In vivo proton HR-MAS NMR metabolic profile of the freshwater cladoceran Daphnia magna
Andrei
Bunescu
*ab,
Jeanne
Garric
a,
Bernard
Vollat
a,
Emmanuelle
Canet-Soulas
c,
Danielle
Graveron-Demilly
c and
Florence
Fauvelle
b
aCemagref, UR MALY, Laboratory of Ecotoxicology, 3 bis quai Chauveau, Lyon, France
bMolecular and Cellular Biophysics, NMR Laboratory, CRSSA, 24, Avenue des maquis du Grésivaudan, BP 87 – 38702, La Tronche Cedex, France. E-mail: andrei.bunescu@gmail.com; Fax: +33 4 7663 6922; Tel: +33 4 7663 6884
cLaboratoire CREATIS-LRMN, Université Claude Bernard Lyon 1, CNRS UMR 5220, Inserm U630, INSA Lyon, Villeurbanne, France
First published on 29th September 2009
Abstract
The method concerning in vivo proton HR-MAS NMR metabolic profiling of the freshwater cladoceran Daphnia magna is presented. Viability tests of D. magna under different spinning rates were performed. All surviving daphnids after analysis have developed eggs and embryos like control animals. Better survival rate at the slowest rotation speed were observed. The maximum length of analysis during which the integrity of the daphnid is maintained was assessed. The recorded proton spectra of in vivo daphnia were attributed to lipids from the triglycerol category. Saturated and unsaturated omega-3 like fatty acid moieties of triacylglycerol were well identified. The relationship between physiological state of daphnids and lipid profile are discussed.
Introduction
An NMR-based metabolomic approach is one of the leading techniques used in the past years for drug safety and toxicology evaluation1,2 as well as for assessment of environmental stressors on organism health.3 The methodology is based on the analysis of the end products of the organism metabolism, e.g.aminoacids, hydrocarbons and lipids, present in biological material such as biofluids, cells, intact tissues or extracts.However, the current procedure for high resolution liquid NMR analysis generally requires chemical extraction of the molecules of interest, which is time consuming and totally destructive for the organism. In the past years, proton high resolution magic-angle spinning (HR-MAS) NMR spectroscopy has been developed as an alternative approach. The intact sample is spun at high speed around an axis at 54.7° from the main static field B0, thus reducing line-broadening effects present in inhomogeneous samples: the resulting 1H NMR spectra are highly resolved and “liquid-like”. Recently, this technique has found numerous applications in the medical field for intact biopsy analysis,4e.g. in cancer research,5 and increasing applications in other research areas are rapidly developing.
However, very few studies are found in literature concerning living organisms. As NMR spectroscopy is a non invasive method, it could be relevant to combine the HR-MAS technique to an in vivo approach.6–8 The very first in vivo proton HR-MAS NMR experiments of invertebrates was carried out on the fruit fly Drosophila melanogaster.9 Nevertheless, the high rotation speeds classically used in HR-MAS NMR (1 to 12 kHz) can irreversibly affect the studied organism. As a consequence, viability tests, following the NMR analysis, are necessary to enable finding the optimal experimental conditions. These viability tests concern basic vital functions. In the case of flies, a survival rate of 67% was observed up to three hours after 20 min at 2 kHz spinning, but no other tests were conducted on surviving flies.
In the present study, we directed our attention on the aquatic crustacean cladoceran Daphnia magna. D. magna is a well-known and widely used aquatic model in ecotoxicology to evaluate the short and long term impact of toxic compounds at physiological and genomic levels.10,11 Indeed, they are easily bred under laboratory conditions as they have a short life cycle and a parthenogenetic reproduction. These enable us to obtain information at different levels of biological organization sub-individual, individual and population, from genetically identical organisms. Moreover, daphnids are sensitive to biotic and abiotic factors e.g. food, temperature, organic and inorganic xenobiotics, which are likely to disrupt their homeostatic metabolism and reproduction. As a consequence, among others, the energetic resources of daphnids are mobilized in order to restore the initial metabolic state or to offset the action of the external stressors.12In vivo31P and 1H NMR studies of D. magnametabolism regarding the evaluation of aquatic toxicity, are found in literature.7,8 However, in these papers, the spectra were obtained for a population of several hundreds of daphnids concentrated in a usual NMR tube, so the data could not be precisely related to the age or the physiological state of individual organisms. Furthermore, 1H NMR spectra of these hundreds of daphnids were poorly resolved and difficult to quantitate.
Our study focuses on an alternative approach based on HR-MAS NMR analyses of alive D. magna organisms in order to describe variations of their metabolic profile at successive life stages. Viability of surviving organisms was assessed by measuring their ability to reproduce. In this paper, we present our first results concerning the optimal experimental conditions as well as the observed changes of the HR-MAS metabolic profile of daphnids at different physiological status. Thus, the feasibility of our approach is demonstrated.
Results
Viability of Daphnia magna under HR-MAS analysis condition
Our previous observation showed that awake daphnids poorly recovered after HR-MAS analysis. Thus, we anaesthetized daphnids during NMR analysis in using ms-222, a compound classically used for aquatic organisms. The anaesthetic conditioning showed that 24 h old daphnids exposed to 1 mg mL−1ms-222 solution for 4 h, recovered without significant effects on their survival.The viability experiments were carried out for different rotation speeds. Thus, it can be noticed from Fig. 1 that the first days after analysis were critical for daphnids. At the beginning of the fourth day, the survival rate had stabilized to 84%; 77%; 75% and 37% for 0 Hz; 1500 Hz; 2000 Hz and 3600 Hz, respectively. From the eighth day, daphnids developed eggs and were used for breeding as control daphnids, e.g. at the age of 7–9 days old.
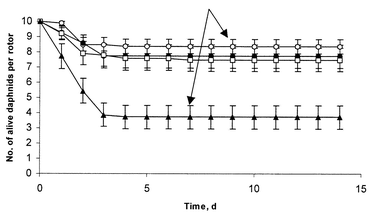 | ||
| Fig. 1 Survival of ten 24 h old daphnids after HR-MAS analysis at different speed during 14 days of observation: 0 Hz (○) n = 8; 1500 Hz (●) n = 7; 2000 Hz (□) n = 10 and 3600 Hz (▲) n = 7, where n is the number of replications of the analysis. The surviving daphnids developed eggs at 7–9 days after analysis (arrow) similar with control daphnids. Data are represented as mean ± SEM. | ||
The integrity of a 7 day old daphnid during long time rotation at 2000 Hz was also verified by acquiring several spectra after 11.5 h. In the first spectrum acquired, immediately after insertion of the daphnid in the rotor, only lipidic resonances were observed (see Fig. 2, bottom), like in spectra presented in Fig. 3 considered as reference. The spectra started to evolve after 3.5 h of analysis, i.e. some additional sharp peaks appeared in the spectra. The intensity of these signals increased until 11.5 h (Fig. 2, top) of analysis, after that no evolution was observed. At 3600 Hz spinning rate, similar signals appeared earlier after about 2.0 h of analysis.
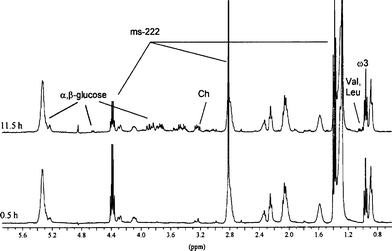 | ||
| Fig. 2 Evolution with time of the in vivo proton HR-MAS NMR spectra of a 7 day old D. magna (with eggs in brood pouch). | ||
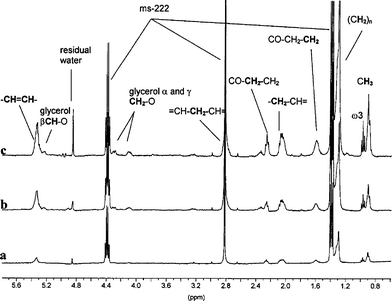 | ||
| Fig. 3 In vivo proton HR-MAS NMR spectra of daphnids at different stage of development: (a) ten daphnids, 24 h old, (b) one daphnid, 7 day old with and (c) one daphnid, 7 day old without eggs in the brood pouch. The spinning rate of the rotor was 2000 Hz. | ||
Attribution of signals in the proton spectrum
The attribution of resonances was made according to literature.13 All peaks arise from lipid molecules. In the region of methyl groups (around 0.9 ppm), two peaks are distinguished (see Fig. 3). The well resolved triplet at 0.95 ppm is due to polyunsaturated fatty acids (PUFA) from the omega-3 category, while the broad resonance at 0.9 ppm is classically attributed to all other terminal methyl groups from aliphatic chains. Next to these resonances, other peaks are found at 1.3, 1.6, 2.1, 2.3 and 2.8 ppm which differ in intensity and correspond to methylenyl protons. The resonances at 1.3 ppm and 2.8 ppm are superposed with peaks of ms-222. The differences in chemical shifts of these methylenyl groups are due to different neighbour functional groups (double bond or carboxyl, see Fig. 3). Close to 5.4 ppm can be found resonances of double bond protons. The 4.1, 4.3 and 5.2 ppm resonances arise from protons of a glycerol molecule, which is completely esterified by fatty acids, e.g.triglycerideslipids.It is well known that daphnids cannot synthesise high quantities of lipidsde novo, so they achieve dietary lipids by feeding algae. So, lipid resonances measured in our first experiments could have arisen from algae located in a daphnid’s gut. To test such a possibility, daphnids were exposed for about 4 h to a suspension of micro-latex beads14 to clear their gut prior to HR-MAS NMR analysis. The HR-MAS NMR spectra of daphnids treated as described above are identical to spectra of non-treated daphnids (data not presented).
Thus, all proton HR-MAS NMR spectra contain the same signals (in terms of chemical shift) regardless of the presence or not of algae, whatever their age or their physiological status, e.g. with or without eggs in their brood pouch.
Lipidic profile of daphnids at different physiological states
Proton HR-MAS NMR spectra of daphnids of different physiological status are displayed in Fig. 3. As noted above, the same peaks were observed on spectra obtained from juvenile organisms and from adults with and without eggs. However, their intensities widely varied. Thus, the spectrum of 7 day old daphnids with eggs (Fig. 3c) was much more intense than those of daphnids without eggs (Fig. 3b), and of 24 h old (Fig. 3a). Moreover, in this latter case, 10 organisms were used while only one adult was analysed (Fig. 3b and c).Discussion
A first in vivo proton HR-MAS NMR approach for analysis of the crustacean Daphnia magnametabolism has been presented.Our early results show that D. magna could be recuperated alive after HR-MAS NMR analysis under several experimental conditions. After a 15 min HR-MAS NMR analysis, a good survival rate was observed, which however decreased in the next 2–3 days for daphnids spun at the highest tested speeds (77%—1500 Hz; 75%—2000 Hz; 37%—3600 Hz). This could be explained by damages caused by spinning and related constraints15 (centrifugation forces). However, the effect of sample preparation (zero spinning) cannot be neglected as we observed also some mortalities (16%). Nevertheless, the survival rates become stable after the fourth day. The surviving daphnids present a normal life cycle, as they developed eggs and embryos as control organisms did.
We observed that the HR-MAS NMR profile remains invariable until 2–3 h of rotation. Thus, the analysis length has an important role for survival and must be taken into account if long lasting acquisitions are required for more complex NMR experiments. Since organisms are completely enclosed in a small water volume (50 μl), a possible depletion of dissolved oxygen cannot be neglected, even if metabolism activity of daphnid is slowed by sedation. The sharp peaks that appear approximately after 3.5 h at 2000 Hz spinning rate could arise from contributions of aminoacids, carbohydrates and other small organic molecules, that are released in solution by mechanical lyses of the organs and cells of daphnids.15 These small molecules probably become observable because they are less restricted and they diffuse from cells into interstitial space. This hypothesis was confirmed by acquiring HR-MAS NMR spectra of the mechanically homogenised daphnid. Hence, the NMR profile obtained was identical to those measured after 11.5 h of spinning. Moreover, microscopic analysis of daphnids after 11.5 h has shown that the organs were altered by rotation. Thus, the apparition of sharp resonances could reflect the degradation of the analysed organism, closely related to its death. However, we can consider that in most acquisitions, which last 15 min, hypoxia is reduced.
According to these results, the optimal conditions for the in vivoHR-MAS NMR approach can be stated as follows, use: (i) a sedative; (ii) a rotation speed close to 2000 Hz or less; (iii) an optimal acquisition time of about 15 min, a maximum acquisition time of 2–3 h.
The proton NMR spectra of D. magna correspond to lipids, in particular neutral lipids like triglycerides from droplets stored within cells in the daphnid body cavity.16 The resonances corresponding to saturated and unsaturated fatty acid moieties like omega-3 are well identified, so it is possible to quantify the lipid unsaturation index. This is a clue parameter to follow the life cycle of daphnids since it has an important role in the reproduction capability of daphnids. The accumulation of lipids in the adult daphnids has been previously reported16–18 Thus the essential fatty acids are allocated to the eggs and are necessary for embryonic development. Beside reproduction, triacylglycerol and phospholipids have an important role for energetic metabolism of daphnids.19 The lipid level inside daphnids tends to increase between molts in a cyclic manner and is the highest just before egg laying.16 Hence, in the spectrum of the 7 day old daphnids with eggs lipidic resonances are more intense than in the spectrum of the 7 day old daphnids without eggs, due to the contribution of the eggs. Indeed, variations in the spectra of daphnids at different stage of development have been shown in Fig. 3.
Similar results were obtained by gas chromatography (GC) analysis of the lipid content of daphnids.20 The study showed that the total quantity of fatty acids was different in juvenile and adult daphnids, and moreover, was proportional to the age. But, in order to quantify fatty acids by GC analysis, they must be transesterified in the fatty acid methyl esters and hence the information about the nature of lipids is lost e.g.triacylglycerol, phospholipids or free fatty acids. However, both GC and HR-MAS NMR methods are complementary, since the latter gives access to the part of lipids that are mobile, on the NMR timescale, and not to the complete set of lipids measured21 by GC.
Beside demonstrating the feasibility of in vivoHR-MAS NMR of Daphnia magna, we showed that the spectra are closely related to their physiological state. More generally, the in vivoHR-MAS NMR profiling of D. magna rapidly enables us to follow up the metabolism of daphnids during their entire life cycle. Further work will concern quantitation of resonances in order to obtain data regarding the lipidic profile of the organisms, as well as their inter-individual variability depending on the development stage.
This will offer the opportunity to assess modifications of the lipidic profile generated by natural or anthropogenic stressors, like chemical stressors, at the individual level. The consequences of these perturbations on major life traits like growth and reproduction could next be measured.
Experimental section
Animal culture
D. magna clones were cultured at 20 ± 1 °C in 2 L bottles filled with Evian® mineral water and a 16 h light: 8 h dark photoperiod and fed with the green alga Pseudokirchineriella subcapitata (about 6.7 × 104 cells per mL). The population density was 12 daphnids per litre of culture medium. All neonates less than 24 h old, were collected from second brood females. Each week a half of the culture medium was renewed.Anaesthetic conditioning
A solution of anaesthetic, 1 mg mL−1, was prepared by dissolving ethyl 3-aminobenzoate methanesulfonic acid (ms-222) in the ISO medium22 in D2O. The pH of the solution was adjusted to 7.8–8.2 using 0.1 M sodium hydroxide.In order to evaluate the maximal exposure time to the anaesthetic which did not lead to any effect on the organisms, five 24 h old daphnids were maintained 0 h, 0.5 h, 1 h, 1.5 h, 2.0 h, 3.0 h and 4 h (n = 3) in 5 mL of anaesthetic solution. In about 7 min, all daphnids were immobilised. At the end of exposure, daphnids were rinsed and transferred in a 20 mL test tube, then kept in the dark for 24 h to measure the survival rate.
Gut clearance
Gut clearance was done as described previously12 by exposing D. magna to 10 mL suspension of micro-latex beads for 2–4 h (106 beads mL−1, Latex beads, Sigma-Aldrich).Organism preparation
For HR-MAS NMR investigation, daphnids of different age and state of development have been used. Firstly, we focused our attention on the juvenile 24 h old and adult 7 day old daphnids. Secondly we investigated adult 7 day old daphnids with and without eggs in their brood pouch. As the reproduction of daphnids is parthenogenetic, all analysed organisms were females. Each HR-MAS rotor contained one organism, except the analyses made with 24 h old daphnids. In the latter case, 10 organisms have been used in order to obtain a signal-to-noise ratio and a biomass similar to the 7 day old daphnids. The day of analysis, selected daphnids were transferred from the culture medium into a 100 mL bottle with clean Evian® water without food.Prior to HR-MAS analysis, daphnids were anaesthetized with the anaesthetic solution directly in the 50 μL zirconium rotor. Before being put in the rotor, daphnids were placed on a microscope lamella using Pasteur’s pipettes, and rinsed twice with 40 μl ISO medium in D2O (11) in order to eliminate H2O and contamination from culture medium. Next, the rotor was filled with 10 μL of 1 mM 3-(trimethylsylyl)propionic acid (TSP-d4, as chemical shift reference with δ = 0.0 ppm) and 80 μL of the sedative solution. Daphnids were aspired with Pasteur’s pipettes from microscope lamella and let to swim from droplet into the rotor. The 40 μL excess of solution was removed with a micropipette from the rotor. After about 7 min, the organism was anaesthetised and the rotor was sealed with insert and cap.
Viability assessment under different rotation speeds
Following the HR-MAS NMR analysis, the survival and the reproduction ability of the analysed organisms have been assessed. Therefore, 10 daphnids 24 h old (n = 7) per zirconium rotor were spun during 15 min at three different rotation speeds: 1500, 2000 and 3600 Hz. The complete protocol was also realized without spinning the rotor (indicated 0 Hz for spinning rate on Fig. 1), to test the sole effect of manipulating and inserting the daphnids into the rotor. Afterwards, surviving organisms were placed in a 1 L culture medium. Surviving daphnids were counted and fed daily as standard culture for 14 days. The dead daphnids were eliminated from the culture. The control group consisted in daphnids in their standard breeding conditions.NMR Spectroscopy
All samples were analysed using a Bruker Avance 400 DMX spectrometer with triple-tuned 1H–13C–31P HR-MAS probe operating at a proton frequency of 400.13 MHz. The proton spectra were acquired in 15 min and 256 transients (16k data-points) using a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with an echo-time 30 ms to attenuate water resonance and macromolecules peaks. The inter-pulse delay was synchronised with the rotation speed. The spinning rate was set between 1500 and 3600 Hz. The temperature was adapted to the rotation speed in order to obtain 20 °C (293 K) inside the rotor, a temperature similar to that of D. magna culture medium.To assess the sample integrity under continuous rotation at 2000 Hz, the same acquisition conditions were used but with 512 transients during 30 min.
Acknowledgements
The authors are thankful for Andrei Bunescu’s post-doctoral fellowship supported by Cemagref, Lyon and to CRSSA for supplying the NMR equipmentReferences
- J. L. Griffin, Drug Discovery Today: Technol., 2004, 1, 285 CrossRef CAS.
- M. E. Lenz, J. M. Weeks, J. C. Lindon, D. Osborn and J. K. Nicholson, Metabolomics, 2005, 1, 123 CrossRef.
- M. R. Viant, E. S. Rosenblum and R. S. Tjeerdema, Environ. Sci. Technol., 2003, 37, 4982 CrossRef CAS.
- H. Rabeson, F. Fauvelle, G. Testylier, A. Foquin, P. Carpentier, F. Dorandeu, D. van Ormondt and D. Graveron-Demilly, Magn. Reson. Med., 2008, 59, 1266 CrossRef CAS.
- B. Sitter, T. F. Bathen, M.-B. Tessem and I. S. Gribbestad, Prog. Nucl. Magn. Reson. Spectrosc., 2009, 54, 239 CrossRef CAS.
- J.-P. Grivet and A.-M. Delort, Prog. Nucl. Magn. Reson. Spectrosc., 2009, 54, 1 CrossRef CAS.
- T. W. Waller and A. D. Sherry, Bull. Environ. Contam. Toxicol., 1981, 26, 73 CrossRef.
- Z. Shirzadi and A. Simson, In vivo NMR of Daphnia magna. MOOT XIX NMR Symposium, University of Guelph, Guelph, Ontario, September 23–24, 2006.
- D. Minitzopoulos, Y. Apidianakis, M. N. Minidrinos, R. G. Tompkins, L. G. Rahme and A. A. Tzika, In vivo proton HRMAS detects effects of trauma in Drosophila melanogaster in Proceedings of the Joint Annual Meeting ISMRM-ESMRMB, Berlin, Germany, 2007, vol. 15, p. 3175 Search PubMed.
- L.-H. Heckmann, R. M. Sibly, R. Connon, H. L. Hooper, T. H. Hutchinson, S. J. Maund, C. J. Hill, A. Bouetard and A. Callaghan, GenomeBiology, 2008, 9, R40 CrossRef.
- V. Tonkopii and I. Iofina, AATEX 14, 2007, Special issue, 565.
- W. M. De Coen and C. R. Janssen, Hydrobiologia, 1998, 367, 199 CrossRef CAS.
- F. Desmoulin, D. Bon, R. Martino and M. Malet-Martino, C. R. Chimie, 2008, 11, 423 Search PubMed.
- P. L. Gillis, P. Chow-Fraser, J. F. Ranville, P. E. Ross and C. M. Wood, Aquat. Toxicol., 2005, 71, 143 CrossRef CAS.
- J. L. Taylor, C.-L. Wu, D. Cory, R. G. Gonzalez, A. Bielecki and L. L. Cheng, Magn. Reson. Med., 2003, 50, 627 CrossRef.
- A. J. Tessier and C. E. Goulden, Limnol. Oceanogr., 1982, 27, 707.
- A. Wacker and D. Martin-Creuzberg, Funct. Ecol., 2007, 21, 738 CrossRef.
- C. Becker and M. Boersma, Limnol. Oceanogr., 2005, 50, 388 CAS.
- P. M. M. Weers and R. D. Gultati, Limnol. Oceanogr., 1997, 42, 1584 CAS.
- C. Barata, J. C. Navarro, I. Varo, M. C. Riva, S. Arun and C. Porte, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2005, 140, 81 CrossRef.
- O. Cruciani, L. Mannina, A. P. Sobolev, C. Cametti and A. L. Segre, Molecules, 2006, 11, 334 Search PubMed.
- ISO (International Organization for Standardization). Water quality – Determination of the mobility of Daphnia magna Straus (Cladocera, Crustacea), ISO 6341-19892, 1989.
| This journal is © The Royal Society of Chemistry 2010 |
