-kaempferol and myricetin (70–73): the ratio of flavonol to anthocyanin is lower in the magenta petals than in the bluish petals. They proposed a co-pigment effect of the flavonols, but could not reconstitute the same blue color by adding flavonols (2–4 eq. to anthocyanin) at pH 5.5, the same as that of the petal juice. However, a similar blue color was obtained at pH values of 6.6–6.8; therefore, the authors concluded that the vacuolar pH may be higher than 5.5.74
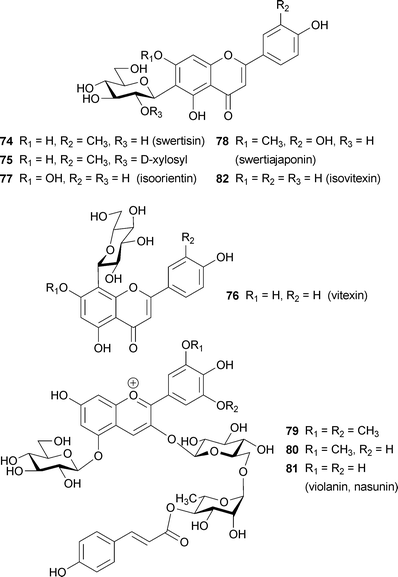
For a long time, the color of blue iris flowers has been attributed to a co-pigmentation effect.75 When C-glycosylflavones, such as swertisin (74), O-xylosylswertisin (75), vitexin (76), isoorientin (77) and swertiajaponin (78), are added to iris anthocyanin, a blue color develops in the absence of metal ions.75 Yabuya et al. reported that Iris ensata contains the 3-O-(4-O-p-coumaroylrhamnosyl-6-O-glucoside)-5-O-glucosides of malvidin (79), petunidin (80), and delphinidin (81), along with isovitexin (82); the delphinidin derivative is known as violanin or nasunin. Addition of 2–10 equiv. of 82 to 0.1 mM solutions of each of the anthocyanins 79–81 yields a blue coloration with a bathochromic shift of λmax of more than 30 nm.76
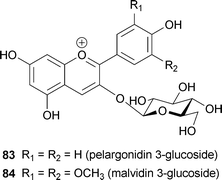
Petals of Anagallis monelli contain 3-glucosides of pelargonidin (83), delphinidin (49) and malvidin (84).77,78 Blue- and red-coloured forms have upper and lower surfaces of the same color, but an intermediate hybrid with violet-and-lilac petals shows different colors; the petals are blue in the adaxial epidermis and red in the abaxial epidermis. The pigment in the blue surface was identified as 84 and that in the red surface as 49; this is in contrast to the standard assumption that methylation of the –OH residue at the B-ring causes a hyperchromic shift.78 The violet-and-lilac petal surface has a mixture of blue and red cells, with the red cells containing a higher level of 49. Furthermore, the upper surface tissue, composed of bluer cells, is more acidic than the lower surface.78 This is an unusual phenomenon considering that the color change of anthocyanins depends on pH, although the authors did not provide any conclusive mechanism. Resolving this question may require single-cell analysis.
5 Anthocyanins with intramolecular stacking
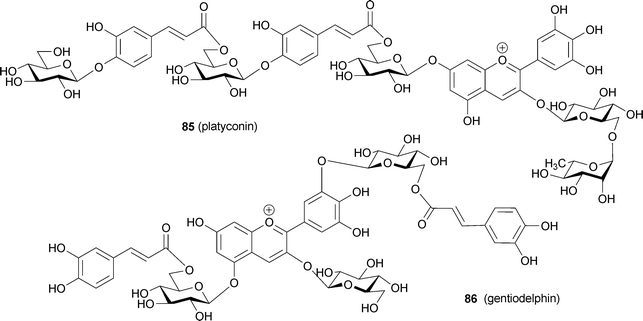
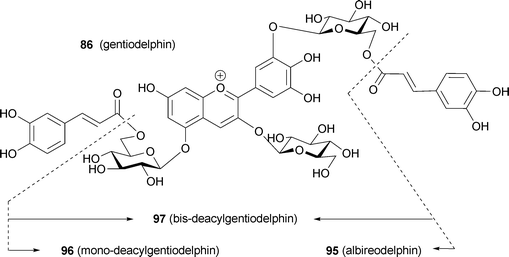
Polyacylated anthocyanins, which are anthocyanins with two or more aromatic acyl residues, such as p-coumaroyl, caffeoyl, feruloyl, and p-hydroxybenzoyl moieties, are found mainly in bluish flower petals (Fig. 21).1,3,7,8,15,16 Therefore, polyacylated anthocyanins have been thought to possess some kind of special mechanism for blue color development. In 1971 platyconin (85) from the bluish-purple Chinese bellflower Platycodon grandiflorum, was described,79 and the first complete structure of a polyacylated anthocyanin, that of gentiodelphin (86) from blue Gentiana makinoi, was published in 1982.80 Until 1992, Goto and Kondo published many structures of polyacylated anthocyanins,81–87 and they proposed intramolecular sandwich-type stacking of aromatic acyl residues onto the anthocyanidin chromophore in order to account for the stability and blue color.3,15,16 Yoshida et al. reported evidence for intramolecular stacking in gentiodelphin; they observed that a caffeoyl residue in the B-ring stacks on top of the anthocyanidin nucleus, even under strongly acidic conditions.88 So far, hundreds of polyacylated anthocyanins have been identified,5 but no X-ray structures have been solved because of the difficulties in crystallizing these molecules. Therefore, very little direct chemical structural evidence exists to explain the unique characteristics of polyacylated anthocyanins. In the following discussion, the cause of petal color change from red to blue during flower opening of the morning glory is described. We then mention recently reported characteristics of polyacylated anthocyanins in blue flower color development and newly identified anthocyanins covalently linked to flavonoids.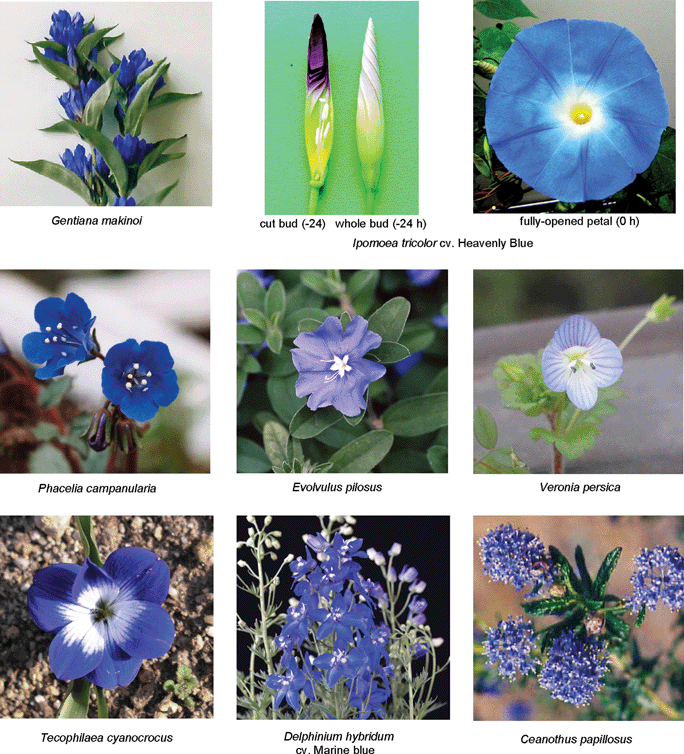 | ||
| Fig. 21 Various blue flowers containing polyacylated anthocyanins. | ||
5.1 Blue morning glories
Petals of the blue morning glory (Ipomoea tricolor cv. Heavenly Blue) contain only one tricaffeoylated anthocyanin, the heavenly blue anthocyanin (87).83 Since the chromophore of 87 is peonidin, blueing due to metal complexation is impossible. The petal color of the morning glory changes from red to blue during the flower-opening period, leading Asen et al. to suggest the cause to be a pH increase in the cell sap.39 Yoshida et al. reported that the anthocyanin component does not change before or after petal blooming. They measured the vacuolar pH (pHv) of surface-colored cells directly using a pH-sensitive microelectrode, and revealed that the pHv of red cells at the bud stage is 6.6 and that of blue cells at the fully-opened stage is 7.7 (Fig. 22).18 Such a high vacuolar pH lies far outside the values known to occur commonly in plants, but this nevertheless provides solid evidence for the pH theory. Interestingly, the pHv increase is observed only in the colored epidermal cells, and not in the inner colorless parenchyma cells; in the latter cells, the pHv remains constant at approximately 6.0.18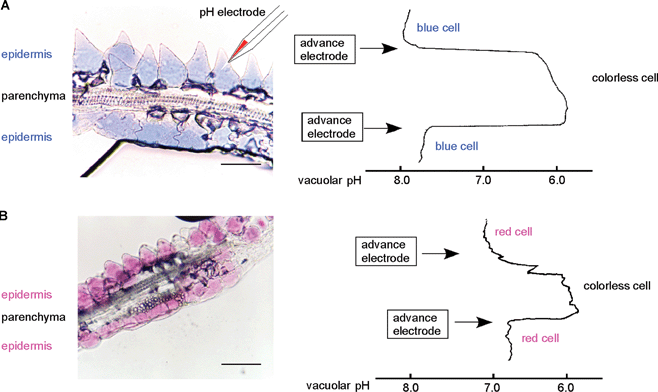 | ||
| Fig. 22 A transverse section of a morning glory petal (I. tricolor cv. Heavenly Blue) and the corresponding profiles of vacuolar pH measurement. A: A fully-opened blue morning glory petal and its vacuolar pH values. B: A red bud-petal and its vacuolar pH values. Bars: 50 µm. | ||
In 2000 a similar pHv increase was reported by Fukada et al. in Japanese blue morning glory petals.89 The group cloned the InNHX1 gene responsible for this pHv increase; it encodes an Na+/H+ antiporter gene.90 However, NHX1 has also been reported to be a salt-tolerance-related gene.91 Thus the role of NHX1 in blue morning glory petals is controversial. In I. tricolor petals, expression of the ItNHX1 protein dramatically increases at the last stage of flower opening, with a simultaneous increase in the activity of both of the proton pumps in the vacuolar membrane, namely V-ATPase and V-PPase.92 Very recently, Yoshida et al. revealed that in colored cells, the K+ ion content increases with increasing pHv and ItNHX1 activation, though Na+ remains undetectable throughout the flowering process.93 Thus, they proposed that ItNHX1 exchanges K+ with H+, but not Na+ with H+, in order to accumulate an ionic osmoticum in the vacuole, which is followed by cell expansion, leading to the full opening of petals with a characteristic blue color (Fig. 23).
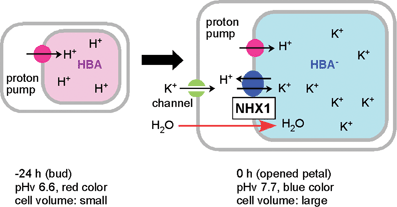 | ||
| Fig. 23 A proposed mechanism for the color change in morning glory petal cells during the flower-opening period. At the bud stage, vacuolar pH is maintained at 6.6 by proton pumps. At the flowering stage, NHX1 is expressed and exchanges H+ for K+, increasing the vacuolar pH to 7.7. The accumulated K+ causes an osmotic imbalance, which allows the cells to incorporate water and expand, facilitating the opening of the flower. | ||
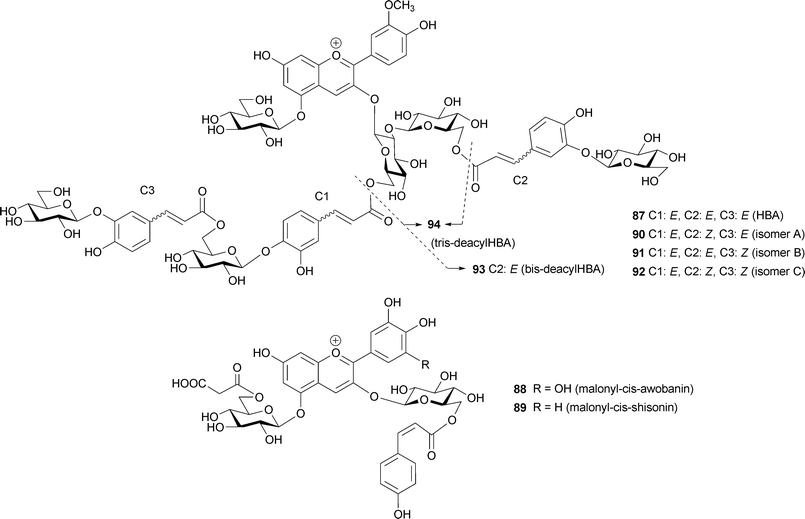
In monoacylated anthocyanins, acyl residues with a Z-configuration at the α,β-double bond, i.e. malonyl-cis-awobanin (88)94 and malonyl-cis-shisonin (89),95 are found in natural plant tissues and are also easily obtained by UV irradiation of the corresponding E-isomers (8, 15).96 However, in polyacylated anthocyanins, neither isolation nor detection of pigments with Z-configured cinnamoyl derivatives has been reported.3,5 Yoshida et al. proposed a mechanism for this prevention of E-to-Z isomerization of polyacylated anthocyanins, gentiodelphin (86) and HBA (87) involving intramolecular stacking.96–98 In strongly acidic methanol solutions, HBA (87) gives three isomerized compounds (90–92) by UV irradiation, and their structures have been determined: two outer caffeoyl residues (C2 and C3) assume the Z-configuration, but the innermost caffeoyl moiety (C1) does not appear to isomerize.98 Furthermore, at the physiological pH of blue petals (pH 7.5), no isomerization reaction occurs, even under strong UV irradiation.98 The stability of 87 under UV irradiation has also been studied. It is most stable in aqueous solutions at pH 7.5, and it decomposes most rapidly in acidic methanol solutions (Fig. 24); the results run counter to the general understanding that anthocyanins are extremely stable under strongly acidic conditions, but unstable in weakly acidic, neutral, or alkaline conditions.98 At pH 7.5, 87 emits strong fluorescence following excitation with UV-B, but neither 87 in acidic conditions, nor the deacylated pigments 93 and 94 at pH 7.5, emit such fluorescence (Fig. 25).98 Intramolecular stacking of caffeoyl residues onto the chromophore of HBA (87) may be stronger under physiological conditions (pH 7.5) than under acidic conditions, thus preventing the E-to-Z isomerization reaction. The light energy absorbed by the caffeoyl residues of 87 may be transferred to the anthocyanidin nucleus, which emits red fluorescence and quenches the light energy.98 Deacylated HBAs, bis-deacyl HBA (93) and tris-deacyl HBA (94) in aqueous solution at pH 7.5 exhibit very little fluorescence.98 This mechanism may function as an effective photo-resistant system in flower petals under strong sunlight.99
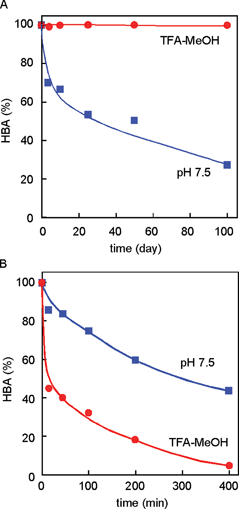 | ||
| Fig. 24 Stability of HBA (87) in the dark (A) and under UV-B irradiation (B) (87: 50 µM, 25 °C). | ||
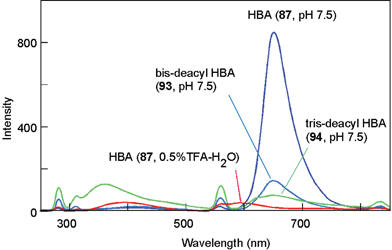 | ||
| Fig. 25 Fluorescence spectra of HBA (87), bis-deacyl HBA (93), and tris-deacyl HBA (94) in buffered solutions with a pH of 7.5, as well as HBA in acidified water irradiated with UV-B at 280 nm (pigment concentration: 50 µM, 25 °C). | ||
5.2 Other polyacylated anthocyanins
Until 1992, degradation reactions were sometimes used for unambiguous structural determination of complex polyacylated anthocyanins. Now, however, connections between sugars and acyl moieties can be determined using various 2D-NMR experiments, such as HMBC and HMQC, using an NMR instrument with a high-field magnet.5,86 In 2002, a review by Honda et al. contained more than 100 structural types of polyacylated anthocyanin.100 Several compounds were isolated from blue petals, and some new observations on the mechanism of stabilization of blue colors by hydrophobic stacking were obtained.Yoshida et al. reported that in gentiodelphin (86), the two caffeoyl residues make different contributions to the blue color.101 Blue coloration experiments using mono-deacylated pigments (95, 96) prepared from 86 revealed that 96, which has an acyl moiety at the B-ring, is more stable than 95 and shows a bluer color in the same neutral buffered solution; in 95, the acyl moiety is attached to the 5-OH.101 However, 95 is more stable under the same conditions than the bis-deacyl pigment (97). They carried out computer-assisted molecular modeling studies, and explained that the gt conformation of the C5–C6 bond in the sugar in the B-ring may contribute to the stacking of the caffeoyl acyl residue onto the delphinidin chromophore (Fig. 26).101
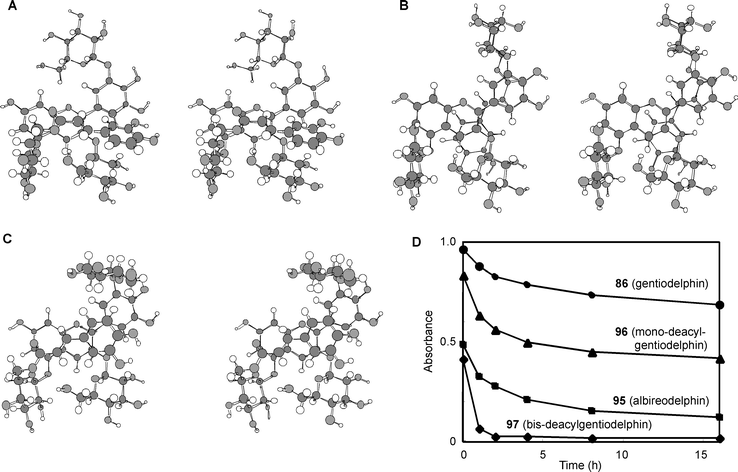 | ||
| Fig. 26 The optimized conformation of mono-deacylated gentiodelphins (95 and 96) by molecular modeling and their stability. A: The optimized conformation of 95 in acidic water. B: The optimized conformation of 96 in acidic water. C: The optimized conformation of 96 in neutral water. D: Stability of 86, 95–97 in buffered solution (pigment concentration: 50 µM, pH 6.0, 25 °C, path length 10 mm). (From K. Yoshida et al., Phytochemistry, 2000, 54, 85–92, with permission). | ||
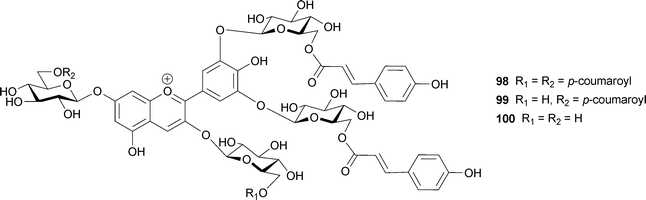
Similar phenomena, namely that aromatic acyl residues in the B-ring may stack more strongly than those in the A-ring, have been observed in anthocyanins (98–100) isolated from blue Dianella nigra berries.102 In a tetraacylated pigment (98), signals of p-coumaroyl residues attached to the 3′- and 5′-O-sugars were observed upfield of signals of residues attached to the 7-O-sugar, followed by signals due to residues attached to the 3-O-sugar. Thus, they concluded that the effectiveness of the intramolecular stacking can be ranked in the order: 3′,5′-GC > 7-GC > 3-GC.102 The author reported that 98 is insoluble in neutral aqueous solutions, but the triacylated pigment (99) is water-soluble and displays a blue color in buffered solutions above pH 4.0. They concluded that the blue color is developed by a combination of blue particles (aggregates of 98) and the blue soluble pigment (99).102
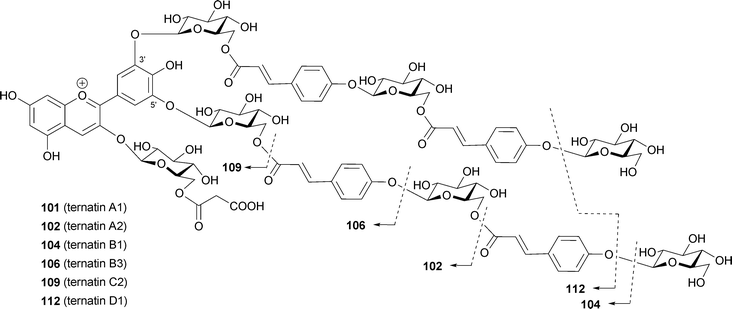
Blue flowers of the butterfly pea Cliteria ternatea are used as a very stable blue food colorant in south-east Asia. Ternatins consist of 15 kinds of malonylated pigments, 14 of which ternatin A1–3 (101–103), B1–4 (104–107), C1–4 (108–111) and D1–3 (112–114) are acylated with p-coumaroyl residue(s).86,103–108 In ternatins, the 3′- and 5′-OH on the B-ring are substituted with a series of chains with alternating D-glucosyl and p-coumaroyl units; each ternatin differs in the number of its sugar and acyl residues. The largest compound among them is ternatin A1 (101)106 with four p-coumaroyl residues; ternatin B1 (104)86 and D1 (112)104 also contain four aromatic acyl residues. The number of p-coumaroyl residues affects on its stability in neutral aqueous solution. Terahara et al. reported that ternatins may assume an intramolecular, sandwich-type stacking conformation like that of gentiodelphin (86), though the group carried out all of their NMR experiments in strongly acidic DMSO-d6.103
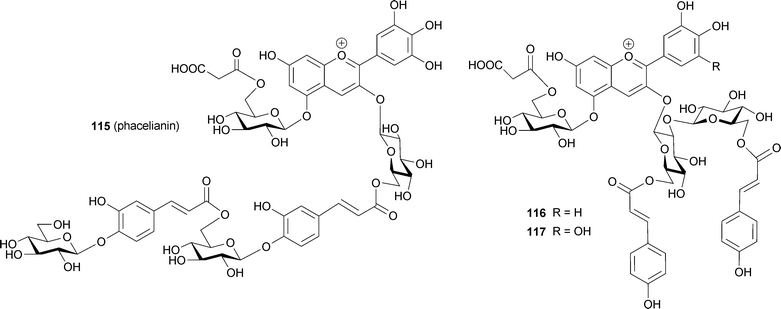
Since the above-mentioned intramolecular stacking of aromatic acyl moieties onto the anthocyanidin chromophore occurs from both sides of the anthocyanidin nucleus, direct interactions between chromophores, or self-association, was previously thought not to exist. Polyacylated anthocyanins reported earlier (85–114) did not show any exciton-type Cotton effect in the visible region by CD.80–87 Recently, however, some polyacylated anthocyanins that exhibit a strong exciton-type Cotton effect in their CD spectra have been found (Fig. 27). Mori et al. reported that phacelianin (115) from blue petals of Phacelia campanularia contains a delphinidin chromophore, and that the 3-OH in 115 is substituted with a glucosyl-caffeoyl-glucosyl-caffeoyl-glucosyl chain and the 5-OH is malonylglucosylated.109 In buffered aqueous solutions at weakly acidic pH, 115 gives the same Vis and CD spectra as the petals, but most of the 115 precipitates within 24 h.109 Addition of small amounts (<0.02 eq.) of Al3+ or Fe3+ to phacelianin improves the stability of the blue solution; therefore, in Phacelia petals, small amounts of trivalent metal ions may stabilize 115 to produce anticlockwise-stacked anthocyanidin chromophores that stack intramolecularly onto caffeoyl residues.109
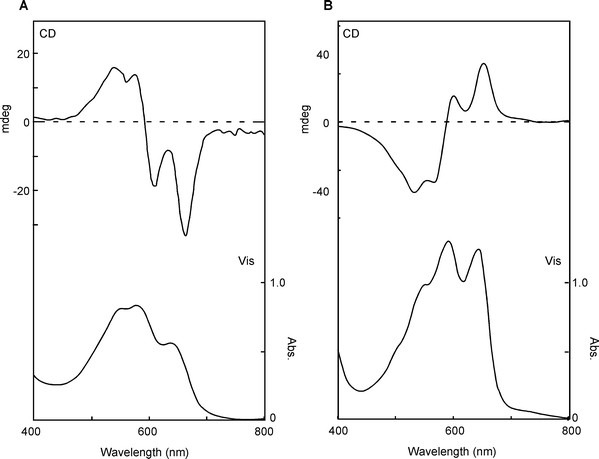 | ||
| Fig. 27 Vis and CD spectra of intact blue petals containing polyacylated anthocyanins. A: P. campanularia. B: T. cyanocrocus. | ||
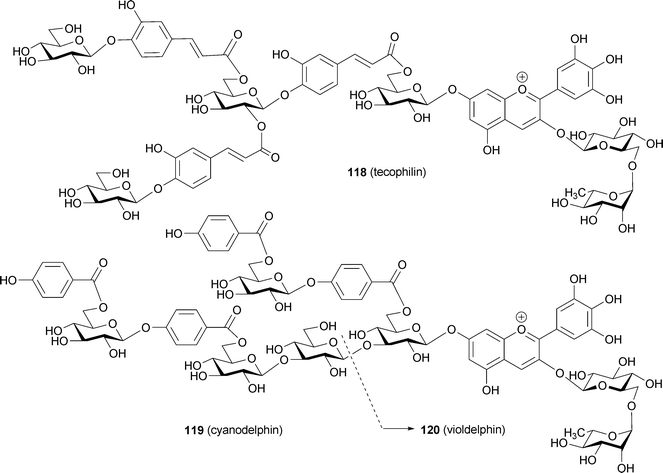
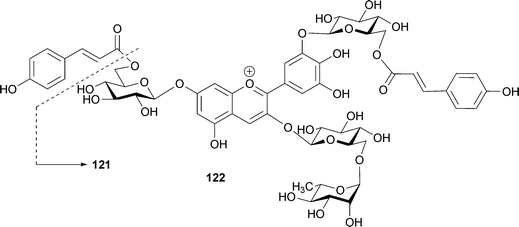
Measurement of the Vis and CD spectra of intact petals is a powerful tool for screening these phenomena.19,110 Using this method, Yoshida and Mori found some polyacylated anthocyanins that may have intra- and intermolecular stacking that plays a role in blue coloration (Fig. 28). Blue petals of Evolvulus pilosus contain the same molecule (115) as P. campanularia,109 and the two petals show similar negative CD spectra, but they differ in color.109 This difference in color may be due to differences in pHv or metal ions. Purplish-blue petals of Ajuga reptans contains two di-acylated anthocyanins (116, 117).111 The pigment 117 was also found in blue petals of Veronica persica (M. Mori, Thesis, Nagoya University, 2005), therefore, the differences between purple A. reptans and blue V. persica may be caused by vacuolar conditions. In contrast to these blue flowers, the blue petals of Tecophilaea cyanocrocus and blue Delphinium hybridum cv. Marine Blue show a positive Cotton effect. Mori et al. isolated a tricaffeoyl anthocyanin, tecophilin (118), from the petals of T. cyanocrocus and determined that the structure contains an additional glucosylcaffeoyl residue attached to platyconin (85, M. Mori and K. Yoshida, unpublished). In the blue cells of T. cyanocrocus, no significant metal ions have been detected; thus, the color development of the petal may not involve metal ions. Kondo et al. isolated cyanodelphin (119)87 from blue delphinium petals and violdelphin (120)85 from violet delphinium petals cv. Black night. The former contains four p-hydroxybenzoic acid residues, and the latter has two. In the same pH-buffered solution, 120 displays a slightly bluer color than 119. Therefore, something else must be responsible for the development of blue-colored petal cells. Microscopic observations revealed blue particles in blue delphinium petal cells. Thus, a similar mechanism to that observed in Dianella berries102 may be responsible for blue color development in this case.
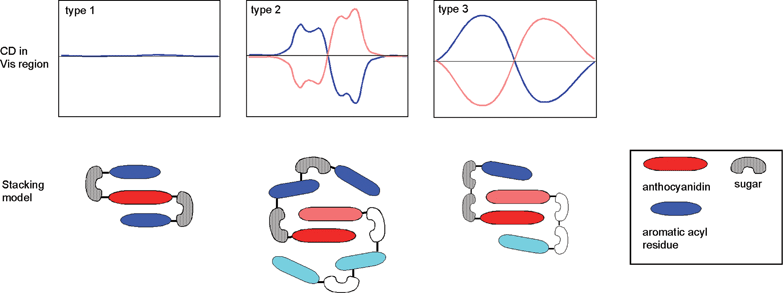 | ||
| Fig. 28 Clarification of intra- and inter-molecular stacking proposed by CD in the visible region. Type 1: Intra-molecular sandwich-type stacking, as found in gentiodelphin (86), HBA (87), and ternatins (101 and 104). Type 2: Pigments with intra- and inter-molecular stacking, as found in phacelianin (115); cyanodelphin (119); and tecophilin (118). Type 3: Nested inter-molecular stacking, as found in alatanin C (126) and the Eichhorinia anthocyanins (123 and 124). | ||
In petals of Ceanothus papillosus, Bloor reported the existence of one monoacylated (121) and one diacylated (122) anthocyanin.112122 alone does not exhibit a blue color at pH 5.5, but addition of eight equivalents of kaempferol 3-glycoside causes a blue color similar to that of the petals. Therefore, the investigators concluded that both intramolecular stacking of the p-coumaroyl acyl residues and co-pigmentation of the special flavonol are essential for blue coloration.112
5.3 Anthocyanins covalently linked to another flavonoid
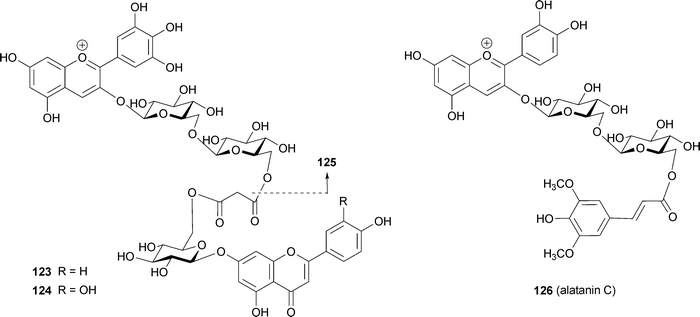
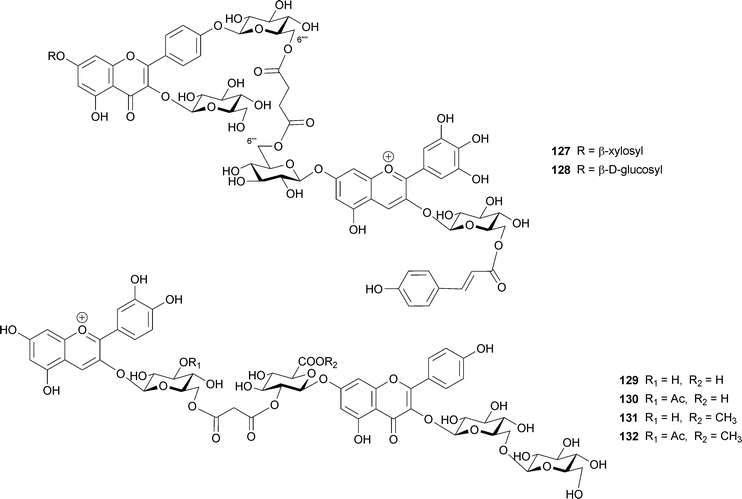
In 1989 Strack and his group isolated from orchids a pigment presumed to be an oxalic diester of cyanin and keampferol 7-O-glucoside.113 Based on the isolation of malonylated anthocyanins and flavones from lupin, Takeda et al. predicted the existence of a similar pigment that forms a covalent bond, producing a diester.114 The first evidence for this was reported by Toki et al. in 1994; the group isolated a malonic diester of delphinidin 3-O-gentiobioside and apigenin 7-O-glucoside at the 6‴-position of the anthocyanin and 6′-position of the flavone (123) from blue-purple flowers of Eichhorinia crassipes.115 The same authors isolated a similar pigment (124) from the same flower as a minor component.116123 and 124 showed a bathochromic shift (λmax 548 nm) even under strongly acidic conditions (0.1% HCl–MeOH), relative to the deacylated pigment delphinidin 3-O-gentiobioside (125, λmax 538 nm).115,116123 and 124 exhibited a negative Cotton effect in their CD spectra at 535 nm and 537 nm, respectively, the authors erroneously supposing that the Cotton effect indicates intramolecular co-pigmentation between the chromophores of the anthocyanin and the flavone. In fact, the negative Cotton effect in 123 and 124 indicates the existence of self-association of anthocyanin chromophores, similar to the cases of phacelianin (115)109 and tecophilin (118). In the event of stacking between two chromophores with λmax values of 548 nm and 300 nm, corresponding respectively to the anthocyanin chromophore and the flavone chromophore, no Cotton effect would be observed, as in the cases of HBA (87) and gentiodelphin (86).15,88 Figueiredo et al. reported that the stability of such a covalent-linked anthocyanin with flavone might be due to similar intramolecular stacking with aromatic rings of flavone like polyacylated anthocyanins.117 Thus, these pigments may assume a nested, intra- and intermolecular stacking conformation, similar to that proposed for the yam anthocyanin, alatanin C (126) (Fig. 28).118Other covalently-linked pigments (127, 128) have been isolated from blue flowers of Agapanthus praecox.119 Their structure consists of a succinic diester of delphinidin 3-O-(6-O-p-coumaroylglucoside)-7-O-glucoside and kaempferol 3,4′-O-diglucoside-7-O-xyloside or kaempferol 3,7,4′-O-triglucoside with the 6‴-OH of the anthocyanin part and the 6⁗-OH of the flavone part.119 The authors describing this work did not offer a mechanism to explain the blue coloration, but the 1H NMR spectrum shows that the signals due to H-4, H-6, and H-8 of the anthocyanin are shifted toward higher field, indicating the presence of an intramolecular stacking conformation.
Four similar pigments (129–132) have been isolated from pale-purple chive flowers, Allium schoenoprasum.120 These include the malonic diesters of cyanidin 3-glucoside or cyanidin 3-(3-acetylglucoside) and kaempferol 3-sophoroside-7-glucosiduronic acid or kaempferol 3-sophoroside-7-methylglucosiduronic acid; two methyl esters might be artifacts during isolation. The anthocyanin and flavonol parts are linked at the 6′-OH of the glucosyl residue of anthocyanin and the 2′-OH of the 7-glucosyl residue of the flavonol.120 A bathochromic shift (16–18 nm) and a high-field shift of proton signals in NMR analysis are observed, relative to the corresponding data for the anthocyanin not linked to the flavonol. How these covalently linked pigments are biosynthesized, and how their intra- and intermolecular stacking structures lead to their blue color, remains to be studied.
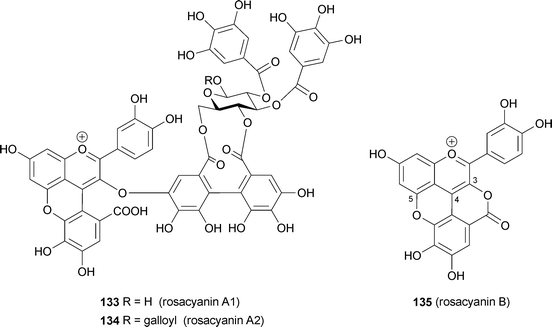
From petals of the so-called traditional blue rose, Rosa hybrida cv. M'me. Violet, Fukui et al. isolated rosacyanin A1 and A2 (133, 134), in which anthocyanins are covalently linked to an ellagitannin moiety.121 The same group reported isolation of a purple pigment, rosacyanin B (135), from the same petals in 2002.122 The pigment 135 is a C-4- and O-5-substituted cyanidin, with gallate and O-3 forming a lactone ring with the gallic acid residue. 133 and 134 have similar substituted cyanidin chromophores; a galloyl group is linked to C-4 and O-5 and ellagitannin is connected to C-3 of the cyanidin nucleus. 133 and 134 show blue color in an HCl–MeOH solution (λmax 585 nm), and did not show decoloration in weakly acidic to neutral aqueous solutions. Both the 4- and 5-substitution may provide greater resistance to hydration. The 3D-conformations of rosacyanins remain unknown, but these pigments may be among the molecules targeted in efforts to breed a blue rose.
6 Molecular breeding
Roses, carnations, and chrysanthemums are the three major cut flowers. However, none of the three has blue flowers; therefore, to find blue-petalled varieties of these plants has been one of the great dreams of breeders.123,124 As described in the previous sections, true blue-colored flower colors are developed through various mechanisms, but polyacylated anthocyanins with delphidin chromophores are considered to be the most promising options in these flowers by breeders. Analysis of petal anthocyanins and the genetic background of anthocyanin biosynthesis demonstrates that the above three species lack F3′,5′-hydroxylase,125,126 which is a member of the P450 oxidase family and which catalyzes the hydroxylation reaction at the 3′- and 5′-OH of the B-ring in dihydrokaempferol to give dihydromyricetin.4,127–129 Thus, petals of these three flowers contain only cyanidin and/or pelargonidin chromophores. Furthermore, petals of these species accumulate relatively simple anthocyanins without aromatic acyl residues. These results indicate that a traditional cross-breeding method would not yield blue roses, carnations, or chrysanthemums. By 1990, cloning of the anthocyanin synthetic genes was nearly completed, and all the structures of the enzymes involved in the early part of anthocyanin biosynthesis (from chalcone to anthocyanidin) had been solved (Fig. 29).4,127,129 Since that time, several genes coding for glycosyltransferases and acyltransferases in flower petals have been cloned. Transcriptional factors that affect anthocyanin biosynthesis have also been reported. Today, we have several new flowers with a bluish hue; all of these are transgenic plants. In this section, genes contributing to petal blueing are described.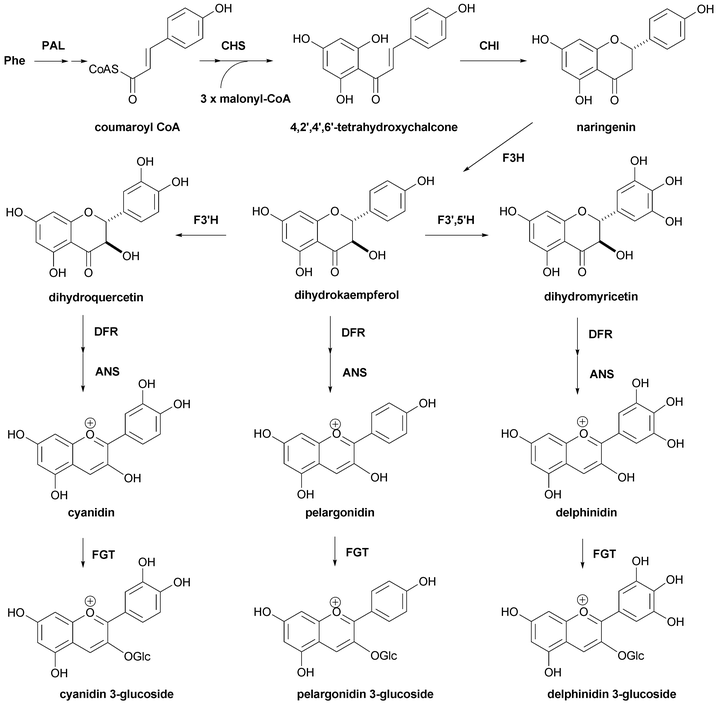 | ||
| Fig. 29 The biosynthetic pathway for the formation of the anthocyanidin 3-glucoside derived from phenylalanine. | ||
6.1 Genes relating to blue coloration of petals
The early part of anthocyanin biosynthesis, from chalcone to anthocyanidin 3-glucoside, is well conserved in higher plants and has been studied in detail.4,127–129 However, after anthocyanidin 3-glucoside, various glycosylation and acylation patterns exist in nature and a wide variety of structural diversity is found in flower petals, depending on the plant species and cultivars. Therefore, the pathway is still not completely elucidated. Even in this situation, several key enzymes responsible for blue coloration have been indicated. One of the most important is F3′,5′-hydroxylase. This gene has been cloned from several plants,130–133 and introduction of this gene into red-colored petals produces a blue hue.125,131,132,134Found in Gentiana and Clitoria flowers, polyacylation of the glucose in the B-ring efficiently produces blue during flower color development. Thus, the enzymes that catalyze the glucosylation reaction at the 3′- and/or 5′-OH of the B-ring and the acylation reaction at the glucose attached to the B-ring have attracted significant attention. The former enzyme, UDP-glucose:anthocyanin 3′-O-glucosyltransferase, has been cloned from a Gentiana flower by Fukuchi-Mizutani et al. and the cDNA has been co-expressed with a 5-GT gene to give a 3,5,3′-triglucosyldelphinidin.135 UDP-glucose:anthocyanin 3′,5′-O-glucosyltransferase was cloned from C. ternatea and its activity and contribution for blue coloration were demonstrated.136–139 Efforts have been made to clone cDNAs coding for aromatic acyltransferase,140 and in 1998 Fujiwara et al. reported cloning the gene from Gentiana triflora petals,141 while in 2000, Yonekura-Sakakibara et al. reported cloning the corresponding gene from Perilla leaves.142 Noda et al. isolated a new acyltransferase from C. ternatea that does not use acyl-CoA, but instead utilizes acylglucoside as the high-energy acyl donor.143
The co-pigmentation effect also plays a role in flower blueing; therefore, accumulation of co-pigments such as flavones and/or flavonols causes blue coloration. Biosyntheses of anthocyanin and flavones/flavonols share the same part of the pathway; therefore, it is difficult to estimate the resulting composition ratio of anthocyanin and co-pigment, and the resulting effect on blue coloration. Aida et al. reported that in Torenia petals, the antisense DFR gene produces a bluer-colored flower by reducing the anthocyanin content without changing the flavone content.144
The expression of structural genes in anthocyanin biosynthesis is regulated by transcriptional factors. Two transcriptional factors, basic helix-loop-helix (b-HLH) and the Myb family, are well-studied and known to be conserved in many plant species.145,146 Therefore, these factors have also become a target for transformation.
Genes related to metal transport are promising for the development of blue flowers because several blue colorations correlate closely with metal complexation. In petals of Tulipa gesneriana cv. Murasakizuisho, a vacuole-localized iron transporter plays a critical role in blue coloration and the gene responsible has been cloned by Momonoi et al.73 Al3+ is essential for blue coloration in hydrangea, so an Al3+ transporter presumably exists there.
6.2 Carnations
The traditional flower colors of the carnation, Dianthus caryophyllus, are white, yellow, pink, and red, but not blue. The anthocyanins in pink and red petals contain cyanidin-based pigments with an unusual malyl diester structure (136).147 The reason why carnations lack blue flowers was originally thought to be due to the absence of a delphinidin derivative caused by the lack of a 3′,5′-hydroxylase.126 Suntory and Florigene cloned the 3′,5′-H gene from petunias, and transformed it into a DFR-deficient white carnation. They obtained several cultivars of purple carnations. All of the purple-colored petals contained an anthocyanin with the delphinidin chromophore with the same substitution pattern (137)148 as that in red petals. The expression of these transformed genes is usually promoted with the cauliflower mosaic virus 35S promoter (CaMV 35S).124 For efficient transgene expression, the location where they integrate into the genome should be controlled. However, in some cases it is very difficult to introduce the exogenous gene into a suitable site; therefore, in practice, many transgenic plants are needed in order to obtain the desired one.123,1246.3 Roses
Roses (Rosa hybrida) lack blue flowers, too.149 The term “blue rose” means “impossible” or “an unrealized dream”. As mentioned previously, blue petals often contain a delphinidin derivative with a relatively high vacuolar pH value (pH > 6). Metal complexation and the existence of co-pigments also affect blue coloration. However, the rose does not satisfy any of these conditions. The major anthocyanin in red and pink rose petals is a simple cyanin (1),23,149 reflecting the lack of the 3′,5′-H gene. It seems that the rose is also deficient in aromatic acyltransferase activity, and the pHv value of red rose cells is very low, generally below 4.0 (K. Yoshida, unpublished). Large amounts of rosmarinic acid are found in rose petals, but it works much less effectively as a co-pigment than do flavones and flavonols. To address these problems, Suntory and Florigene screened many rose cultivars in the hope of breeding a blue rose.21 They described four criteria for selection: accumulation of flavonols, higher pHv, F3′H deficiency, and accumulation of pelargonidin rather than cyanidin. Then, the Viola-derived F3′5′-H gene, the Torenia-derived 5-aromatic acyltransferase (5AT) gene, and the iris-derived DFR gene were transformed into rose plants using various vectors in several combinations.21 At the same time, the expression of the endogenous DFR gene was suppressed. Expression of the F3′5′-H and 5AT genes resulted in increased production of anthocyanins with the delphinidin chromophore; the highest value was 95%, and acylated anthocyanins contained approximately 40%. Expression of F3′5′-H from Viola and DFR from iris with suppression of endogenous rose DFR yielded a petal that contains 90% of delphin (138) and 10% of delphinidin 3-glucoside (49). These transgenic petals show a bluer hue than that of petals obtained by traditional breeding methods.217 Perspectives
Over the past 15 years, mechanisms of blue flower coloration have been clarified to a considerable extent. This progress is due to development of instrumental analysis and new techniques of single-cell treatment and analysis. In the case of metalloanthocyanins, we expect that more varieties of such pigments will continue to be found. The mechanism of blue coloration may be common among blue flowers, and so by screening such pigments by direct Vis and CD measurements of intact petals, researchers should be able to find new metalloanthocyanins. The effects of structural differences on the formation of metalloanthocyanins – a combination of matching pair and non-matching pair – should extend our understanding of the molecular recognition chemistry involved.However, ‘fuzzy’ unstable blue coloration may remain a problem. Structural determination of such weakly associated pigments in aqueous solutions and a ‘breakthrough idea’ is sorely needed. Petals of blue clover and blue Muscari species also contain simple anthocyanins; therefore, these cases of blue flower coloration may be due to a similar ‘fuzzy’ metal complex. Polyacylated anthocyanins seem to be classified into two types. One is a group consisting of gentiodelphin, HBA, and ternatins; aromatic acyl residues may stack onto both sides of the anthocyanidin chromophore. The other is a group including tecophilin, phacelianin and cyanodelphin, in which intramolecular stacking of aromatic acyl residues onto the chromophore and intermolecular stacking of chromophores occur simultaneously. Except for morning glory petals, the true blueing mechanisms have not yet been clarified; therefore, physicochemical studies on the charge transfer effect on the bathochromic shift need to be undertaken. The atomic structure of intra- and intermolecular stacking also needs to be resolved. In addition to chemical studies, mechanisms of metal and pigment transport into vacuoles remain a major unresolved question, and we expect that molecular biological studies related to the chemical mechanisms for blue coloration will progress in the next decade. For a true blue rose to be developed, a multilateral strategy is necessary.
8 Acknowledgments
We thank Prof. Atsushi Nakagawa of Osaka University for valuable suggestions and for offers of several figures on metalloanthocyanins. We acknowledge Ms. Naoko Miki of Nagoya University for technical assistance.9 References
- Plant pigments – An introduction to research and experiments, ed. K. Hayashi, Yokendo, Tokyo, 1988 (in Japanese) Search PubMed.
- D. Strack and V. Wray, in The Flavonoids – Advances in Research since 1986, ed. J. B. Harborne, Chapman & Hall, London, 1994, pp. 1–22 Search PubMed.
- T. Goto and T. Kondo, Angew. Chem. Int. Ed. Engl., 1991, 30, 17–33 CrossRef.
- E. Grotewold, Ann. Rev. Plant Biol., 2006, 57, 761–780 CrossRef CAS.
- Ø. M. Andersen and M. Jordheim, in Flavonoids – Chemistry, Biochemistry and Applications, ed. Ø. M. Andersen and K. R. Markham, CRC Press, Boca Raton, 2006, pp. 471–551 Search PubMed.
- J. B. Harborne and C. A. Williams, Phytochemistry, 2000, 55, 481–505 CrossRef CAS.
- R. Brouillard, in The Flavonoids – Advances in Research since 1980, ed. J. B. Harborne, Chapman & Hall, London, 1988, pp. 525–538 Search PubMed.
- R. Brouillard and O. Dangles, in The Flavonoids – Advances in Research since 1986, ed. J. B. Harborne, Chapman & Hall, London, 1994, pp. 565–588 Search PubMed.
- J. B. Harborne and C. A. Williams, Nat. Prod. Rep., 1995, 12, 639–657 RSC.
- J. B. Harborne and C. A. Williams, Nat. Prod. Rep., 1998, 15, 631–651 RSC.
- J. B. Harborne and C. A. Williams, Nat. Prod. Rep., 2001, 18, 310–333 RSC.
- C. A. Williams and R. J. Grayer, Nat. Prod. Rep., 2004, 21, 539–573 RSC.
- C. V. Nigel and R. J. Grayer, Nat. Prod. Rep., 2008, 25, 555–611 RSC.
- R. Brouillard, in Anthocyanins as Food Colors, ed. P. Markakis, Academic Press, New York, 1982, pp. 1–40 Search PubMed.
- T. Goto, H. Tamura, T. Kawai, T. Hoshino, N. Harada and T. Kondo, Ann. N.Y. Acad. Sci., 1986, 471, 155–173 CAS.
- T. Goto, Progress in the Chemistry of Organic Natural Products, 1987, 52, 113–158 Search PubMed.
- T. Kondo, K. Yoshida, A. Nakagawa, T. Kawai, H. Tamura and T. Goto, Nature, 1992, 358, 515–518 CrossRef CAS.
- K. Yoshida, T. Kondo, Y. Okazaki and K. Katou, Nature, 1995, 373, 291 CAS.
- K. Yoshida, M. Mori, Y. Shinkai, Y. Toyama and T. Kondo, 44th Symposium on The Chemistry of Natural Products (Symposium papers), Tokyo, 2002, pp. 409–414 Search PubMed.
- D. Ito, Y. Shinkai, N. Miki, Y. Kato, T. Kondo and K. Yoshida, 49th Symposium on The Chemistry of Natural Products (Symposium papers), Sapporo, 2007, pp. 455–460 Search PubMed.
- K. Katsumoto, M. Fukuchi-Mizutani, Y. Fukui, F. Brugliera, T. A. Holton, M. Karan, N. Nakamura, K. Yonekura-Sakakibara, J. Togami, A. Pigeaire, G.-Q. Tao, N. S. Nehra, C.-Y. Lu, B. K. Dyson, S. Tsuda, T. Ashikari, T. Kusumi, J. G. Mason and Y. Tanaka, Plant Cell Physiol., 2007, 48, 1589–1600 CrossRef.
- R. Willstätter and A. E. Everest, Jus. L. Ann. Chem., 1913, 401, 189–232 Search PubMed.
- R. Willstätter and T. J. Nolan, Jus. L. Ann. Chem., 1915, 408, 1–14 Search PubMed.
- R. Willstätter and H. Mallison, Jus. L. Ann. Chem., 1915, 408, 147–162 Search PubMed.
- K. Shibata, Y. Shibata and I. Kasiwagi, J. Am. Chem. Soc., 1919, 41, 208–220 CrossRef CAS.
- R. Brouillard and B. Delaporte, J. Am. Chem. Soc., 1977, 99, 8461–8468 CrossRef CAS.
- R. Robinson, Ber., 1934, 67A, 85–105 CAS.
- A. Robertson and R. Robinson, J. Chem. Soc., 1927, 242–247 RSC.
- S. Murakami, A. Robertson and R. Robinson, J. Chem. Soc., 1931, 2665–2671 RSC.
- G. M. Robinson and R. Robinson, Biochem. J., 1931, 25, 1687–1705 CAS.
- K. Hayashi, Y. Abe and S. Mitsui, Proc. Japan Acad., 1958, 34, 373–378 Search PubMed.
- S. Mitsui, K. Hayashi and S. Hattori, Proc. Japan Acad., 1959, 35, 169–174 Search PubMed.
- E. Bayer, Chem. Ber., 1958, 91, 1115–1122 CrossRef CAS.
- E. Bayer, H. Egeter, A. Fink, K. Nether and K. Wegmann, Angew. Chem. Int. Ed., 1966, 5, 791–798 CrossRef CAS.
- T. Kondo, M. Ueda, H. Tamura, K. Yoshida, M. Isobe and T. Goto, Angew. Chem., 1994, 33, 978–979.
- S. Asen, R. N. Stewart and K. H. Norris, Phytochemistry, 1972, 11, 1139–1144 CrossRef.
- T. Goto, K. Yoshida, M. Yoshikane and T. Kondo, Tetrahedron Lett., 1990, 31, 713–716 CrossRef CAS.
- T. Hoshino and T. Goto, Tetrahedron Lett., 1990, 31, 1593–1596 CrossRef CAS.
- S. Asen, R. N. Stewart and K. H. Norris, Phytochemistry, 1977, 16, 1118–1119 CrossRef CAS.
- K. Takeda, Proc. Jpn. Acad., Ser. B, 2006, 82, 142–154 Search PubMed.
- M. Shiono, N. Matsugaki and K. Takeda, Nature, 2005, 436, 791 CrossRef CAS.
- K. Takeda, M. Yanagisawa, T. Kifune, T. Kinoshita and C. F. Timberlake, Phytochemistry, 1994, 35, 1167–1169 CrossRef CAS.
- T. Kondo, K. Oyama and K. Yoshida, Angew. Chem. Int. Ed. Engl., 2001, 40, 894–897 CrossRef CAS.
- M. Mori, T. Kondo and K. Yoshida, Phytochemistry, 2008, 69, 3151–3158 CrossRef CAS.
- T. Goto, T. Kondo, H. Tamura and S. Takase, Tetrahedron Lett., 1983, 24, 4863–4866 CrossRef CAS.
- H. Tamura, T. Kondo and T. Goto, Tetrahedron Lett., 1986, 27, 1801–1804 CrossRef CAS.
- K. Takeda and K. Hayashi, Proc. Japan Acad., 1977, 53(B), 1–5 Search PubMed.
- A. Nakagawa, J. Cryst. Soc. Japan, 1993, 35, 327–333 Search PubMed.
- T. Kondo, M. Ueda, K. Yoshida, K. Titani, M. Isobe and T. Goto, J. Am. Chem. Soc., 1994, 116, 7457–7458 CrossRef CAS.
- K. Hayashi, N. Saito and S. Mitsui, Proc. Jpn. Acad., 1961, 37, 393–397 Search PubMed.
- S. Asen and L. Jurd, Phytochemistry, 1967, 6, 577–584 CrossRef CAS.
- H. Tamura, T. Kondo, Y. Kato and T. Goto, Tetrahedron Lett., 1983, 24, 5749–5752 CrossRef CAS.
- T. Kondo, M. Ueda, M. Isobe and T. Goto, Tetrahedron Lett., 1998, 39, 8307–8310 CrossRef CAS.
- K. Takeda, A. Osakabe, S. Saito, D. Furuyama, A. Tomita, Y. Kojima, M. Yamadera and M. Sakuta, Phytochemistry, 2005, 66, 1607–1613 CrossRef CAS.
- T. Ishikawa, T. Kondo, T. Kinoshita, H. Haruyama, S. Inaba, K. Takeda, R. J. Grayer and N. C. Veitch, Phytochemistry, 1999, 52, 517–521 CrossRef CAS.
- J. F. Ma, P. R. Ryan and E. Delhaize, Trends Plant Sci., 2001, 6, 273–278 CrossRef CAS.
- K. Hayashi and Y. Abe, Misc. Rep. Res. Inst. Nat. Resour., 1953, 29, 1–8 Search PubMed.
- T. Kondo, M. Yoshikane, K. Yoshida and T. Goto, Tetrahedron Lett., 1989, 30, 6729–6732 CrossRef CAS.
- E. M. Chenery, J. Roy. Hort. Soc., 1937, 62, 604–620 Search PubMed.
- R. C. Allen, Boyce Thompson Institute, 1943, 13, 221–242 Search PubMed.
- S. Asen, H. W. Siegelman and N. W. Stuart, Proc. Am. Soc. Hort. Sci., 1957, 69, 561–569 Search PubMed.
- K. Takeda, R. Kubota and C. Yagioka, Phytochemistry, 1985, 24, 1207–1209 CrossRef CAS.
- K. Takeda, M. Kariuda and H. Itoi, Phytochemistry, 1985, 24, 2251–2254 CrossRef CAS.
- K. Takeda, T. Yamashita, A. Takahashi and C. F. Timberlake, Phytochemistry, 1990, 29, 1089–1091 CrossRef CAS.
- K. Yoshida, Y. Toyama-Kato, K. Kameda and T. Kondo, Plant Cell Physiol., 2003, 44, 262–268 CrossRef CAS.
- Y. Toyama-Kato, K. Yoshida, E. Fujimori, H. Haraguchi, Y. Shimizu and T. Kondo, Biochem. Eng. J., 2003, 14, 237–241 CrossRef CAS.
- T. Kondo, Y. Toyama-Kato and K. Yoshida, Tetrahedron Lett., 2005, 46, 6645–6649 CrossRef CAS.
- Y. Toyama-Kato, T. Kondo and K. Yoshida, Heterocycles, 2007, 72, 239–254 CrossRef CAS.
- K. Takeda, S. Yamaguchi, K. Iwata, Y. Tsujino, T. Fujimori and S. Z. Husain, Phytochemistry, 1996, 43, 863–865 CrossRef.
- M. Tanaka, T. Fujimori, I. Uchida, S. Yamaguchi and K. Takeda, Phytochemistry, 2001, 56, 373–376 CrossRef CAS.
- K. Yoshida, S. Kitahara, D. Ito and T. Kondo, Phytochemistry, 2006, 67, 992–998 CrossRef CAS.
- K. Shoji, N. Miki, N. Nakajima, K. Momonoi, C. Kato and K. Yoshida, Plant Cell Physiol., 2007, 48, 243–251 CAS.
- K. Momonoi, K. Yoshida, S. Mano, H. Takahashi, C. Nakamori, K. Shoji, A. Nitta and M. Nishimura, Plant J. DOI:10.1111/j.1365-313X.2009.03879.x.
- K. R. Markham, K. A. Mitchell and M. R. Boase, Phytochemistry, 1997, 45, 417–423 CrossRef CAS.
- S. Asen, R. N. Stewart, K. H. Norris and D. R. Massie, Phytochemistry, 1970, 9, 619–627 CrossRef CAS.
- T. Yabuya, M. Nakamura, T. Iwashina, M. Yamaguchi and T. Takehara, Euphytica, 1997, 98, 163–167 CrossRef CAS.
- R. Freyre and R. J. Griesbach, HortScience, 2004, 39, 1220–1223 Search PubMed.
- A. Quintana, J. Albrechtova, R. J. Griesbach and R. Freyre, Sci. Hortic., 2007, 112, 413–421 CrossRef CAS.
- N. Saito, Y. Osawa and K. Hayashi, Phytochemistry, 1971, 10, 445–447 CrossRef CAS.
- T. Goto, T. Kondo, H. Tamura, H. Imagawa, A. Iino and K. Takeda, Tetrahedron Lett., 1982, 23, 3695–3698 CrossRef CAS.
- T. Goto, T. Kondo, H. Tamura and K. Kawahori, Tetrahedron Lett., 1983, 24, 2181–2184 CrossRef CAS.
- T. Goto, T. Kondo, T. Kawai and H. Tamura, Tetrahedron Lett., 1984, 25, 6021–6024 CrossRef CAS.
- T. Kondo, T. Kawai, H. Tamura and T. Goto, Tetrahedron Lett., 1987, 28, 2273–2276 CrossRef CAS.
- T. Kondo, J. Yamashiki, K. Kawahori and T. Goto, Tetrahedron Lett., 1989, 30, 6055–6058 CrossRef CAS.
- T. Kondo, K. Oki, K. Yoshida and T. Goto, Chem. Lett., 1990, 137–138 CAS.
- T. Kondo, M. Ueda and T. Goto, Tetrahedron, 1990, 46, 4749–4756 CrossRef CAS.
- T. Kondo, K. Suzuki, K. Yoshida, K. Oki, M. Ueda, M. Isobe and T. Goto, Tetrahedron Lett., 1991, 32, 6375–6378 CrossRef CAS.
- K. Yoshida, T. Kondo and T. Goto, Tetrahedron, 1992, 48, 4313–4326 CrossRef CAS.
- S. Fukada-Tanaka, Y. Inagaki, T. Yamaguchi, N. Saito and S. Iida, Nature, 2000, 407, 581 CrossRef CAS.
- T. Yamaguchi, S. Fukada-Tanaka, Y. Inagaki, N. Saito, K. Yonekura-Sakakibara, T. Tanaka, T. Kusumi and S. Iida, Plant Cell Physiol., 2001, 42, 451–461 CrossRef CAS.
- T. Yamaguchi and E. Blumwald, Trends Plant Sci., 2005, 10, 615–620 CrossRef CAS.
- K. Yoshida, M. Kawachi, M. Mori, M. Maeshima, M. Kondo, M. Nishimura and T. Kondo, Plant Cell Physiol., 2005, 46, 407–415 CrossRef CAS.
- N. Miki, K. Katou, Y. Okazaki and K. Yoshida, Plant Cell Physiol., 2007, 48, s121.
- T. Kondo, K. Yoshida, M. Yoshikane and T. Goto, Agric. Biol. Chem., 1991, 55, 2919–2921 CAS.
- K. Yoshida, T. Kondo, K. Kameda and T. Goto, Agric. Biolog. Chem., 1990, 54, 1745–1751 Search PubMed.
- K. Yoshida, R. Okuno, K. Kameda, M. Mori and T. Kondo, Biochem. Eng. J., 2003, 14, 163–169 CrossRef CAS.
- K. Yoshida, R. Okuno, K. Kameda and T. Kondo, Tetrahedron Lett., 2002, 43, 6181–6184 CrossRef CAS.
- K. Yoshida, M. Mori, M. Kawachi, R. Okuno, K. Kameda and T. Kondo, Tetrahedron Lett., 2003, 44, 7875–7880 CrossRef CAS.
- M. Mori, K. Yoshida, Y. Ishigaki, T. Matsunaga, O. Nikaido, K. Kameda and T. Kondo, Bioorg. Med. Chem., 2005, 13, 2015–2020 CrossRef CAS.
- T. Honda and N. saito, Hetercycles, 2002, 56, 633–692 Search PubMed.
- K. Yoshida, Y. Toyama, K. Kameda and T. Kondo, Phytochemistry, 2000, 54, 85–92 CrossRef.
- S. J. Bloor, Phytochemistry, 2001, 58, 923–927 CrossRef CAS.
- N. Terahara, M. Oda, T. Matsui, Y. Osajima, N. Saito, K. Toki and T. Honda, J. Nat. Prod., 1996, 59, 139–144 CrossRef CAS.
- N. Terahara, N. Saito, T. Honda, K. Toki and Y. Osajima, Tetrahedron Lett., 1989, 30, 5305–5308 CrossRef CAS.
- N. Terahara, N. Saito, T. Honda, K. Toki and Y. Osajima, Heterocycles, 1990, 31, 1773–1776 CrossRef CAS.
- N. Terahara, N. Saito, T. Honda, K. Toki and Y. Osajima, Tetrahedron Lett., 1990, 31, 2921–2924 CrossRef CAS.
- N. Terahara, N. Saito, T. Honda, K. Toki and Y. Osajima, Phytochemistry, 1990, 29, 949–953 CrossRef CAS.
- N. Terahara, K. Toki, N. Saito, T. Honda, T. Matsui and Y. Osajima, J. Nat. Prod., 1998, 61, 1361–1367 CrossRef CAS.
- M. Mori, T. Kondo, K. Toki and K. Yoshida, Phytochemistry, 2006, 67, 622–629 CrossRef CAS.
- T. Hoshino, Phytochemistry, 1986, 25, 859–832 CrossRef.
- N. Terahara, A. Callebaut, R. Ohba, T. Nagata, M. Ohnishi-Kameyama and M. Suzuki, Phytochemistry, 1996, 42, 199–203 CrossRef CAS.
- S. Bloor, Phytochemistry, 1997, 45, 1399–1405 CrossRef CAS.
- D. Strack, E. Busch and E. Klein, Phytochemistry, 1989, 28, 2127–2139 CrossRef CAS.
- K. Takeda, J. B. Harborne and P. G. Waterman, Phytochemistry, 1993, 34, 421–423 CrossRef CAS.
- K. Toki, N. Saito, K. Iimura, T. Suzuki and T. Honda, Phytochemistry, 1994, 36, 1181–1183 CrossRef CAS.
- K. Toki, N. Saito, S. Tsutsumi, C. Tamura, A. Shigihara and T. Honda, Heterocycles, 2004, 63, 899–902 CrossRef CAS.
- P. Figueiredo, M. Elhabiri, K. Toki, N. Saito, O. Dangles and R. Brouillard, Phytochemistry, 1996, 41, 301–308 CrossRef CAS.
- K. Yoshida, T. Kondo, K. Kameda, S. Kawakishi, A. J. M. Lubag, E. M. T. Mendoza and T. Goto, Tetrahedron Lett., 1991, 32, 5575–5578 CrossRef CAS.
- S. J. Bloor and R. Falshaw, Phytochemistry, 2000, 53, 575–579 CrossRef CAS.
- T. Fossen, R. Slimestad, D. O. Ÿvstedal and O. M. Andersen, Phytochemistry, 2000, 54, 317–323 CrossRef CAS.
- Y. Fukui, K. Nomoto, T. Iwashita, K. Masuda, Y. Tanaka and T. Kusumi, Tetrahedron, 2006, 62, 9661–9670 CrossRef CAS.
- Y. Fukui, T. Kusumi, K. Masuda, T. Iwashita and K. Nomoto, Tetrahedron Lett., 2002, 43, 2637–2639 CrossRef CAS.
- Y. Tanaka, Y. Katsumoto, F. Brugliera and J. Mason, Plant Cell, Tiss. Org. Cul., 2005, 80, 1–24 Search PubMed.
- C. Stephen and Y. Tanaka, Crit. Rev. Plant Sci., 2007, 26, 169–197 CrossRef.
- Y. Tanaka, S. Tsuda and T. Kusumi, Plant Cell Physiol., 1998, 39, 1119–1126 CAS.
- J. Mol, E. Cornish, J. Mason and R. Koes, Curr. Opin. Biotech., 1999, 10, 198–201 CrossRef CAS.
- W. Heller and G. Forkmann, in The Flavonoids – Advances in Research since 1986, ed. J. B. Harborne, Chapman & Hall, London, 1994, pp. 499–535 Search PubMed.
- T. A. Holton and E. C. Cornish, Plant Cell, 1995, 7, 1071–1083 CrossRef CAS.
- K. M. Davies and K. E. Schwinn, in Flavonoids – Chemistry, Biochemistry and Applications, ed. Ø. M. Andersen and K. R. Markham, CRC Press, Boca Raton, 2006, pp. 143–218 Search PubMed.
- S. Mori, M. Nakano, M. Kondo, Y. Hoshi and H. Kobayashi, Acta Hortic., 2005, 673, 429–435 Search PubMed.
- T. Holton, F. Brugliera, D. Lester, Y. Tanaka, G. Hyland, J. Menting, C.-Y. Lu, E. Farcy, T. Stevenson and E. Cornish, Nature, 1993, 366, 276–279 CrossRef CAS.
- Y. Shimada, R. Nakano-Shimada, M. Ohbayashi, Y. Okinaka, S. Kiyokawa and Y. Kikuchi, FEBS Lett., 1999, 461, 241–245 CrossRef CAS.
- C. Seitz, C. Eder, B. Deiml, S. Kellner, S. Martens and G. Forkmann, Plant Mol. Biol., 2006, 61, 365–381 CrossRef CAS.
- Y. Tanaka, Phytochem. Rev., 2006, 5, 283–291 CrossRef CAS.
- M. Fukuchi-Mizutani, H. Okuhara, Y. Fukui, M. Nakao, Y. Katsumoto, K. Yonekura-Sakakibara, T. Kusumi, T. Hase and Y. Tanaka, Plant Physiol., 2003, 132, 1652–1663 CrossRef CAS.
- N. Noda, N. Kato, K. Kogawa, K. Kazuma and M. Suzuki, Plant Cell Physiol., 2004, 45, s132.
- K. Kazuma, K. Kogawa, N. Noda, N. Kato and M. Suzuki, Chem. Biodiv., 2004, 1, 1762–1770 CrossRef CAS.
- K. Kazuma, N. Noda and M. Suzuki, Phytochemistry, 2003, 64, 1133–1139 CrossRef CAS.
- K. Kogawa, N. Kato, K. Kazuma, N. Noda and M. Suzuki, Planta, 2007, 226, 1501–1509 CrossRef CAS.
- T. Nakayama, H. Suzuki and T. Nishino, J. Mol. Cat. B, 2003, 23, 117–132 CrossRef CAS.
- H. Fujiwara, Y. Tanaka, K. Yonekura-Sakakibara, M. Fukuchi-Mizutani, M. Nakano, Y. Fukui, M. Yamaguchi, T. Ahikari and T. Kusumi, Plant J., 1998, 16, 421–431 CrossRef CAS.
- K. Yonekura-Sakakibara, Y. Tanaka, M. Fukuchi-Mizutani, H. Fujiwara, Y. Fukui, T. Ashikari, Y. Murakami, M. Yamaguchi and T. Kusumi, Plant Cell Physiol., 2000, 132, 1652–1663.
- N. Noda, K. Kazuma, N. Kogawa and M. Suzuki, Plant Cell Physiol., 2006, 47, s109.
- R. Aida, K. Yoshida, T. Kondo, S. Kishimoto and M. Shibata, Plant Sci., 2000, 160, 49–56 CrossRef CAS.
- J. Mol, E. Grotewold and R. Koes, Trends Plant Sci., 1998, 3, 212–217 CrossRef.
- K. Springob, J. Nakajima, M. Yamazaki and K. Saito, Nat. Prod. Rep., 2003, 20, 288–303 RSC.
- S. J. Bloor, Phytochemistry, 1998, 49, 225–228 CrossRef CAS.
- Y. Fukui, Y. Tanaka, T. Kusumi, T. Iwashita and K. Nomoto, Phytochemistry, 2003, 63, 15–23 CrossRef CAS.
- C. H. Eugster and E. Märki-Fischer, Angew. Chem. Int. Ed. Engl., 1991, 30, 654–672 CrossRef.
Footnote |
| † Present address: Kitasato Institute for Life Sciences, Kitasato University, 5-9-1 Shirokane, Minato, Tokyo 108-8641, Japan |
| This journal is © The Royal Society of Chemistry 2009 |