Use of synchrotron- and plasma-based spectroscopic techniques to determine the uptake and biotransformation of chromium(III) and chromium(VI) by Parkinsonia aculeata
Yong
Zhao
a,
Jason G.
Parsons
a,
Jose R.
Peralta-Videa
a,
Martha L.
Lopez-Moreno
a and
Jorge L
Gardea-Torresdey
*ab
aDepartment of Chemistry, University of Texas at El Paso, El Paso, TX 79968, USA
bEnvironmental Science and Engineering PhD Program, University of Texas at El Paso, El Paso, TX 79968, USA. E-mail: jgardea@utep.edu; Fax: +1 (915) 747-5748; Tel: +1 (915) 747-5359
First published on 8th June 2009
Abstract
In this study, a combination of inductively coupled plasma optical emission spectroscopy and X-ray absorption spectroscopy (XAS) was used to study the uptake and speciation of chromium in Parkinsonia aculeata, commonly known as Mexican Palo Verde. Plants were treated for 14 days in a modified Hoagland solution containing chromium(III) or chromium(VI) at several concentrations. The results showed that plants treated with 70 mg Cr(III) L−1 and 30 mg Cr(VI) L−1 had similar Cr concentrations in leaves (∼200 mg kg−1 dry weight, DW). The results also showed that neither Cr(III) nor Cr(VI) affected the uptake of phosphorus and sulfur. However, the concentration of calcium in the stems of plants treated with Cr(VI) at 40 mg L−1 (about 6000 mg Ca kg−1 DW) was significantly higher compared to the Ca concentration (about 3000 mg kg−1 DW) found in the stems of plants treated with 150 mg Cr(III) L−1. However, no differences were observed in potassium and magnesium concentrations. The iron concentration (about 1000 mg kg−1 DW) in roots treated with 40 mg Cr(VI) L−1 was similar to the iron concentration found in the roots of plants treated with 110 mg Cr(III) L−1. The XAS data showed that Cr(VI) was reduced to Cr(III) in/on the plant roots and transported as Cr(III) to the stems and leaves. The XAS studies also showed that Cr(III) within plants was present as an octahedral complex.
1. Introduction
Heavy metals are of major concern to environmental and human health due to their toxicity and interference in the absorption of nutrients. Heavy metals enter the environment through weathering and dissolution of minerals and through anthropogenic activities such as mining and smelting.1 More important than the total concentration, however, is element speciation. For, example arsenic(III) is much more toxic than arsenic(V) and trivalent chromium [Cr(III)] is actually an essential nutrient for animals, whereas hexavalent chromium [Cr(VI)] is highly toxic and a known carcinogen.2–4It has been shown that living plants and inactivated plant tissues are able to reduce Cr(VI) to the less toxic Cr(III).5–14 However, the mechanism of reduction is not fully understood. It has been suggested that reducing sugars or small organic acids such as citrate are responsible for the reduction due to electron transfer reactions on the plant surface or inside plants.5 It has also been suggested that the reduction of Cr(VI) to Cr(III) by living plants may be an enzymatic process.15
The speciation of Cr in plant samples has been studied using techniques such as liquid chromatography–inductively coupled plasma-mass spectrometry (LC–ICP MS) and X-ray absorption spectrometry (XAS). However, XAS seems to be the simplest and easiest technique. XAS has been used to show the reduction of Cr(VI) to Cr(III) in/on the inactivated biomass of Agave lechugilla (lechuguilla), Medicago sativa (alfalfa), Avena sativa (oat), and salt bush (Atriplex canescens), among others.9–14 Also, the reduction of Cr(VI) to Cr(III) in living samples of Mesquite (Prosopis sp.), field bindweed (Convolvulus arvensis), pea (Pisum sativum), and clover (Trifolium sp.), among others has been determined using XAS.5–8,16 All of these studies have shown that the Cr reduction occurs at the root level and only Cr(III) is found within the plants.5–8 The references cited above suggest that the reduction of Cr is due to an enzymatic process. The reduction of Cr would remove electrons from compounds such as ascorbic acid and different sugars present in/on the root, altering physiological processes in the plant system. These alterations are noticed through growth reduction and changes in nutrient uptake pattern. For instance, Cary et al.17 reported that leafy vegetables that tend to accumulate Fe are the most effective in translocating Cr to the edible tops of the plant. In addition, Gardea-Torresdey et al.18 reported that C. arvensis plants treated with Cr(VI) increased Fe accumulation in all plant tissues, whereas the accumulation of K and Mg increased in the leaves. In this plant, Cr(VI) did not affect the accumulation of B, Mn, Mo and Zn. On the other hand, in tumbleweed, Cr(III) and Cr(VI) increased Ca concentration in the roots but reduced the concentration of K, P, Mg, Cu and Zn. However, in this plant, Cr(III) at 10 mg L−1 increased P concentration in leaves.19 Similar results have been reported for maize (Zea mays),20 tomato (Lycopersicon esculentum)21 and other crop plants.22
In this study, inductively coupled plasma optical emission spectroscopy (ICP OES) was used to determine the total amount of Cr, micro, and macro nutrients taken up by hydroponically grown Palo Verde (Parkinsonia aculeata L.) plants. In addition, XAS was used to determine the oxidation state and coordination environment of Cr taken up by Palo Verde plants treated with Cr(III) and Cr(VI). Palo Verde is one of the desert plants included in an EPA-NSF grant to study metal accumulation in local desert plant species exposed to metal nanoparticles and metal ions.
Methodology
Seed treatment and plant growth conditions
All materials, e.g. paper towels, tweezers and water, were autoclaved prior to use. Seeds of the Mexican Palo Verde were collected from the region of El Paso, TX, USA, from a site with no previous reports on metal contamination. All seeds were immersed in concentrated sulfuric acid for 3 h, rinsed and immersed in deionized water (DI) for 24 h. Then, seeds were wrapped in paper towels soaked with an antibiotic-antimycotic solution (Sigma A5955, St Louis, MO), placed in darkness for 7 d and then exposed to light for 1 d. Seedlings were set for 7 d in 200-mL Mason jars containing a modified Hoagland nutrient solution. After that, seedlings were treated for 7 d in freshly prepared Hoagland solution containing Cr(III) [from Cr(NO3)3] at 0, 35, 70, 110, 150 mg L−1, and Cr(VI) [from K2Cr2O7] at 0, 10, 20, 30, and 40 mg L−1. Solutions containing Cr(III) and Cr(VI) were pH adjusted to 5.0 and 5.3, respectively with NaOH or HCl as needed. Concentrations of Cr were selected based on a preliminary experiment designed to test the tolerance of Palo Verde to Cr(III) and Cr(VI) stress. Jars with experimental plants were set for two weeks at 25 ± 2 °C, a light/dark cycle of 12/12 h, and irradiation of 53 μmoles m−2 s−1. The system was continuously aired using aquarium pumps. Plants were harvested after 14 d of treatment. At harvest, samples of 10 plants/treatment were randomly selected and measured to determine the effect of treatments on plant elongation (data not shown). Plants were then washed with 0.01 M HNO3, rinsed with DI, separated into roots, stems, and leaves and oven dried at 60 °C for 72 h. Samples were then digested in a CEM microwave oven (CEM MarsX, CEM Corporation, Mathews, NC) with 3 mL trace pure HNO3 (SCP Science, NY, USA) and diluted to 25 mL with double DI. Total Cr, macro, and micronutrient concentrations in roots, stems, and leaves were determined using an ICP/OES Optima 4300 DV (Perkin Elmer, Shelton, CT).XAS studies
Samples for XAS analysis were frozen in liquid nitrogen for 45 min and lyophilized to remove any free water using a Freezone 4.5 freeze dryer at −45 °C and 70 × 10−3 mbar (Labconco, Kansas City, MO). The lyophilized samples were ground with a mortar and pestle, loaded in aluminum sample holders with Kapton® tape and examined on beam line 7–3 at Stanford Synchrotron Radiation Laboratories (SSRL, Palo Alto, CA). Spectra of plant samples and Cr model compounds were collected using the Cr K edge (E0 5.989 keV). All samples were run at ambient temperature. The beamline was operated with a Si (111 with a φ0 orientation) double crystal monochromator and a 1 × 10 mm slit (which resulted in a resolution of approximately 1–2 eV). The output was detuned by 30% to reduce higher order harmonics. Sample spectra were collected in fluorescence mode using a 13 element Canberra Ge detector. Two to three scans of each sample were collected to improve the signal to noise ratios. Additionally, a chromium foil [Cr(0)] was used as a calibration standard to determine the correct edge energy of samples. The foil was placed between the I1 and I2 ion chambers and collected simultaneously with each sample spectra. The model compound Cr(III) acetylacetonate was prepared through the dilution of the Cr salt in boron nitrideviagrinding and homogenizing using a mortar and pestle.XAS data analysis
The XAS data were analyzed with the WinXAS software package using standard methods.23–24 Sample and model compound spectra were calibrated using a second degree derivative of the internal Cr foil (E0 5.989 keV). Spectra were then background corrected with a one degree polynomial fitting of the pre-edge region and a fourth degree polynomial fitting of the post-edge region, and subsequently normalized to one absorption unit across the edge. The XANES spectra were then extracted from 5.95 to 6.12 keV.The extended X-ray absorption fine structure (EXAFS) analysis was performed by first converting spectra of samples and model compounds into k space, or wave vector space (Å−1). Following the conversion, EXAFS data were extracted using a cubic spline of four knots with a k weight of three from 2.0 to 12.2 Å−1. Spectra were then Fourier transformed from 2.0 to 12.2 Å−1 and subsequently back transformed to extract the EXAFS of the first three coordination shells. The back transformed EXAFS were then fitted using calculations from the ab initio multiple-scattering code FEFF V8.00.24 Parameters determined using the FEFF 8.00 calculations were interatomic distances, coordination numbers, Debye–Waller factors, and energy shifts. Crystallographic inputs used in the FEFF fitting were created using the ATOMS software and based on crystallographic data from the literature for Cr(III) acetylacetonate.25–27
Statistical analysis
Data of total Cr, macro, and micronutrient concentrations were analyzed with one-way analysis of variance (ANOVA) using SPSS software, version 12.0 (SPSS Inc., Chicago, IL). Significant differences between treatment means were detected using the Tukey-HSD (honestly significant difference) test. Any reference to a significant difference between data is based on a probability of p < 0.05 unless otherwise stated.Indexes
Indexes such as translocation factor (TF = Concentration in shoot/conc. in root), enrichment coefficient (EC = Concentration in shoot/conc. in medium), and bioconcentration factor (BCF = Concentration in plant/conc. in medium) were calculated to compare the efficiency of Cr uptake from Cr(III) and Cr(VI).Results and discussion
Chromium uptake
At harvest, the amount of biomass samples (dry weight base) collected for elemental uptake ranged between 0.05 and 0.10 g. Concentrations of Cr in roots, stems, and leaves for Cr(III) and Cr(VI) treated plants are shown in Fig. 1A and 2A, respectively. As can be seen in these figures, in both cases (except in the case of Cr(III) at 110 mg L−1 and Cr(VI) at 30 mg L−1) Cr in tissues increased as the external Cr increased. As reported for other plants, most of the absorbed Cr was kept in the roots.5–7,23–24 The low translocation of chromium from the roots to the leaves has been linked to the precipitation of Cr as Cr(III) hydroxide in roots.16 However, the formation of Cr(III) hydroxide does not fully explain results obtained in studies where XAS have been used to determine the uptake of Cr. Several researchers have reported that a Cr(III)–organic acid species is formed in some plant species.5–8 This complex precipitates in vacuoles while hydroxides are trapped in the root cell walls.28 A high concentration of an element on the roots with poor translocation to the stems and leaves is indicative of non-active transport. A further examination of Cr absorption data shows that Palo Verde absorbed Cr(III) more efficiently. Comparing Cr absorption by plants treated with Cr(III) at 35 mg L−1 and plants treated with Cr(VI) at 30 and 40 mg L−1, one can see that the BCF and EC were higher in Cr(III) treated plants (Table 1). In addition, EC and BCF were higher in plants treated with Cr(III) at 150 mg L−1, compared to plants treated with Cr(VI) at 40 mg L−1. However, in all cases the TF were higher in plants treated with Cr(VI), as shown in Table 1. It is very likely that the reduction process in roots takes some time and the Cr(VI) anions (e.g. CrO4−2) move faster through the plant transport system because Cr(III) interacts with cell walls.19 | ||
| Fig. 1 Concentrations of chromium (A), calcium (B), magnesium (C), and potassium (D) in Palo Verde plants treated for 14 days in hydroponics with varying concentrations of chromium(III). Error bars represent SE. Upper case letters indicate statistical differences in roots, lower case letters in stems, and numbers in leaves, (p < 0.05). | ||
 | ||
| Fig. 2 Concentrations of chromium (A), calcium (B), magnesium (C), and potassium (D) in Palo Verde plants treated for 14 days in hydroponics with varying concentrations of chromium(VI). Error bars represent SE. Upper case letters indicate statistical differences in roots, lower case letters in stems, and numbers in leaves, (p < 0.05). | ||
| a TF | EC | BCF | |
|---|---|---|---|
| a TF = Concentration in shoot/conc. in root; EC = Concentration in shoot/conc. in the medium; BCF = Concentration in plant/conc. in the medium. | |||
| Cr(III) | |||
| 35 ppm | 0.071 | 24.382 | 38.899 |
| 70 ppm | 0.124 | 39.502 | 58.285 |
| 110 ppm | 0.113 | 21.656 | 31.778 |
| 150 ppm | 0.104 | 19.616 | 28.921 |
| Cr(VI) | |||
| 10 ppm | 0.114 | 24.548 | 39.793 |
| 20 ppm | 0.137 | 20.043 | 29.843 |
| 30 ppm | 0.199 | 22.803 | 30.078 |
| 40 ppm | 0.168 | 15.898 | 22.272 |
Macronutrient uptake
The absorption of some macronutrients in living plants has been shown to be affected by both Cr(III) and Cr(VI). In this study the concentrations of Ca, K, Mg, P, and S were determined in tissues of Palo Verde plants treated with Cr(III) and Cr(VI). Data showed that phosphorus (P) and sulfur (S) were unaffected by the presence of Cr in the growth media (data not shown). However, Ca, K and Mg were differentially affected by Cr(III) and Cr(VI), as shown in Fig. 1B–D and Fig. 2B–C. In plants treated with Cr(III), the accumulation of Ca in roots and stems was lower compared to plants treated with Cr(VI). In addition, Cr(III) treated roots had less Ca than control roots, while Cr(VI) treated roots had more Ca than control roots. Moreover, the concentration of Ca in the roots of Cr(VI) treated plants was higher compared to the Ca concentration in control roots. However, in leaves, all Cr treated plants had significantly less Ca than the control plants (Fig. 1B and 2B). Due to the important role of Ca in the preservation of plant homeostasis, its increase in Cr(VI) treated plants could indicate a detoxification strategy.29 Similarly, in the roots of all Cr treated plants, the concentration of Mg decreased compared to the control plants (Fig. 1C and 2C). In addition, the roots of Cr(VI) treated plants had more Mg than the roots of Cr(III) treated plants. No conspicuous differences in Mg concentrations were observed in the aerial parts. The accumulation of K in the roots of all Cr treated plants was dramatically reduced compared to the control plants, mainly in the Cr(III) treated plants. The reduction in K accumulation in the stems and leaves of Cr treated plants was not clear. However, the stems of plants treated with Cr(VI) accumulated more K than Cr(III) treated plants, but this difference was not seen in the leaves. The reduction in K accumulation could be the result of a possible competition with Cr ions.25 A further explanation of the effect of Cr on K accumulation in roots of Palo Verde may be a simple ion exchange. Chromium generally has a higher affinity to carboxylic acids and sugars than K. The presence of Cr in the nutrient medium may be displacing K from the root surface.Micronutrient uptake
The concentrations of iron (Fe) in plants were shown to be affected greatly by the presence of Cr ions (Fig. 3). All Cr(III) treated plants and plants treated with Cr(VI) at 30 mg L−1 had significantly more Fe in the roots than control plants. However, plants treated with Cr(VI) at 10 and 20 mg L−1 had significantly less Fe compared to control plants. In the stems, Cr(III) treated plants and control plants showed similar Fe concentrations, although, the stems and leaves of Cr(VI) treated plants and leaves of Cr(III) treated plants had significantly less Fe than the control plants (Fig. 3A and C). It has been reported that the accumulation of Cr is accompanied by the accumulation of Fe,17; however, it has also been reported that Cr(VI) reduces the uptake of the essential elements Fe, K, Mg, Mn, P, and Ca.18 Because of their similar ionic ratios, Cr(III) replaces Fe(III) in heme proteins, decreasing their activity,30 which reduces the accumulation of Fe. In addition, reports indicate that Cr increases the activity of root-associated Fe(III) reductase and that there is an interconversion of CrO42− and Cr3+ in roots, which could explain the different trend in Fe accumulation under Cr(III) and Cr(VI) stress.31 The absorption of Zn was severely reduced by both Cr ions, but more by Cr(III). None of the Cr(III) treated plants showed Zn in the roots. There was also a drastic reduction in Zn concentration in the stems of Cr(III) treated plants. However, no changes were observed in the leaves of Cr(III) treated plants, but the leaves of Cr(VI) treated plants showed an increase in Zn concentration. This response is opposed to the response reported by Sharma and Pant32 in maize (Zea mays), where Cr reduced Zn concentrations in leaves but increased them in stems and roots. It is very likely that precipitation of Cr species on the roots of the Palo Verde plants may act like Fe and Mn plaques, which inhibit the uptake of nutrients.33,34 The precipitation of Cr species or the formation of Cr compounds on the roots of Palo Verde would also help to explain why the translocation of Cr from roots to stems was low, as was shown in Fig. 1A and 2A. In addition, the formation of Cr coordination compounds outside or within roots may also affect the uptake of nutrients from solution by changing the charge on the surface of the plant roots. This would prevent the electrostatic attraction between cations and roots, which may result in lower Fe and Zn concentrations within roots compared to control plants. | ||
| Fig. 3 Concentration of iron (A and C) and concentration of Zn (B and D) in Palo Verde plants treated for 14 days in hydroponics with varying concentrations of Cr(III) and Cr(VI), respectively Error bars represent SE. Upper case letters indicate statistical differences in roots, lower case letters in stems, and numbers in leaves, (p < 0.05). | ||
XAS study
The XANES spectra shown in Fig. 4 demonstrate that, irrespective of the supplied Cr form, Palo Verde plant samples contained trivalent Cr. The oxidation state is seen through the edge position of the spectra, which is the same in all samples. Additionally, the pre-edge features shown in plant samples (including samples treated with Cr(VI)) confirms Cr(III) bound to six oxygen ligands, although the spectra are slightly distorted for Cr(VI) shoot samples (the Cr(III) pre-edge feature located at 5.989 keV is present in the sample, although the low Cr concentration produces a high amount of scatter). Consequently, the Cr(III) pre-edge feature in Cr(VI) treated shoots is not as a prominent as that found in the remaining samples. However, additional evidence supporting the presence of Cr(III) in Cr(VI) treated shoot samples may be found in the energy of the photoelectron ejected from the sample, 6.002 keV, identical to that of Cr(III) treated roots and shoots, and Cr(VI) root samples. While the low Cr concentration in Cr(VI) shoot samples precluded their extraction and fitting viaEXAFS, there does exist a precedent of Cr(VI) to Cr(III) reduction in mesquite plants, water hyacinths and C. arvensis.5–7 Additionally, Cr(VI) to Cr(III) reduction has been shown to occur in inactivated tissues of oat, saltbush, and lechugilla biomass , among others.9–15 | ||
| Fig. 4 XANES spectra of Cr(III) (A) and Cr(VI) (B) samples of Palo Verde plants treated for 14 days in hydroponics with trivalent and hexavalent chromium. (C) XANES spectra of Cr(III) acetylacetonate and potassium dichromate model compounds. | ||
Fig. 5 shows EXAFS of samples and model compounds, while Table 2 presents the fittings of the EXAFS. The EXAFS of Cr(III) treated roots and shoots, Fig. 5, display very similar spectra in regards to both their amplitude and the positions of their oscillations. The fitting of the root and shoot samples revealed coordination numbers of six oxygen ligands at approximately 1.98 Å in the first coordination shell, and coordination of six carbon atoms in the second shell at approximately 2.90 Å, corroborating the XANES results. This coordination environment is similar to the first two shells of the Cr(III) acetylacetonate model compound (Fig. 4); however, Cr(III) treated samples are missing both the third shell coordination at 3.20 Å and the multiple scattering at 3.27 Å. The local coordination environment of the Cr(III) ion in the Cr(III) acetylacetonate model is found in Fig. 6, showing that the Cr(III) ion is directly bound to six oxygen ligands in the first shell, six carbon ligands in the second shell, and three carbons in the third shell, creating a ring structure. However, due to the absence of the third coordination shell in Cr(III) treated samples, this ring structure was not observed in the EXAFS of Cr(III) treated roots and shoots, although the presence of the second shell of six carbon atoms at an interatomic distance of 2.80–2.90 Å indicates that the Cr(III) ion may be octahedrally bound to small organic acids in the roots and shoots of Cr(III) samples. The EXAFS of Cr(VI) treated roots are shown in Fig. 5. The Fourier transformed EXAFS of this sample is very similar to the EXAFS of Cr(III) treated plants shown in Fig. 5. The fitting of Cr(VI) treated plant root EXAFS are shown in Table 2. The fittings of Cr in Palo Verde roots treated with Cr(VI) showed a coordination of 6 oxygen ligands at approximately 1.98 Å, and an interaction of 4 carbon atoms at approximately 2.83 Å. The Cr(VI) Palo Verde root sample suggests that Cr is bound to four small organic acids and two water molecules. The results of the EXAFS fittings for Palo Verde plant roots treated with Cr(VI) corroborate the results from the XANES analysis.
 | ||
| Fig. 5 Fourier transform of raw EXAFS of Cr(III) treatment of Palo Verde plants (dotted line) and the Fourier transform of the fitting of the back transformed EXAFS (solid line) on/in (A) roots and (B) shoots. (C) Fourier transform of raw EXAFS of Cr(VI) treatment in shoots of Palo Verde plants (dotted line) and the Fourier transform of the fitting of the back transformed EXAFS (solid line). (D) Fourier transform of raw EXAFS of Cr(III) acetylacetonate model compound (dotted line) and the Fourier transform of the fitting of the back transformed EXAFS (solid line). | ||
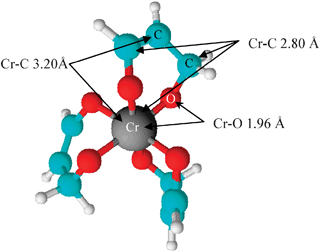 | ||
| Fig. 6 Graphical representation of the local coordination environment of chromium(III) ions in the Cr(III) acetylacetonate model compound. | ||
| Sample | Interaction | CN | R/Å | σ 2/Å2 | S 0 2 |
|---|---|---|---|---|---|
| Cr(III) acetylacetonate | Cr–O | 6.0 | 1.96(3) | 0.0022 | 0.90 |
| Cr–C | 6.0 | 2.79(1) | 0.0030 | ||
| Cr–C | 3.0 | 3.20(3) | 0.0093 | ||
| MS | 12 | 3.27(4) | 0.010 | ||
| Palo Verde roots treated with Cr(III) | Cr–O | 6.0 | 1.98(0) | 0.0031 | 0.85 |
| Cr–C | 6.0 | 2.98(6) | 0.0056 | ||
| Palo Verde shoots treated with Cr(III) | Cr–O | 6.0 | 1.98(3) | 0.0041 | 0.82 |
| Cr–C | 6.0 | 2.94(5) | 0.0073 | ||
| Palo Verde roots treated with Cr(VI) | Cr–O | 5.9 | 1.98(3) | 0.0049 | 0.87 |
| Cr–C | 4.0 | 2.83(2) | 0.0091 |
The octahedral coordination environment for Cr(III) in live plants has been previously observed in mesquite and water hyacinth. Mesquite and water hyacinth, exhibit a very similar first-shell coordination to Palo Verde plants, which possessed Cr–O interaction distances ranging from 1.95 to 2.0 Å.6,7 In mesquite plant, Cr(III) and Cr(VI) were found to have octahedral arrangements of oxygen ligands in the first shell at an interatomic distance of 1.98 to 2.0 Å, nearly identical to the results of the Palo Verde plant, although the interatomic distance of the second shell of the Cr–C interaction was determined to be at 3.41 Å, a greater distance than the second shell of the Palo Verde, approximately 2.90 Å. However, water hyacinth plants showed a second shell coordination at an interatomic distance of approximately 2.7 Å, slightly shorter than in Palo Verde, and a third shell at approximately 3.8 Å, although the data were not fitted with EXAFS.5 The authors of that study suggested that this coordination was indicative of Cr being bound by some type of oxalate ligand, which is the base of oxalic acid.5 In the present study, the EXAFS oscillations allowed only the fitting of the second shell of six carbons, and it is possible a third shell exists, not determined by EXAFS due to concentration effects.
The results in this study show a strong similarity to other studies depicting a partial or complete reduction of Cr(VI) to Cr(III), with Cr ions exhibiting a first shell coordination of six oxygen ligands. Chromium uptake studies on C. arvensis produced XANES spectra demonstrating fully the reduction of Cr(VI) to Cr(III).7 Additional evidence may be found from examining the XANES spectra of C. arvensis, which shows the presence of the Cr(III) pre-edge feature, found only when Cr(III) is bound to six oxygen ligands.7 The results obtained from the Palo Verde plant are strikingly similar to the results obtained in numerous live-plant/Cr interactions, where octahedral coordination of Cr ions was observed, coupled with the reduction of Cr(VI) to Cr(III) in roots and the subsequent presence of only Cr(III) in the aerial portion of the plants.
Conclusions
The uptake of Cr by Palo Verde plants closely matches the results of chromium uptake in other plant species. The Cr ion is found in an octahedral conformation bound to six oxygen atoms, with an absence of Cr(VI) in the shoots and leaves due to reduction to Cr(III) in the roots. The study of Palo Verde grown in hydroponics demonstrates that Cr(III) fails to impart toxicity, e.g. necrosis and/or chlorosis to plants with Cr(III) levels up to 150 mg L−1. However, the presence of Cr(III) significantly reduced overall plant growth. On the other hand, Cr(VI) treated plants in concentrations up to 40 mg L−1 showed significant signs of toxicity and plant growth was reduced in comparison to Cr(III) treated plants.With regards to macro- and micronutrient accumulation, while it’s apparent that the presence of Cr has a significant effect on the uptake and distribution of most of the metal ions examined, the data are not fully clear. Interactions between Cr and macro- and micronutrient ions examined in this study may be based on a variety of sources, including their physicochemical properties, charge, or their effective ionic or metal radii, possibly leading to complex formation between Cr and the metal ions. Chromium/plant interactions may alter plant metabolism, thereby affecting the uptake of metal ions.30 Alternatively, Cr may simply compete with metal ions for uptake. Whether the changes observed are due to a single mechanism or a combination of mechanisms, given the complex relationships observed, remains unknown. Further studies investigating the mechanism of chromium/plant and chromium/metal ion interactions are necessary before the potential of Cr phytoremediation by Palo Verde is fully understood. Studies currently performed in soil will shed more light on this potential.
Acknowledgements
The authors acknowledge the National Science Foundation NSF #s 0723115 and 0521650. This material is based upon work supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number EF 0830117. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to EPA review and no official endorsement should be inferred. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program. J. Gardea-Torresdey acknowledges the Dudley family for the Endowed Research Professorship in Chemistry and the LERR and STARs programs of the University of Texas System.References
- H. B. Bradl, Interface Sci. Technol., 2005, 6, 1–27 Search PubMed.
- J. G. Parsons, A. Martinez-Martinez, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Chemosphere, 2008, 70, 2076–2083 CrossRef CAS.
- S. De Flora, Carcinogenesis, 2000, 21, 533–541 CrossRef CAS.
- D. Ryberg and J. Alexander, Chem.-Biol. Interact., 1990, 75, 141–51 CrossRef CAS.
- C. M. Lytle, F. C. Lytle, N. Yang, J. H. Qian, D. Hansen, A. Zayed and N. Terry, Environ. Sci. Technol., 1998, 32, 3087–3093 CrossRef CAS.
- M. V. Aldrich, J. L. Gardea-Torresdey, J. R. Peralta-Videa and J. G. Parsons, Environ. Sci. Technol., 2003, 37, 1859–1864 CrossRef CAS.
- M. O. Montes-Holguin, J. R. Peralta-Videa, G. Meitzner, A. Martinez-Martinez, G. de la Rosa, H. A. Castillo-Michel and J. L. Gardea-Torresdey, Environ. Toxicol. Chem., 2006, 25, 220–226 CrossRef CAS.
- Hiram A. Castillo-Michel, Jason Parsons, Jose R. Peralta-Videa, Alejandro Martinez and Jorge L. Gardea-Torresdey, Proceeding 231st ACS National Meeting, Atlanta GA, 2006 Search PubMed.
- J. G. Parsons, K. Dokken, J. R. Peralta-Videa, J. Romero-Gonzalez and J. L. Gardea-Torresdey, Appl. Spectrosc., 2007, 61, 338–45 CrossRef CAS.
- J. L. Gardea-Torresdey, K. J. Tiemann, V. Armendariz, L. Bess-Oberto, R. R. Chianelli, J. Rios, J. G. Parsons and G. Gamez, J. Hazard. Mater., 2000, 80, 175–188 CrossRef CAS.
- M. F. Sawalha, J. L. Gardea-Torresdey, J. G. Parsons, G. Saupe and J. R. Peralta-Videa, Microchem. J., 2005, 81, 122–132 CrossRef CAS.
- J. G. Parsons, M. Hejazi, K. J. Tiemann, J. Henning and J. L. Gardea-Torresdey, Microchem. J., 2002, 71, 211–219 CrossRef CAS.
- K. J. Tiemann, A. E. Rascon, G. Gamez, J. G. Parsons, T. Baig, I. Cano-Aguilera and J. L. Gardea-Torresdey, Microchem. J., 2002, 71, 133–141 CrossRef CAS.
- D. Park, Y. S. Yun and J. M. Park, J. Colloid Interface Sci., 2008, 317, 54–61 CrossRef CAS.
- F. Battaglia-Brunet, S. Foucher, D. Morin and I. Ignatiadis, Water, Air, Soil Pollut., 2004, 4, 127–135 CAS.
- J. A. Howe, R. H. Loeppert, V. J. DeRose, D. B. Hunter and P. M. Bertsch, Environ. Sci. Technol., 2003, 37, 4091–4097 CrossRef CAS.
- E. E. Cary, W. H. Allaway and O. E. Olson, J. Agric. Food Chem., 1997, 25, 300–304.
- J. L. Gardea-Torresdey, J. R. Peralta-Videa, M. Montes, G. de la Rosa and B. Corral, Bioresour. Technol., 2004, 92, 229–235 CrossRef CAS.
- J. L. Gardea-Torresdey, G. de la Rosa, J. R. Peralta-Videa, M. Montes, G. Cruz-Jimenez and I. Cano, Arch. Environ. Contam. Toxicol., 2005, 48, 225–232 CrossRef CAS.
- D. C. Sharma and R. C. Pant, J. Environ. Sci. Health, Part A, 1994, 29, 941–948.
- R. Mora, I. Gomez and J. N. Pedreno, J. Mataix. Agrochimica, 1996, 40, 132–138 Search PubMed.
- A. K. Shanker, C. Cervantes, H. Loza-Tavera and S. Avudainayagam, Environ. Int., 2005, 31, 739–753 CrossRef CAS.
- T. Ressler, J. Synchrotron Radiat., 1998, 5, 118–122 CrossRef CAS.
- J. G. Parsons, M. V. Aldrich and J. L. Gardea-Torresdey, Appl. Spectrosc. Rev., 2002, 37, 187–222 CAS.
- A. L. Ankudinov, B. J. Ravel, J. J. Rehr and S. D. Conradson, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 7565 CrossRef CAS.
- B. Ravel, J. Synchrotron Radiat., 2001, 8, 314–316 CrossRef CAS.
- B. Morosin, Acta Crystallogr., 1965, 19, 131–137 CrossRef CAS.
- D. Liu and I. Kottke, Cell Biol. Toxicol., 2003, 19, 299–311 CrossRef CAS.
- B. R. James and R. J. Bartlett, J. Environ. Qual., 1984, 13, 67–70 CAS.
- N. Pandey and C. P. Sharma, Environ. Exp. Bot., 2003, 49, 195–200 CrossRef CAS.
- A. Zayed, C. M. Lytle, J.-H. Qian and N. Terry, Planta, 1998, 206, 293–299 CrossRef CAS.
- D. C. Sharma and R. C. Pant, J. Environ. Sci. Health, Part A, 1994, 29, 941–948.
- D. Liu, J. Zou, M. Wang and W. Jiang, Bioresour. Technol., 2008, 99, 2628–2636 CrossRef CAS.
- N. R. Bishnoi, L. K. Chugh and S. K. Sawhney, J. Plant Physiol., 1993, 142, 25–30 CAS.
| This journal is © The Royal Society of Chemistry 2009 |
