A perspective on the role of metals in diabetes: past findings and possible future directions
Jennifer A.
Meyer
and
Dana M.
Spence
Department of Chemistry, Michigan State University, East Lansing, MI 48824, USA. E-mail: dspence@chemistry.msu.edu; Fax: +1 517 353 1793; Tel: +1 517 355 9715 x174
First published on 2nd December 2008
Abstract
In this review, the authors present a brief overview of metals and their possible roles as determinants in the pathogenesis of diabetes and complications. Of course, due to the complexity of diabetes and its far-reaching complications, it would be difficult to cover every metal that has been implicated in diabetes. Therefore, this review has two main objectives, the first of which is to educate the reader with regards to the types of diabetes and complications, especially in relation to hyperglycemia and anti-oxidant properties. Following an overview of the more cited metals in diabetes, the second objective of this review is to offer some opinions about current needs in the area of metal analysis. Specifically, the challenges for scientists to perform quantitative determinations on biological samples in near-real time with subcellular-level spatial resolution.
 Jennifer A. Meyer Jennifer A. Meyer | Jennifer Meyer got her bachelor’s degree in chemistry and mathematics from Central Michigan University in 2005. She began her doctoral studies in the fall of 2005 and is currently a graduate student in the labs of Dr Dana Spence at Michigan State University. Jennifer’s research has focused on defining new roles for C-peptide and its effect on erythrocytes as it pertains to diabetic complications. Her work has been published in both clinical and chemical journals. After graduation, she hopes to pursue a career in academia. |
 Dana M. Spence Dana M. Spence | Dana Spence is an associate professor in the Department of Chemistry at Michigan State University. He received his PhD working with Dr Stanley Crouch at Michigan State University in 1997. The analyses performed in the Spence group are designed to enable communication between various cell types in real-time and monitor competing metabolic pathways within cells simultaneously. Quantitative determinations on samples performed in his research group are largely inspired by health problems related to diabetes, multiple sclerosis, and cardiovascular disease. Efforts relating to metals in diabetes stem from recent studies involving the role of zinc in the stimulation of C-peptide. |
Diabetes and its complications
According to the World Health Organization, diabetes mellitus is a disease that affects an estimated 180 million people worldwide, and this number is expected to double by the year 2030. Various forms of hyperglycemia are characteristic of diabetes and are thought to result from a lack of insulin production by the pancreatic β-cells or the inability of the body to efficiently use the insulin that is produced. While diabetes may result from different origins, it is generally classified into 3 forms, namely type 1 diabetes or juvenile diabetes, type 2 diabetes, and gestational diabetes.Type 1 diabetes generally occurs during early childhood or adolescence and is characterized by a lack of insulin production due to the destruction of the β-cells. It is thought to be an autoimmune disease and is fatal without regular insulin injections. Type 2 diabetes is often referred to as adult-onset diabetes or non-insulin dependent diabetes, and often results when the insulin being produced in the pancreas cannot be used effectively by the body. This form of diabetes is thought to result from a lack of physical activity and excessive body weight. Until recently, this form of diabetes was only observed in middle-aged adults and the elderly; however, it is becoming more common in obese children. The third form of diabetes is referred to as gestational diabetes, and is characterized by hyperglycemia during pregnancy. It is similar to type 2 diabetes and generally ceases after pregnancy.
Diabetes is typified by hyperglycemia, although the resulting complications are much more serious than simply an elevated level of blood glucose. Interestingly, the observed complications among the various forms of diabetes are remarkably similar and manifold. Diabetes is classified as a single disease, although it results in a wide variety of complications such as retinopathy, which causes blindness in 2% and visual impairment in 10% of patients with diabetes. A comparison of diabetes traits and complications are shown in Fig. 1.
 | ||
| Fig. 1 Characteristics and side effects associated with type 1 and type 2 diabetes. | ||
Additionally, diabetic neuropathy occurs in over 50% of patients with diabetes. Patients with diabetic neuropathy often experience pain, tingling, or numbness in the extremities. The numbness due to neuropathy, in combination with poor blood flow, results in a higher than average foot amputation. Unfortunately, there is currently no medication available for the treatment of diabetic neuropathy.
Patients with diabetes also experience a high rate of nephropathy, which eventually results in fatal kidney failure for 10–20% of people with diabetes. Finally, diabetes also increases the risk of heart disease and stroke. Over 50% of people diagnosed with diabetes will die of a cardiovascular disease.
Metals as determinants of oxidative stress and glycemic control
At the molecular level, patients with diabetes often have high levels of intracellular oxidative stress as a result of hyperglycemia. High levels of reactive oxygen species are often present as a result of a reduced oxidant defense system.1,2 Increased oxidative stress has been reported to reduce insulin secretion and increase insulin resistance in certain diabetic models, thereby playing a possible role in the pathogenesis of diabetes.3 Moreover, it has been reported that the levels of glutathione, the most abundant non-enymatic antioxidant in red blood cells, are lower in people with diabetes in comparison to healthy controls.4 In addition, patients with type 2 diabetes have lower levels of glucose-6-phosphate dehydrogenase, an important enzyme in the pentose phosphate pathway, a pathway that is a key contributor in the defense against oxidant insult.5–9 Moreover, it has been demonstrated that improving glycemic control results in an improved oxidant defense system.10With all of the available current research indicating the importance of the regulation and maintenance of glycemic control to reduce oxidative stress, there has been a significant amount of reported research indicating the potential for antioxidant metals as adjunct therapy for diabetes.11 While the maintenance of proper blood glucose levels is essential for people with diabetes, the use of certain metals can be justified to reduce or prevent the harmful side effects of having elevated blood glucose levels.12
The metals that would have the most potential to elicit beneficial effects are those metals requiring small quantities for proper metabolic function. Many metals are able to indirectly participate in the reversal of oxidant stress by improving glycemic control or exhibiting effective antioxidant properties (see Table 1).
| Metal/mineral | Glycemic control | Antioxidant | Concentration In vivo type 2 diabetics (males)/μg g−1 or mg L−1* | Concentration In vivo controls (males)/μg g−1 or mg L−1* |
|---|---|---|---|---|
| Copper | X | Blood: 1.8 ± 0.5921 | Blood: 1.4 ± 0.3221 | |
| Urine: 0.19 ± 0.04321 | Urine: 0.15 ± 0.02321 | |||
| Hair: 12.7 ± 1.821 | Hair: 11.5 ± 0.921 | |||
| Manganese | X | Blood: 43.4 ± 8.821 | Blood: 54.7 ± 6.1221 | |
| Urine: 1.8 ± 0.3221 | Urine: 1.4 ± 0.1721 | |||
| Hair: 2.7 ± 0.921 | Hair: 3.8 ± 0.6321 | |||
| Magnesium | X | Serum: 13.60 ± 4.8*152 | Serum: 14.10 ± 4.80*152 | |
| Urine: 11.55 ± 6.40*152 | Urine: 5.80 ± 4.00*152 | |||
| Iron | Blood: 656.9 ± 72.421 | Blood: 708.4 ± 51.321 | ||
| Urine: 1.8 ± 0.721 | Urine: 2.4 ± 0.521 | |||
| Hair: 38.5 ± 2.821 | Hair: 31.9 ± 1.421 | |||
| Selenium | X | X | Serum: 0.065 ± 0.023*153 | Serum: 1.075 ± 0.027*153 |
| Urine: 0.019 ± 0.01*153 | Urine: 0.020 ± 0.0101*153 | |||
| Vanadium | X | 0.00001–0.001*154 | Same as diabetics | |
| Chromium | X | Blood: 49.9 ± 4.621 | Blood: 58.9 ± 2.6121 | |
| Urine: 12.5 ± 3.5821 | Urine: 8.5 ± 2.4621 | |||
| Hair: 2.3 ± 0.4321 | Hair: 3.5 ± 0.2821 | |||
| Zinc | X | X | Blood: 6.9 ± 2.0621 | Blood: 10.4 ± 1.3521 |
| Urine: 1.4 ± 0.621 | Urine: 0.8 ± 0.321 | |||
| Hair: 169.8 ± 15.821 | Hair: 212.1 ± 8.521 |
There is mounting evidence to demonstrate that metals may have the ability to play key roles in the pathogenesis and complications of the disease. Chromium and zinc, for example, have been shown to play key roles in the pathogenesis of glucose intolerance.13,14 Despite the accumulating evidence suggesting that certain metals may be able to ease the complications associated with diabetes, the American Diabetes Association does not recommend the supplementation of metals by patients with diabetes. Instead, it is recommended that various metals are supplemented in the form of changing dietary habits to include foods that are richer in metals. This is suggested because an oral supplement of a particular metal (such as those metals able to become redox active) may be at such a high dose that it will actually become pro-oxidant in the body and have the opposite effect. The metals or minerals (in the case of selenium) that have been suggested to play an important role in the pathogenesis and complications associated with diabetes and that will be reviewed here are copper, manganese, magnesium, iron, selenium, vanadium, chromium, and zinc.
Brief examinations of various metals in diabetes
Copper
Copper is an essential metal that is found in various enzymes throughout the body. Importantly, copper is a center in the enzymes cytochrome c oxidase and superoxide dismutase. Both of these enzymes are thought to be determinants in diabetes by their roles in regulating oxidant status. Copper deficiency has been linked to a decrease in glucose tolerance, and an increase in lipid peroxidation due to elevated hemoglobin A1C levels.15,16 An increase in copper concentration has been linked to disorders in the structure of arterial walls, stress, infection, and diabetes.17 There have also been reports on increased concentrations of copper in the liver and kidney of streptozotocin-induced diabetic rats.18 However, the reason for this increase and its resultant implications has not been fully explained, and it is not yet known whether the abnormalities in copper metabolism are a consequence of the disease, or whether they are key in the progression of the disease.Manganese
Similarly to copper, manganese is the center of manganese superoxide dismutase and plays an important role in the regulation of antioxidant defense functions. Appropriate levels of manganese are required for insulin synthesis and secretion,19 and a deficient level of manganese could result in poor glucose handling. It has been reported that people with diabetes who have been deemed insulin resistant respond well to oral doses of manganese.20 This may be due, in part, to the lower basal levels of manganese measured in the blood and hair of patients with diabetes compared to controls,21 although other reports determining the levels of manganese in the whole blood of diabetics and controls have not reported a deficiency compared to controls.22Magnesium
Magnesium is one of the most abundant intracellular ions and is a cofactor in more than 300 essential metabolic reactions. Type 2 diabetes is characterized by cellular and extracellular magnesium depletion.23–25 Magnesium has been shown to play an important role in insulin action. Also, insulin can stimulate the transport of magnesium from the extracellular to the intracellular compartment of a cell, thereby increasing the magnesium content available for producing energy using magnesium-dependent adenosine triphosphate (ATP).26 In addition to being critical for cellular energy regulation, red blood cell-derived ATP is a recognized stimulus of nitric oxide synthase and subsequent production of nitric oxide, a potent vasodilator.27 Such a role for Mg would have important implications in the microvasculature of people with diabetes. While it is difficult to provide a direct relationship between type 2 diabetes, ATP, and magnesium, it is interesting to note that our own group reported that the red blood cells of people with type 2 diabetes release less ATP than controls, although no studies involving magnesium were performed in association with the report.5A magnesium deficiency may also result in disorders of tyrosine kinase activity on the insulin receptor.28 This deficiency may be related to the development of insulin resistance and decreased cellular glucose utilization. Lower basal levels of magnesium would necessitate more insulin to metabolize the same glucose load, hence a decrease in insulin sensitivity. Furthermore, the link between insulin resistance and magnesium deficiency has been strengthened by the observation that several medications currently prescribed to treat type 2 diabetes also increase magnesium levels. For example, metformin, has been shown to increase levels of magnesium in the liver.29 Additionally, pioglitazone, an anti-diabetes drug that has been reported to increase insulin sensitivity, has also been shown to have positive actions on magnesium metabolism.30,31
Magnesium deficiency extends itself into the vascular complications often associated with diabetes. It has been reported that lower magnesium levels were associated with ischemic heart disease and severe retinopathy in patients with diabetes.32 Even in patients with type 1 diabetes, serum magnesium levels have been associated with early atherosclerosis.33 In patients with type 2 diabetes, low levels of magnesium have been associated with a more rapid decline of renal function.34
Oral supplementation of magnesium has also reportedly resulted in an improvement in insulin-mediated glucose uptake.35,36 Therefore, the literature does support the idea that a deficiency in magnesium is associated with complications associated with diabetes, the exact mechanism of how magnesium supplementation improves these complications is not clear.
Iron
Iron overload is a genetic disorder that results in increased levels of iron being produced within the body, which may have a role in type 2 diabetes. This phenomenon was first observed when it was noted that the frequency of diabetes was increased among people with hereditary hemochromatosis. Hemochromatosis is a hereditary condition, which is characterized by excessive absorption of dietary iron, resulting in a pathological increase in total body iron stores. Among individuals with type 1 hereditary hemochromatosis, up to 60% develop diabetes,37,38 which is characterized by a decrease in insulin production and a resistance to the insulin that is produced. The exact mechanism of iron-induced diabetes is still uncertain, however, a link has been made suggesting improvements in insulin sensitivity and insulin secretion with frequent blood donation or iron chelation therapy.39,40Iron overload also affects complications associated with diabetes. Several studies have reported increased amounts of iron in the kidneys of both animals and humans.41–43 Animal studies have provided evidence for the role of iron and oxidants in diabetic nephropathy. Various forms of oxidative stress have further contributed to the availability of intracellular iron that can worsen oxidative stress and renal damage. Once renal damage has occurred, there is considerable evidence to suggest that the damage continues to worsen over time. To slow the progression of kidney failure/disease or prevent initial kidney damage, an iron-deficient diet or chelators are prescribed.44–46
An increased iron level also places people with diabetes at a higher risk of cardiovascular disease than their healthy counterparts.47 As previously mentioned, people with diabetes suffer from cardiovascular disease at a much higher rate than the general population. There is a clear association between iron stores and total cholesterol, triglycerides, blood pressure, and glucose, factors that often are negatively impacted by diabetes.48 Similar to the treatment of nephropathy observed due to iron overload, the cardiovascular problems can be treated through blood donation or chelation.49–51 It is clear that iron overload plays an important role in the development of diabetes, however, similarly to the other metals discussed above, the exact mechanisms by which iron exerts its affects remain to be completely understood.
Selenium
Selenium is an essential trace element that is actively involved in defending against oxidative stress via selenium-dependent glutathione peroxidases and other selenoproteins. While it is not a metal, it has the ability to exhibit properties of metals and has become extremely important in the field of inorganic complexes for diabetes research. Because of its antioxidant defense properties, selenium may help prevent the development of diabetes due to oxidative stressors, although this hypothesis has been recently refuted by data that suggests that selenium supplementation may increase the risk for type 2 diabetes.52 Selenium is present in two major forms in the human diet; it can be derived from animal sources mainly in the form of selenocysteine, whereas selenium derived from plants is present predominantly in the form of selenomethionine. When taking selenium as a supplement or suggesting that selenium be used to treat certain ailments associated with diabetes, it is important to note that selenium levels over 900 μg day−1 are considered toxic, which can lead to health problems. As a supplement, selenium is frequently added in the form of an inorganic salt, sodium selenite (selenium oxidation state of +4) and sodium selenate (oxidation state of +6). To date, the inorganic form of selenium, selenate, has been shown to have beneficial effects for the treatment of diabetes.53–59There have been several reports describing the ability of selenate to have insulin-mimetic properties via its ability to increase glucose uptake into cells. Ezaki demonstrated that selenate stimulated glucose transport activity dose-dependently in isolated rat adipocytes.60 Similar to insulin, selenate was able to increase the glucose transport within two minutes after administration. Further demonstrating its insulin-mimetic effects, it was observed that the increase in glucose transport was due to translocation of the glucose transporters to the membrane surface. Similar studies have also been performed in rat muscle with comparable results.54
In addition to increasing glucose transport in in vitro studies, selenate has also been reported to increase glucose transportin vivo.56 Using streptozotocin-induced diabetic animals, the administration of selenate improved food and water intake and facilitated a decrease in plasma glucose levels within two weeks.
Several studies have also demonstrated selenate’s ability to mimic insulin with regard to glycolysis, gluconeogenesis, fatty acid synthesis, and the pentose phosphate pathway.61–64 When given oral supplementation, diabetic animals partly reversed abnormal liver expression of both glycogenic and gluconeogenic enzymes.61
Patients with diabetes often have depressed levels of lipid metabolism, which is believed to be a contributing factor to a higher risk of stroke and heart disease. Studies performed using streptozotocin-induced diabetic rats demonstrated the ability of selenate to improve glucose tolerance and normalize plasma glucose levels. Untreated diabetic rats had developed an increase in left ventricular pressure in comparison to the treated diabetic rats. Treatment with selenate was able to normalize this parameter to a level that was equal to controls suggesting that selenate may have the ability to improve heart function in people with diabetes.57
Selenate has also been shown to have insulin-mimetic effects on the expression of glucose-6-phosphate dehydrogenase (G6PD) in diabetic rats,54 which is important because G6PD activity is significantly lower in diabetes in comparison to controls.5–9G6PD is an important enzyme in maintaining the overall antioxidant defense status in cells. Upon treating diabetic rats with selenate, the G6PD levels were restored to about 80–90% of controls.
Like insulin, selenate has the ability to mediate signal transduction of an insulin signaling pathway. More specifically, selenate has been shown to stimulate phosphorylation of the β subunit of the insulin receptor.65 To observe these results, incubation of the cells with selenate took one hour as compared to insulin, whereas phosphorylation can be observed in minutes. This suggested that time was needed for selenate to enter a cell and mediate insulin-regulated processes through a post-insulin receptorkinase mechanism. To support these claims, evidence has also been presented demonstrating selenate’s ability to increase phosphorylation of the insulin receptor without directly increasing insulin receptor tyrosine kinase activity. Additionally, selenate has been shown to stimulate the phosphorylation of epidermal growth factor, and stimulate kinase activityin several other venues.66
Vanadium
Physiologically, vanadium can exist in two forms, vanadate, which is the anionic form, or vanadyl, which is the cationic form.67,68 Most of the intracellular vanadium is present as vanadyl, where it is bound to proteins and peptides , such as glutathione.68,69 The plasma concentration of vanadium is relatively low at 20 nM, and the total amount of vanadium in the body is estimated to be approximately 100–200 μg.67,70It has been known for over 100 years that vanadium can exhibit antidiabetic properties when administered to people with diabetes, and its first discovery as an orally active insulin-mimetic agent was described in 1985.71 Unlike other metals, vanadium has the ability to be beneficial to people with type 1 and people with type 2 diabetes.
Vanadium has been found to be insulin-mimetic and/or enhancing and is considered a potential candidate for oral therapy in type 1 diabetes. There have been several reports that have shown that prolonged treatment with vanadate or vanadyl restores plasma glucose levels and corrects hyperlipidemia in animal models of type 1 diabetes without a significant effect on plasma insulin levels.71–75 Additionally, it has been determined that vanadate has no significant effects on plasma insulin and glucose levels in control animals.71 Studies performed using the euglycemic hyperinsulinemic clamp technique have also observed improvement in the overall metabolic state of diabetic animals after treatment with vanadyl or vanadate. More specifically, it was determined that the vanadyl or vanadate treatment resulted in a significant increase in peripheral glucose utilization and complete normalization of elevated hepatic glucose output.76–78 The improvement in peripheral glucose uptake was determined to be associated with the correction of the GLUT4 transporter, a major glucose transporter regulated by insulin.75,79,80 In addition, it has been reported that streptozotocin-induced diabetic rats who were given an organic vanadium complex (vanadyl) in their drinking water for a period of 8 weeks saw an enhancement in the GLUT4 translocation to the plasma membrane in the cardiac muscle.81
Vanadium has also been shown to be beneficial for patients with type 2 diabetes. Various rat models of type 2 diabetes and insulin resistance, when treated with organic and inorganic vanadate or vanadyl, demonstrated significantly lower plasma insulin levels and improved insulin sensitivity.82–84 When using Zucker diabetic fatty rats, whose characteristics closely resemble type 2 diabetes in humans, it was determined that chronic treatment with vanadyl reduced the elevated plasma glucose levels.85 Similar to the results obtained from experiments of vanadate or vanadyl on type 1 rat models, when using Zucker fatty rats and the euglycemic hyperinsulinemic clamp, there has been an improvement in glucose homeostasis without inhibiting the hepatic glucose output and improving insulin sensitivity in the skeletal muscle.86 It is hypothesized that vanadium complexes improve insulin sensitivity by mimicking and enhancing the metabolic effects of insulin on these tissues. Vanadium has also been shown to restore the activity of key enzymes in glycogen metabolism, lipogenic enzymes, and is able to effectively preserve the pancreatic β-cell function in animal models.84,85,87–89
Vanadate has been shown to act on signaling pathways involved in glucose disposal, more specifically the phopsphatidylinositol-3-kinase (PI3-K) pathway and the mitogen-activated protein kinase (MAPK) pathway. It is believed that the PI3-K pathway mediates most of the metabolic effects of insulin, and the MAPK pathway is involved in mediating the mitogenic effects of insulin.90 Because, like insulin, vanadate stimulates both glucose uptake and glycogen synthesis, several insulin-receptor and post-receptor sites have been suggested as potential sites for vanadate action.91–94
Vanadate is recognized as a nonspecific protein tyrosine phosphatase inhibitor; therefore a possible mechanism for vanadate action would be that vanadate enhances insulin receptorphosphorylation indirectly.95,96 Specifically, two non-receptor protein tyrosine kinases were suggested to mediate some of the insulin-mimetic effects of vanadate.97,98
Currently the molecular mechanism by which vanadium mediates metabolic effects in rat models of diabetes is not well understood despite the established antidiabetic effects of vanadium. It has been suggested that vanadate or vanadyl has selective effects on the mechanisms responsible for exaggerated metabolic pathways during diabetes because of its ability to lower plasma glucose levels and restore diabetes-induced metabolic disorders without demonstrating the same effects on control animals. Unfortunately, most of the insulin-like effects of vanadium demonstrated in vitro are observed only in the presence of high (millimolar) concentrations of vanadium compounds, which is much higher than what is observed in vivo suggesting that the effects of vanadium may not have therapeutic relevance due to toxicity.
Chromium
Recently chromium has gained popularity as a carbohydrate burning supplement as well as a nutritional supplement for diabetic patients. While its role as a micronutrient has been known for over four decades, progress in elucidating its exact role has been slow. There are several different oxidation states of chromium, although the biologically important form of chromium is the trivalent form Cr3+.During the 1950s, Mertz and Schwarz discovered that rats fed a Torula yeast diet developed impaired glucose tolerance when given an intravenous glucose load.99 This resulted in the identification of a new dietary requirement called the glucose tolerance factor.100 This phenomenon was reversed when given a diet that was rich in chromium or when given inorganic chromium salts. These researchers determined the active ingredient of the glucose tolerance factor to be Cr3+.101 Natural sources of glucose tolerance factor were identified in brewer’s yeast and acid-hydrolyzed porcine kidney powder. Concentrates of the natural sources had the ability to restore proper glucose metabolism in deficient rats. While the separation and purification protocol was not specific, glucose tolerance factor was determined to be water-soluble, extractable with phenol and isobutanol, and absorbable on charcoal and ion exchange resins. Several additional inorganic compounds containing nearly all other transition metals were tested and none could restore glucose tolerance.101
The mechanism of action has been hypothesized to involve the interaction of Cr3+ with the activity of insulin. Several attempts were made over the course of decades to try to synthesize and characterize glucose tolerance factor. It was postulated that the glucose tolerance factor is a glutathione complex; however several inorganic chromium containing complexes seem to improve glucose tolerance.
During the 1980s a breakthrough occurred in establishing the mechanism of chromium at a molecular level. Wada and coworkers discovered a low molecular weight chromium binding substance termed chromodulin.102 It was determined to be a peptide containing only glycine, cysteine, glutamate, and aspartate with carboxylates composing more than half of the amino acid residues.103,104 This substance was determined to have a molecular weight of only 1500 kDa. Despite this, the peptide is able to bind four equivalents of chromic ions. To date this oligopeptide has been isolated and purified from rabbit liver,105 bovine liver,106 porcine kidney and kidney powder,107 dog liver,108 and mouse and rat liver.109 Based on the isolation and purification data, it appears that chromodulin is widely distributed among mammals. The most important attribute of chromodulin is its ability to potentiate the effects of insulin during the conversion of glucose into carbon dioxide or lipid as determined using isolated rat adipocytes.110–112 This stimulation of insulin is accomplished without changing the concentration of insulin required, suggesting that chromodulin plays an intrinsic role in the adipocytes.113
Chromodulin has also been implicated in signal transduction. Recently chromodulin has been shown to result in the activation of a membrane phosphotyrosine phosphatase114 and an insulin-sensitive stimulation of insulin receptortyrosinekinase activity.103,110 The activation of these signal transduction pathways was determined to be dependent on the chromium content of the oligopeptide. Substituting chromium for another transition metal was shown to be ineffective in restoring the ability of apochromodulin to stimulate kinase activity, indicating that the activation is chromium specific.
Chromodulin has also been suggested to play a role in insulin signaling.110,115 It has been reported that chromodulin is stored in the cytosol of insulin-sensitive cells and is able to move from the blood to insulin-dependent cells in the presence of increasing plasma insulin.116,117 It has been suggested that this movement is due to the metal transport protein transferrin.
With all of the data suggesting a role for chromium in glucose metabolism, chromium has been marketed as a nutritional supplement, used frequently during weight training and exercise. It is sold as chromium picolinate, which has become extremely profitable and is available over the counter in the form of pills, chewing gum, sports drinks, and nutrition bars. Chromium picolinate is absorbed relatively well in the body with 2–5% efficiency compared to dietary chromium, which is absorbed with only 0.5% efficiency.118,119 While chromium picolinate has been proposed to be the biologically active form of chromium,120 it has not been shown to exist in vivo.
During a double-blind crossover study that involved volunteers taking 200 μg of chromium as chromium picolinate per day, it was reported that the volunteers had decreased total cholesterol, LDL cholesterol, and apolipoprotein B and increased apolipoprotein A.121 Studies performed using cultured rat skeletal muscle myoblasts found increased insulin internalization and increased glucose and leucine uptake when exposed to media containing chromium picolinate. These results were not obtained when using the same concentration of chromic chloride, chromium nicotinate, or zinc picolinate.120
Recently, studies have made a link between type 2 diabetes and chromium. Specifically, individuals with type 2 diabetes have lower serum and higher urine levels of chromium.122,123 However, many early reports suggest that chromium has the ability to lower blood glucose levels or to have an effect on cholesterol, triglycerides or insulin after several weeks.124,125 More recently, a study performed in China demonstrated the ability of chromium picolinate to lower fasting serum glucose levels and hemoglobin A1C levels with increasing chromium.126 It has been suggested that a similar study needs to be performed in the United States as the body mass indexes of the Chinese patients differs significantly from American type 2 diabetics.127 A comprehensive study on the effectiveness of chromium supplementation as it pertains to diabetes by Balk concluded that chromium supplementation did not have an effect on patients without diabetes; however chromium supplementation significantly improved glycemia among patients with diabetes.128
Recently, our group has shown that chromium activated C-peptide had the ability to increase the levels of ATP released by red blood cells obtained from people with type 2 diabetes to levels that were statistically equivalent to controls.129 This is important because it has previously been reported that the ATP release of red blood cells obtained from type 2 diabetics was less than their healthy human counterparts.5,130 As previously mentioned, ATP is a recognized stimulus of nitric oxide synthase, the enzyme responsible for subsequent production of nitric oxide, a potent vasodilator.
C-peptide is a peptide that is secreted in equimolar amounts to insulin and has negatively charged glutamates at four positions that are responsible for the metal–C-peptide interaction. C-peptide has been shown to ameliorate several complications associated with diabetes, although a successful long-term study has not been demonstrated.131–136 It has been reported that without a metal for activation, C-peptide cannot elicit an ATP release from red blood cells. However, it was determined that chromium is not the only metal that is capable of activating C-peptide. Even the slightest contamination in the purification process of C-peptide can result in the presence of trace metals, especially iron or chromium, both of which have been reported to elicit ATP release from red blood cells in combination with C-peptide. However, it has not been determined which metal, if any, is bound to C-peptide in vivo. It has been speculated that the lack of a successful long-term study may be a result of having no metal present or the contamination of a metal that does not possess optimal binding chemistry to C-peptide.
Zinc
Insulin is secreted by the β-cell both tonically and as a spike in response to an increase in blood glucose concentrations. It is well established that there is a relationship between insulin and zinc. Before any evidence of a relationship between zinc and insulin in the β-cell, it was known that the addition of zinc to insulin would extend the effect of a given dose of insulin. When insulin was first prescribed as a treatment for type 1 diabetes, zinc was added in vitro to prolong the duration of the action of insulin by delaying its absorption from the subcutaneous injection site.When produced in the β-cells of the pancreatic islets, insulin is produced in a solid hexamer around zinc.137–142 The levels of zinc in the β-cells at any given time are near millimolar levels, although most of this zinc is not available in the free form. There are two Zn2+ ions per insulin hexamer, however Zn2+ continues to accumulate even after it is bound to the hexamer.142 As shown in Fig. 2, proinsulin, the peptide containing insulin and C-peptide, is located inside vesicles within the pancreas. The zinc transporter, ZnT-8, is a transmembrane transporterprotein that facilitates Zn2+ into the vesicles. Here, the insulin forms a hexamer around the Zn2+, followed by the addition of more Zn2+ into the vesicle. Eventually, the vesicle reaches the surface of the β-cell where insulin, C-peptide, and Zn2+ are released into the β-cell. Insulin is secreted in equimolar amounts to C-peptide, although the concentration present in the bloodstream may not be the same as C-peptide, it may be hypothesized that the biologically active form of C-peptide is likely to be bound to zinc, however this has not been determined to be true. Beyond the physical conformational data demonstrating the importance of zinc in relationship to insulin, there has been data suggesting that the conformational changes that occur in the presence of zinc also affect the receptor binding and antigenic properties of insulin.143 In addition, data suggests that insulin binds to isolated liver membranes to a greater extent and that there is less degradation when zinc is co-administered with insulin.
 | ||
| Fig. 2 Mechanism of insulin/C-peptide secretion and the role of zinc ions in the pancreatic β-cell. Zinc accumulates in the vesicles throughout the insulin maturation, storage, and secretion processes. | ||
Patients with diabetes often suffer from hypozincemia, which may be the result of hyperzincuria or decreased absorption of zinc, or both. While it is clear that patients with diabetes secret more zinc in the urine than individuals without diabetes, there is less data suggesting that the there is malabsorption of zinc. It appears that hyperzincuria is a result of hyperglycemia more than any specific effect of insulin. There is conflicting data as to whether insulin treatment reduces hyperzincuria.144–147
Elevated levels of zinc have been found in the liver, muscle and kidney in streptozotocin-induced diabetic rats.148 This is significant because these are the tissues that are responsive to insulin-mediated glucose transport. However, when using rats that spontaneously become diabetic, hyperglycemia was associated with reduced levels of zinc in the liver, kidney, and muscle.149
Zinc has also been implicated in the cellular oxidant status of people with diabetes. Because type 1 diabetes is thought to be the result of an autoimmune attack on the β-cell, the destruction of these cells results in less bioavailable zinc for use in important antioxidant enzymes. It has been proposed that the lack of zinc available for use in the enzymes may contribute to the tissue damage observed in diabetes.150
Zinc also plays a role in the progression of type 2 diabetes. While there is no solid evidence for oxidative stress as a major factor in the development of either insulin deficiency or islet cell damage, there is evidence of increased levels of insulin secretion, and therefore zinc, in the early stages of the disease.151 It is possible for the pancreas to synthesize more insulin; however, the pancreas cannot synthesize more zinc, which will result in the depletion of zinc from the pancreas. Without zinc, the pancreas may not function correctly and could result in complete destruction of the islet. This hypothesis proves a mechanism by which zinc deficiency may affect the progress of type 2 diabetes.
Challenges involving metals in diabetes
While sufficient evidence exists suggesting that metals are determinants in the development and treatment of diabetes, several mechanistic questions concerning the role of these metals still remain. For example, there is considerable data demonstrating the insulin-mimetic effects of selenate, although a mechanism describing the effect of this metal is not clear, especially when considering that most insulin-mimetic functions do not require selenium.The example involving selenium brings forth another challenge for investigations involving metals in diabetes. That is, although many reports in the literature describe concentration decreases or increases of a particular metal in association with diabetes, there is very little explanation given as to what effect this may have on the pathogenesis of diabetes. Specifically, is the abnormal level of the metal a result of the diabetes, a cause, or perhaps even a homeostatic attempt by the cell, tissue, organelle, or subject to rectify a parallel condition associated with the disease? To answer such questions, experts in the field of measuring metals may have to perform similar measurements over a longer period of time in order to gain some level of understanding as to when abnormal levels of these metals begin to appear in their samples, cultures, or subjects.
Another challenge involving the role of metals in diabetes, and one which may be the most difficult to meet, is the determination of the metal’s location and its availability to participate in certain biological functions. The use of C-peptide for diabetic complications is an excellent representation of such a challenge. As mentioned above, C-peptide has recently been shown to display activity on red blood cells when pre-activated with exogenous metal. Unfortunately, to date, no studies have been performed to identify which metals are bound to C-peptide in vivo. It would seem likely that if a metal were bound to the C-peptide, it would be zinc due to the high concentration of zinc (∼millimolar) in the β-cells. However, confirming that zinc is bound to C-peptide in vivo would be difficult. The concentration of plasma C-peptide is generally thought to be approximately 1–10 nM, a level that is near the lower detection limits of many spectroscopic techniques. Furthermore, this concentration of C-peptide does not guarantee a similar concentration of zinc because many of the zinc ions would be displaced by more highly concentrated metals in the bloodstream such as sodium and potassium. Of course, measuring zinc in whole blood is possible and well within the detection range of most spectroscopic methodologies. However, as mentioned above, such a measurement becomes more difficult if the analyst requires additional knowledge of the zinc’s location; that is, the zinc could be bound to the albumin , a cell membrane, the C-peptide, or a separate peptide or protein. In addition, as shown in Fig. 3, it would be beneficial to have knowledge as to which amino acid the zinc is bound. Studies are already underway in the authors’ laboratories to determine the binding properties. Of course, this binding changes when the studies are performed in water as opposed to a physiological salt solution containing other metals at concentrations found in vivo.
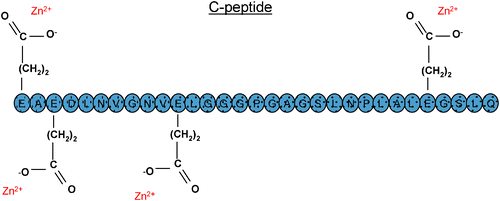 | ||
| Fig. 3 The location of the glutamic acid amino acid residueson C-peptide and their hypothesized interaction with Zn2+. | ||
The challenges listed here are the opinion of the authors and, as such, most readers of this review will undoubtedly be thinking about challenges in their own research endeavors. Overall, it does seem that many of the questions still remaining to be answered would be helped by new detection methods that continue to lower the current detection limits, improve sensitivity, and are robust enough to be performed under physiological conditions. Over the years, spectroscopists involved in metal determinations have always seemed to rise to these challenges and find novel ways of solving problems and making the important measurements. This author is confident that this trend will continue and that the role of metals in diabetes will be further clarified.
References
- D. Bonnefont-Rousselot, J. P. Bastard, M. C. Jaudon and J. Delattre, Diabetes Metab., 2000, 26, 163–176 CAS.
- A. Maritim, B. A. Dene, R. A. Sanders and J. B. Watkins III, J. Biochem. Mol. Toxicol., 2003, 17, 193–199 CrossRef CAS.
- Y. J. Suzuki, H. J. Forman and A. Sevanian, Free Radical Biol. Med., 1996, 22, 269–285.
- M. Raththagala, P. D. Root and D. M. Spence, Anal. Chem., 2006, 78, 8556–8560 CrossRef CAS.
- J. Carroll, M. Raththagala, W. Subasinghe, S. Baguzis, T. D. a. Oblak, P. Root and D. Spence, Mol. BioSyst., 2006, 2, 305–311 RSC.
- C. Costagliola, Clin. Physiol. Biochem., 1990, 8, 204–210 Search PubMed.
- R. S. Gaskin, D. Estwick and R. Peddi, Ethn. Dis., 2001, 11, 749–754 Search PubMed.
- X. M. Leverve, B. Guigas, D. Detaille, C. Batandier, E. A. Koceir, C. Chauvin, E. Fontaine and N. F. Wiernsperger, Diabetes Metab., 2003, 29, 6S88–86S94 CAS.
- W. Subasinghe and D. M. Spence, Anal. Chim. Acta, 2008, 618, 227–233 CrossRef CAS.
- B. Wierusz-Wysocka, H. Wysocki, H. Byks, D. Zozulinska, A. Wykretowicz and M. Kazmierczak, Diabetes Res. Clin. Pract., 1995, 27, 193–197 CrossRef CAS.
- D. Bonnefont-Rousselot, J. Soc. Biol., 2001, 195, 391–398 Search PubMed.
- J. J. Cunningham, J. Am. Coll. Nutr., 1998, 17, 7–10 Search PubMed.
- R. A. Anderson, J. Am. Coll. Nutr., 1998, 17, 548–555 Search PubMed.
- M. J. Salgueiro, N. Krebs, M. B. Zubillaga, R. Weill, E. Postaire, A. E. Lysionek, R. A. Caro, T. De Paoli, A. Hager and J. Boccio, Biol. Trace Elem. Res., 2001, 81, 215–228 CrossRef CAS.
- J. T. Saari, Nutr. Res. (New York, NY, U. S.), 1994, 14, 1689–1699 Search PubMed.
- J. T. Saari, Nutr. Res. (New York, NY, U. S.), 1996, 16, 467–477 Search PubMed.
- D. Beshgetoor and M. Hambidge, Am. J. Clin. Nutr., 1998, 67, 1017S–1021S CAS.
- N. E. Craft and M. L. Failla, Am. J. Physiol., 1983, 244, E122–E128 CAS.
- G. J. Naga Raju, P. Sarita, G. A. V. Ramana Murty, M. Ravi Kumar, B. S. Reddy, M. J. Charles, S. Lakshminarayana, T. S. Reddy, S. B. Reddy and V. Vijayan, Appl. Radiat. Isot., 2006, 64, 893–900 CrossRef CAS.
- C. Ekmekcioglu, C. Prohaska, K. Pomazal, I. Steffan, G. Schernthaner and W. Marktl, Biol. Trace Elem. Res., 2001, 79, 205–219 CrossRef CAS.
- T. G. Kazi, H. I. Afridi, N. Kazi, M. K. Jamali, M. B. Arain, N. Jalbani and G. A. Kandhro, Biol. Trace Elem. Res., 2008, 122, 1–18 CrossRef CAS.
- R. M. Walter, Jr, J. Y. Uriu-Hare, K. L. Olin, M. H. Oster, B. D. Anawalt, J. W. Critchfield and C. L. Keen, Diabetes Care, 1991, 14, 1050–1056 CrossRef CAS.
- M. de Lordes Lima, T. Cruz, J. C. Pousada, L. E. Rodrigues, K. Barbosa and V. Cangucu, Diabetes Care, 1998, 21, 682–686 CrossRef CAS.
- H. W. de Valk, Neth. J. Med., 1999, 54, 139–146 CrossRef CAS.
- N. L. Eibl, H. P. Kopp, H. R. Nowak, C. J. Schnack, P. G. Hopmeier and G. Schernthaner, Diabetes Care, 1995, 18, 188–192 CrossRef CAS.
- M. Barbagallo, R. K. Gupta and L. M. Resnick, Diabetologia, 1993, 36, 146–149 CrossRef CAS.
- R. S. Sprague, M. L. Ellsworth, A. H. Stephenson and A. J. Lonigro, Am. J. Physiol., 1996, 271, H2717–H2722 CAS.
- O. G. Kolterman, R. S. Gray, J. Griffin, P. Burstein, J. Insel, J. A. Scarlett and J. M. Olefsky, J. Clin. Invest., 1981, 68, 957–969 CrossRef CAS.
- S. Ewis and M. S. Abdel-Rahman, J. Appl. Toxicol., 1995, 15, 387–390 CrossRef CAS.
- F. Guerrero-Romero and M. Rodriguez-Moran, Exp. Clin. Endocrinol. Diabetes, 2003, 111, 91–96 Search PubMed.
- J. Nadler and S. Scott, Biochem. Biophys. Res. Commun., 1994, 202, 416–421 CrossRef CAS.
- M. Barbagallo, R. K. Gupta and L. M. Resnick, Diabetes Care, 1996, 19, 1393–1398 CrossRef CAS.
- M. E. Atabek, S. Kurtoglu, O. Pirgon and M. Baykara, Diabetes Res. Clin. Pract., 2006, 72, 42–47 CrossRef CAS.
- P. C. T. Pham, P. M. T. Pham, P. A. T. Pham, S. V. Pham, H. V. Pham, J. M. Miller, N. Yanagawa and P. T. T. Pham, Clin. Nephrol., 2005, 63, 429–436 Search PubMed.
- F. Guerrero-Romero and M. Rodriguez-Moran, Arch. Med. Res., 2005, 36, 250–257 CrossRef CAS.
- G. Paolisso and M. Barbagallo, Am. J. Hypertens., 1997, 10, 346–355 CrossRef CAS.
- I. W. Dymock, J. Cassar, D. A. Pyke, W. G. Oakley and R. Williams, Am. J. Med., 1972, 52, 203–210 CrossRef CAS.
- D. L. Witte, W. H. Crosby, C. Q. Edwards, V. F. Fairbanks and F. A. Mitros, Clin. Chim. Acta, 1996, 245, 139–200 CrossRef CAS.
- J. M. Fernandez-Real, A. Lopez-Bermejo and W. Ricart, Clin. Chem. (Washington, D. C.), 2005, 51, 1201–1205 CrossRef CAS.
- R. Jiang, J. E. Manson, J. B. Meigs, J. Ma, N. Rifai and F. B. Hu, JAMA, J. Am. Med. Assoc., 2004, 291, 711–717 Search PubMed.
- D. C. H. Harris, C. Tay and B. J. Nankivell, Clin. Exp. Pharmacol. Physiol., 1994, 21, 73–81 CrossRef CAS.
- B. J. Nankivell, R. A. Boadle and D. C. Harris, Am. J. Kidney Dis., 1992, 20, 580–584 Search PubMed.
- B. J. Nankivell, J. Chen, R. A. Boadle and D. C. H. Harris, J. Am. Soc. Nephrol., 1994, 4, 1598–1607 CAS.
- A. C. Alfrey, D. H. Froment and W. S. Hammond, Kidney Int., 1989, 36, 753–759 CrossRef CAS.
- K. A. Nath, M. Fischereder and T. H. Hostetter, Kidney Int. Suppl., 1994, 45, S111–115 Search PubMed.
- A. Remuzzi, S. Puntorieri, B. Brugnetti, T. Bertani and G. Remuzzi, Kidney Int., 1991, 39, 647–652 CrossRef CAS.
- A. I. Schafer, R. G. Cheron, R. Dluhy, B. Cooper, R. E. Gleason, J. S. Soeldner and H. F. Bunn, N. Engl. J. Med., 1981, 304, 319–324 CAS.
- S. Kiechl, J. Willeit, G. Egger, W. Poewe and F. Oberhollenzer, Circulation, 1997, 96, 3300–3307 CAS.
- C. Borgna-Pignatti, M. D. Cappellini, P. De Stefano, G. C. Del Vecchio, G. L. Forni, M. R. Gamberini, R. Ghilardi, A. Piga, M. A. Romeo, H. Zhao and A. Cnaan, Blood, 2006, 107, 3733–3737 CrossRef CAS.
- G. M. Brittenham, P. M. Griffith, A. W. Nienhuis, C. E. McLaren, N. S. Young, E. E. Tucker, C. J. Allen, D. E. Farrell and J. W. Harris, N. Engl. J. Med., 1994, 331, 567–573 CrossRef CAS.
- G. Fabio, F. Minonzio, P. Delbini, A. Bianchi and M. D. Cappellini, Blood, 2007, 109, 362–364 CrossRef CAS.
- S. Stranges, J. R. Marshall, R. Natarajan, R. P. Donahue, M. Trevisan, G. F. Combs, F. P. Cappuccio, A. Ceriello and M. E. Reid, Ann. Intern. Med., 2007, 147, 217–223.
- A. S. Mueller and J. Pallauf, J. Nutr. Biochem., 2006, 17, 548–560 CrossRef CAS.
- E. A. Berg, J. Y. Wu, L. Campbell, M. Kagey and S. R. Stapleton, Biochimie, 1995, 77, 919–924 CrossRef CAS.
- A. S. Mueller, J. Pallauf and J. Rafael, J. Nutr. Biochem., 2003, 14, 637–647 CrossRef CAS.
- J. H. McNeill, H. L. M. Delgatty and M. L. Battell, Diabetes, 1991, 40, 1675–1678 CrossRef CAS.
- M. L. Battell, H. L. M. Delgatty, L. M. Delgatty and J. H. McNeill, Mol. Cell. Biochem., 1998, 179, 27–34 CrossRef CAS.
- A. S. Mueller, E. Most and J. Pallauf, J. Anim. Physiol. Anim. Nutr., 2005, 89, 94–104 CrossRef CAS.
- A. S. Mueller, A. Bosse and J. Pallauf, Curr. Nutr. Food Sci., 2006, 2, 151–168 Search PubMed.
- O. Ezaki, J. Biol. Chem., 1990, 265, 1124–1128 CAS.
- D. J. Becker, B. Reul, A. T. Ozcelikay, J. P. Buchet, J. C. Henquin and S. M. Brichard, Diabetologia, 1996, 39, 3–11 CrossRef CAS.
- F. Bosch, M. Hatzoglou, E. A. Park and R. W. Hanson, J. Biol. Chem., 1990, 265, 13677–13682 CAS.
- T. M. Johnson, M. H. Meisler, M. I. Bennett and G. R. Willsky, Diabetes, 1990, 39, 757–759 CrossRef CAS.
- M. Miralpeix, J. F. Decaux, A. Kahn and R. Bartrons, Diabetes, 1991, 40, 462–464 CrossRef CAS.
- S. R. Stapleton, G. L. Garlock, L. Foellmi-Adams and R. F. Kletzien, Biochim. Biophys. Acta, 1997, 1355, 259–269 CAS.
- T. S. Pillay and M. W. Makgoba, FEBS Lett., 1992, 308, 38–42 CrossRef CAS.
- S. M. Brichard and J.-C. Henquin, Trends Pharmacol. Sci., 1995, 16, 265–270 CrossRef CAS.
- K. H. Thompson, J. H. McNeill and C. Orvig, Chem. Rev., 1999, 99, 2561–2571 CrossRef CAS.
- M. C. Cam, R. W. Brownsey and J. H. McNeill, Can. J. Physiol. Pharmacol., 2000, 78, 829–847 CrossRef CAS.
- I. Goldwaser, D. Gefel, E. Gershonov, M. Fridkin and Y. Shechter, J. Inorg. Biochem., 2000, 80, 21–25 CrossRef.
- C. E. Heyliger, A. G. Tahiliani and J. H. McNeill, Science (Washington, D.C.), 1985, 227, 1474–1477 CrossRef CAS.
- S. M. Brichard, W. Okitolonda and J. C. Henquin, Endocrinology, 1988, 123, 2048–2053 CrossRef CAS.
- M. C. Cam, R. A. Pederson, R. W. Brownsey and J. H. McNeill, Diabetologia, 1993, 36, 218–224 CrossRef CAS.
- S. Ramanadham, J. J. Mongold, R. W. Brownsey, G. H. Cros and J. H. McNeill, Am. J. Physiol., 1989, 257, H904–H911 CAS.
- H. V. Strout, P. P. Vicario, C. Biswas, R. Saperstein, E. J. Brady, P. F. Pilch and J. Berger, Endocrinology, 1990, 126, 2728–2732 CrossRef CAS.
- O. Blondel, D. Bailbe and B. Portha, Diabetologia, 1989, 32, 185–190 CrossRef CAS.
- M. Matsuda, L. Mandarino and R. A. DeFronzo, Metab., Clin. Exp., 1999, 48, 725–731 Search PubMed.
- N. Venkatesan, A. Avidan and M. B. Davidson, Diabetes, 1991, 40, 492–498 CrossRef CAS.
- S. J. Kopp, J. Daar, D. J. Paulson, F. D. Romano and R. Laddaga, J. Mol. Cell. Cardiol., 1997, 29, 2355–2362 CrossRef CAS.
- A. Mohammad, V. Sharma and J. H. McNeill, Mol. Cell. Biochem., 2002, 233, 139–143 CrossRef CAS.
- S. H. Li and J. H. McNeill, Mol. Cell. Biochem., 2001, 217, 121–129 CrossRef CAS.
- S. Bhanot, A. Michoulas and J. H. McNeill, Mol. Cell. Biochem., 1995, 153, 205–209 CrossRef CAS.
- J. Meyerovitch, P. Rothenberg, Y. Shechter, S. Bonner-Weir and C. R. Kahn, J. Clin. Invest., 1991, 87, 1286–1294 CrossRef CAS.
- S. Pugazhenthi, J. F. Angel and R. L. Khandelwal, Metab., Clin. Exp., 1991, 40, 941–946 Search PubMed.
- V. G. Yuen, E. Vera, M. L. Battell, W. M. Li and J. H. McNeill, Diabetes Res. Clin. Practice, 1999, 43, 9–19 Search PubMed.
- Y. Shechter and S. J. D. Karlish, Nature (London), 1980, 284, 556–558 CAS.
- R. L. Khandelwal and s. Pugazhenthi, Mol. Cell. Biochem.., 1995, 153, 87–94 CrossRef CAS.
- S. Pugazhenthi, J. F. Angel and R. L. Khandelwal, Mol. Cell. Biochem., 1993, 122, 77–84 CrossRef CAS.
- V. G. Yuen, R. A. Pederson, S. Dai, C. Orvig and J. H. McNeill, Can. J. Physiol. Pharmacol., 1996, 74, 1001–1009 CrossRef CAS.
- Y. J. Hei, J. Pharmacol. Toxicol. Methods, 1998, 40, 123–135 CrossRef CAS.
- A. S. Clark, J. M. Fagan and W. E. Mitch, Biochem. J., 1985, 232, 273–276 CAS.
- A. B. Goldfine, D. C. Simonson, F. Folli, M.-E. Patti and C. R. Kahn, Mol. Cell. Biochem., 1995, 153, 217–231 CrossRef CAS.
- A. Green, Biochem. J., 1986, 238, 663–669 CAS.
- T. K. Jackson, A. I. Salhanick, J. D. Sparks, C. E. Sparks, M. Bolognino and J. M. Amatruda, Diabetes, 1988, 37, 1234–1240 CrossRef CAS.
- I. G. Fantus and E. Tsiani, Mol. Cell. Biochem., 1998, 182, 109–119 CrossRef CAS.
- N. Sekar, J. Li and Y. Schechter, Crit. Rev. Biochem. Mol. Biol., 1996, 31, 339–359 CrossRef CAS.
- G. Elberg, Z. He, J. Li, N. Sekar and Y. Shechter, Diabetes, 1997, 46, 1684–1690 CrossRef CAS.
- A. Shisheva and Y. Shechter, J. Biol. Chem., 1993, 268, 6463–6469 CAS.
- W. Mertz and K. Schwarz, Arch. Biochem. Biophys., 1955, 58, 504–506 CAS.
- K. Schwarz and W. Mertz, Arch. Biochem. Biophys., 1957, 72, 515–518.
- K. Schwarz and W. Mertz, Arch. Biochem. Biophys., 1959, 85, 292–295.
- O. Wada, S. Manabe, N. Yamaguchi, S. Ishikawa and H. Yanagisawa, Ind. Health, 1983, 21, 35–41 CrossRef CAS.
- C. M. Davis and J. B. Vincent, Biochemistry, 1997, 36, 4382–4385 CrossRef CAS.
- A. Yamamoto, O. Wada and H. Suzuki, J. Nutr., 1988, 118, 39–45 CAS.
- A. Yamamoto, O. Wada and T. Ono, Eur. J. Biochem., 1987, 165, 627–631 CrossRef CAS.
- C. M. Davis and J. B. Vincent, Arch. Biochem. Biophys., 1997, 339, 335–343 CrossRef CAS.
- K. H. Sumrall and J. B. Vincent, Polyhedron, 1997, 16, 4171–4177 CrossRef CAS.
- O. Wada, G. Y. Wu, A. Yamamoto, S. Manabe and T. Ono, Environ. Res., 1983, 32, 228–239 CrossRef CAS.
- A. Yamamoto, O. Wada and T. Ono, J. Inorg. Biochem., 1984, 22, 91–102 CrossRef CAS.
- C. M. Davis and J. B. Vincent, JBIC, J. Biol. Inorg. Chem., 1997, 2, 675–679 CrossRef CAS.
- W. Mertz, E. E. Roginski and K. Schwarz, J. Biol. Chem., 1961, 236, 318–322 CAS.
- J. B. Vincent, J. Nutr., 1994, 124, 117–119 CAS.
- A. Yamamoto, O. Wada and S. Manabe, Biochem. Biophys. Res. Commun., 1989, 163, 189–193 CrossRef CAS.
- C. M. Davis, K. H. Sumrall and J. B. Vincent, Biochemistry, 1996, 35, 12963–12969 CrossRef CAS.
- J. B. Vincent, J. Am. Coll. Nutr., 1999, 18, 6–12 Search PubMed.
- B. W. Morris, T. A. Gray and S. Macneil, Clin. Sci., 1993, 84, 477–482 Search PubMed.
- B. W. Morris, S. MacNeil, K. Stanley, T. A. Gray and R. Fraser, J. Endocrinol., 1993, 139, 339–345 Search PubMed.
- R. A. Anderson, N. A. Bryden, M. M. Polansky and K. Gautschi, J. Trace Elem. Exp. Med., 1996, 9, 11–25 CrossRef CAS.
- K. L. Olin, D. M. Stearns, W. H. Armstrong and C. L. Keen, Trace Elem. Electrolytes, 1994, 11, 182–184 Search PubMed.
- G. W. Evans and T. D. Bowman, J. Inorg. Biochem., 1992, 46, 243–250 CrossRef CAS.
- R. I. Press, J. Geller and G. W. Evans, West. J. Med., 1990, 152, 41–45 CAS.
- B. W. Morris, H. Griffiths and G. J. Kemp, Clin. Chem., 1988, 34, 1525–1526 CAS.
- B. W. Morris, G. J. Kemp and C. A. Hardisty, Clin. Chem., 1985, 31, 334–335 CAS.
- L. Sherman, J. A. Glennon, W. J. Brech, G. H. Klomberg and E. S. Gordon, Metab., Clin. Exp., 1968, 17, 439–442 Search PubMed.
- M. I. Uusitupa, J. T. Kumpulainen, E. Voutilainen, K. Hersio, H. Sarlund, K. P. Pyorala, P. E. Koivistoinen and J. T. Lehto, Am. J. Clin. Nutr., 1983, 38, 404–410 CAS.
- R. A. Anderson, N. Cheng, N. A. Bryden, M. M. Polansky, N. Cheng, J. Chi and J. Feng, Diabetes, 1997, 46, 1786–1791 CrossRef CAS.
- M. K. Hellerstein, Nutr. Rev., 1998, 56, 302–306 Search PubMed.
- E. M. Balk, A. Tatsioni, A. H. Lichtenstein, J. Lau and A. G. Pittas, Diabetes Care, 2007, 30, 2154–2163 CrossRef CAS.
- J. A. Meyer, J. M. Froelich, G. E. Reid, W. K. A. Karunarathne and D. M. Spence, Diabetologia, 2008, 51, 175–182 CAS.
- R. S. Sprague, A. H. Stephenson, E. A. Bowles, M. S. Stumpf and A. J. Lonigro, Diabetes, 2006, 55, 3588–3593 CrossRef CAS.
- T. Forst and T. Kunt, Exp. Diabesity Res., 2004, 5, 51–64 CrossRef CAS.
- Z.-g. Li and A. A. F. Sima, Exp. Diabesity Res., 2004, 5, 79–90 CrossRef CAS.
- A. A. F. Sima and H. Kamiya, Sci. Med. (Narberth, PA, U. S.), 2004, 9, 308–319 Search PubMed.
- A. A. F. Sima, H. Kamiya and Z. G. Li, Eur. J. Pharmacol., 2004, 494, 77 CrossRef CAS.
- A. F. Sima Anders, J. Neurol. Sci., 2004, 220, 133–136 CrossRef CAS.
- P. Vague, T. C. Coste, M. F. Jannot, D. Raccah and M. Tsimaratos, Exp. Diabesity Res., 2004, 5, 37–50 CrossRef CAS.
- P. D. Zalewski, S. H. Millard, I. J. Forbes, O. Kapaniris, A. Slavotinek, W. H. Betts, A. D. Ward, S. F. Lincoln and I. Mahadevan, J. Histochem. Cytochem., 1994, 42, 877–884 CAS.
- E. N. Baker, T. L. Blundell, J. F. Cutfield, S. M. Cutfield, E. J. Dodson, G. G. Dodson, D. M. Crowfoot Hodgkin, R. E. Hubbard and N. W. Isaacs et al., Philos. Trans. R. Soc. London, Ser. B, 1988, 319, 369–456 CrossRef CAS.
- M. L. Brader and M. F. Dunn, Trends Biochem. Sci., 1991, 16, 341–345 CrossRef CAS.
- J. Brange and L. Langkjoer, Pharm. Biotechnol., 1993, 5, 315–350 Search PubMed.
- U. Derewenda, Z. Derewenda, G. G. Dodson, R. E. Hubbard and F. Korber, Br. Med. Bull., 1989, 45, 4–18 Search PubMed.
- F. Chimienti, A. Favier and M. Seve, BioMetals, 2005, 18, 313–317 Search PubMed.
- E. R. Arquilla, P. Thiene, T. Brugman, W. Ruess and R. Sugiyama, Biochem. J., 1978, 175, 289–297 CAS.
- A. el-Yazigi, N. Hannan and D. A. Raines, Diabetes Res., 1993, 22, 67–75 Search PubMed.
- V. K. Garg, R. Gupta and R. K. Goyal, J. Assoc. Physicians India, 1994, 42, 720–721 Search PubMed.
- J. Honnorat, M. Accominotti, C. Broussolle, A. C. Fleuret, J. J. Vallon and J. Orgiazzi, Biol. Trace Elem. Res., 1992, 32, 311–316 CrossRef CAS.
- T. Isbir, L. Tamer, A. Taylor and M. Isbir, Diabetes Res., 1994, 26, 41–45 Search PubMed.
- A. Cordova, Biol. Trace Elem. Res., 1994, 42, 209–216 CrossRef CAS.
- I. Raz, J. H. Adler and E. Havivi, Diabetologia, 1988, 31, 329–333 CAS.
- A. Rabinovitch, W. L. Suarez, P. D. Thomas, K. Strynadka and I. Simpson, Diabetologia, 1992, 35, 409–413 CrossRef CAS.
- J. E. Sprietsma and G. E. Schuitemaker, Med. Hypotheses, 1994, 42, 15–23 CrossRef CAS.
- A. C. Nsonwu, C. A. O. Usoro, M. H. Etukudo and I. N. Usoro, Turk Biyokim. Derg., 2006, 31, 107–114 Search PubMed.
- M. Navarro-Alarcon, H. L.-G. De La Serrana, V. Perez-Valero and C. Lopez-Martinez, Sci. Total Environ., 1999, 228, 79–85 CrossRef CAS.
- A. Stern, X. Yin, S. S. Tsang, A. Davison and J. Moon, Biochem. Cell Biol., 1993, 71, 103–112 Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |
