Receptor-independent modulation of reconstituted Gαiprotein mediated by liposomes†
Paola
Luciani
a,
Debora
Berti
*a,
Martina
Fortini
a,
Piero
Baglioni
a,
Carla
Ghelardini
b,
Alessandra
Pacini
c,
Dina
Manetti
d,
Fulvio
Gualtieri
d,
Alessandro
Bartolini
b and
Lorenzo
Di Cesare Mannelli
b
aDepartment of Chemistry and CSGI, University of Florence, Via della Lastruccia 3, 50019 Sesto Fiorentino, Florence, Italy. E-mail: debora.berti@unifi.it
bDepartment of Preclinical and Clinical Pharmacology, University of Florence, Viale Pieraccini 6, 50134, Florence, Italy
cDepartment of Anatomy, Histology and Forensic Medicine, Anatomy Section, University of Florence, Viale Morgagni 85, 50134, Florence, Italy
dDepartment of Pharmaceutical Sciences, University of Florence, Via U. Schiff 6, 50019 Sesto Fiorentino, Florence, Italy
First published on 29th January 2009
Abstract
A cationic amphiphile, BC5 (N-pentadecylpiperidin-4-amine), was recently designed and tested for its ability to directly stimulate the activity of recombinant Gα inhibitory subunits. However, amphiphilic drugs can self-associate and bind to plasma membranes, causing undesired side effects. In this contribution, we report on the incorporation of BC5 in 1,2-dipalmytoyl-sn-glycerophosphocoline (DPPC) liposomes and on the characterization of the mixed DPPC/BC5 systems at various lipid/drug mole ratios by means of dynamic light scattering, differential scanning calorimetry and fluorescence spectroscopy. The myristoylated Gαi subunit (Gα-mir) was reconstituted in 1,2-dimiristoyl-sn-glycerophosphocoline (DMPC) bilayers, as a mimic of the drug target. We compare several reconstitution procedures in liposomes and present for the first time a complete characterization of a Gα subunit reconstitution in model membranes in terms of protein activity as a function of the reconstitution protocol. The incorporation of the drug in DPPC bilayers resulted in enhanced Gi-modulating efficiency (evaluated in terms of binding to GTPγS (guanosine-5′-(γ-thio)-triphosphate)). A correlation of the physico-chemical features and binding activity of protein-containing membrane model is proposed.
Introduction
Heterotrimeric Gαβγ complexes are composed of three subunits associated with the inner surface of cell membranes. The key role of G proteins in the complex intracellular signaling1–3 justifies their importance as pharmaceutical targets.Damaged G proteins are involved in several pathological conditions: mutations in Gα inhibitory subunit (Gαi) codifying genes have been associated with tumors4–6 and there is increasing evidence for implications in infections, inflammations, neurological and cardiovascular diseases and endocrine disorders.7,8 Moreover, a hypofunctionality of Gαi in lymphocytes of cephalalgic and fibromialgic patients was demonstrated.9,10
G-protein activity modulation in these altered conditions can be obtained following two different strategies. The conventional approach, consisting in the pharmacological activation of G-protein-coupled receptors, can produce some drawbacks, due to the recruitment of several effectors11 or based on the fact that some pathological conditions, such as cardiac hypertrophy, are mediated by persistent stimulation of multiple receptors coupled with G proteins.12 The second method consists of the design of compounds able to interact directly with the subunits.13–18 A receptor-independent stimulation can unfold new approaches and offer unprecedented selectivity for drug treatment. The direct targeting of intracellular G proteins would allow modulating individual effector pathways, altering specific signals from particular G protein classes or subclasses, and modifying the kinetics of G protein signalling.
The common structural feature of receptor-independent drugs is the simultaneous presence of a hydrophobic part and a net positive charge. A novel series of low molecular weight derivatives, belonging to the previous class, was found able to stimulate Gi-protein signalling pathway in human lymphocytes and to activate isolated recombinant Gαiproteins.19,20 Among these derivatives, a 4-aminopiperidinic derivative, named BC5 (Chart 1), has been shown to modulate cAMP levels in a recombinant system, reconstituted with the isoform 1 of Gαi subunit (Gαi1) and the intracellular fragments of adenylate cyclase.21
 | ||
| Chart 1 Structure of BC5. | ||
BC5 is a surface-active drug,22 with a hydrophobic portion and a cationic head group at physiological pH. Formulation and screening of surface-active drugs represents a particularly critical issue, considering their concentration-dependent self-assembly and membrane lytic properties.
A drug delivery system can overcome the above side effects and vehicle the drug efficiently and safely to the target. The use of liposomes as biodegradable or biocompatible drug carriers to enhance the efficacy and reduce the toxicity of therapeutics is now well established.23Lipid vesicles provide a lipophilic environment that prevents an indiscriminate direct interaction between the surface-active drug and plasma membranes, together with a remarkable versatility. Incorporation of amphiphilic drugs in the bilayer may induce leakiness affecting its fluidity, shift or even eliminate the gel (quasi-crystalline two-dimensional lattice) to liquid crystalline (two-dimensional fluid bilayer) phase transition. The phase properties of a lipid bilayer determine in turn permeability, aggregation, protein binding and eventually liposomal fusion. Understanding the influence of drug incorporation in the carrier and correlating the physico-chemical characterization with the therapeutical behavior represent an essential achievement in the design of more effective vectors.
A correct physiological functionality of G proteins24 requires anchoring to a lipid bilayer; the co-translational conjugation of a myristoyl group to the N-terminus of the members of Gα family promotes the association of the heterotrimer with the membrane according to a two-signal membrane trapping model.25 Mutations that prevent myristoylation or palmitoylation may severely interfere with protein functionality. A complete study on the Gα subunit functionality should therefore include this fatty acid modifications, and take into proper account the importance of a hydrophobic environment. A reconstitution of the myristoylated Gα subunit in model plasma membranes represents the simplest way to mimic the lipidic milieau. It becomes therefore essential to design supramolecular systems able to host the myristoylated protein so that the secondary structure is retained and protein functionality is preserved.
The aim of this study is twofold: (a) the first purpose is the design of a suitable membrane mimic to host the myristoylated Gαi subunit (Gα-mir), where the effect of several preparation protocols on protein functionality is tested; (b) secondly, we set up and test a drug delivery system able to enhance therapeutic effects, or to lower the proven toxicity26 of cationic surface active drugsin vivo.
In order to improve the protocols of Gα-mir reconstitution in lipid vesicles with preservation of protein functionality, the effects of freeze-thaw cycles, lamellarity, extrusion procedures and lipid chain length on Gαi activity were evaluated. BC5 surface activity was assessed by means of surface tension. Liposomes containing increasing amounts of BC5 were prepared and characterized by means of dynamic light scattering (DLS), differential scanning calorimetry (DSC) and fluorescence. BC5-loaded liposomes were then tested for their ability to stimulate binding of GTPγS to recombinant membrane-bound Gαiproteins, which is a measure of direct interaction with Gαiproteins. Finally, correlations between the physico-chemical and biological features of both drug delivery systems and protein-containing membrane models were established.
Results
DMPC/Gα-mir preparation protocols
In order to produce an intracellular-like environment able to host and possibly enhance protein activity, lipid vesicles were prepared and different protocols of protein association to the bilayer were tested.‡Gα-mir-loaded liposomes were prepared following different procedures, as shown in Fig. 1 (see Experimental for details), to select the best protocol able to leave protein functionality unaltered and to give the best direct activation.
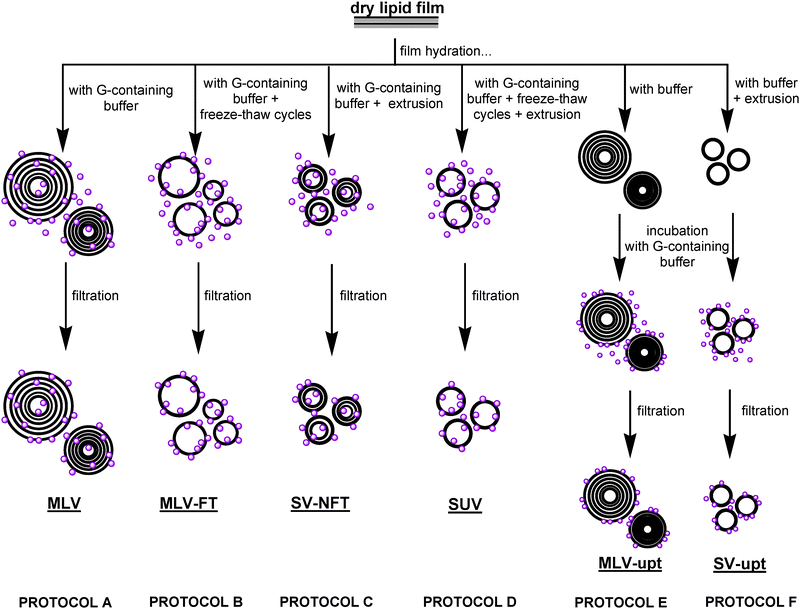 | ||
| Fig. 1 Schematic representation of the different DMPC/Gα-mir preparation protocols. Gα-mir molecules are represented as purple balloons. MLV: multilamellar vesicles hydrated with Gα-mir containing buffer; MLV-FT: multilamellar vesicles hydrated with Gα-mir containing buffer subjected to freeze-thaw cycles; SV-NFT: multilamellar vesicles hydrated with Gα-mir containing buffer subjected to extrusion; SUV: extruded small unilamellar vesicles hydrated with Gα-mir containing buffer; MLV-upt: preformed multilamellar vesicles incubated with Gα-mir containing buffer; SV-upt: preformed small extruded vesicles incubated with Gα-mir containing buffer. | ||
After each preparation protocol, the unbound fraction of Gα-mir was removed by filtration with Vivaspin tubes (Sartorius, as detailed in Experimental).
G protein activity was then evaluated by GTPγS binding at 30 °C both in basal conditions and in the presence of BC5 1 × 10−5M (concentration near the EC50 = 12.02 ± 2.19 μM). The α subunit of G protein binds GTP in the active state; then, through an intrinsic hydrolytic activity, it hydrolyzes GTP in GDP (bound in the inactive state). The amount of the non-hydrolyzable analogous GTPγS bound to the alpha subunit is considered the measure of the G protein activation.
To assess the efficiency of Gα-mir protein modulation in different environments, it is useful to define an adimensional parameter, the stimulation ratio r, which takes into account the variations of the basal activity in the presence of different media and the possible side effects due to the aspecific binding between GTPγS and BC5. r is defined as r = [(substrate bound to protein with BC5) − (substrate bound to BC5)]/(substrate bound to protein without BC5).
Different reconstitution procedures, yielding different protein distributions and accessibilities, yielded different results both in terms of protein basal functionality and addressability by BC5.
As a reference, we take r = 3.62 ± 0.14 for BC5 1 × 10−5 M in TRISBuffer; if “naked” BC5 (i.e. non-liposomal BC5) is then added at the same concentration to solutions of Gα-mir hosted in different liposomal systems, any r values higher than 3.62 would represent an overall improvement of BC5-mediated activation for such drug concentration.
The basal activity mirrors protein availability to GTPγS: Gα-mir-loaded DMPC MLV liposomes (MLV, protocol A) showed a lower basal activity (see Table 1) and a remarkable reduction of the aspecific binding (multilamellar vesicles without protein; data not shown). As a result, a higher BC5 stimulatory activity was observed (r = 4.50 ± 0.21, see Table 1). Extruded vesicles not subjected to freeze-thaw cycles (SV-NFT, protocol C) were able to induce simultaneously a lower aspecific effect and a higher basal activity (r = 2.65 ± 0.05). Protocol D (G protein inserted in extruded vesicles subjected to freeze-thaw cycles, SUV) allowed observing the highest basal activity but an unmodified aspecific effect with respect to the liposome-free condition (r = 1.48 ± 0.09).
| Protocol | Basal activity | r(BC5 = 1 × 10−5 M) | Protocol | Basal activity | r(BC5 = 1 × 10−5 M) | ||
|---|---|---|---|---|---|---|---|
| TRIS | / | 9.16 ± 0.89 | 3.62 ± 0.14 | SUV | D | 17.33 ± 2.27 | 1.48 ± 0.09 |
| MLV | A | 4.73 ± 0,85 | 4.50 ± 0.21 | SUV-upt | F | 14.38 ± 0.79 | 2.28 ± 0.08 |
| MLV-upt | E | 11.82 ± 0.49 | 2.58 ± 0.11 | SV-NFT | C | 11.21 ± 0.34 | 2.48 ± 0.04 |
| MLV-FT | B | 6.40 ± 0.69 | Nd |
Protein uptake on preformed liposomes, both multilamellar and unilamellar (protocol E and F), preserved Gα functionality as well (data not shown). As a consequence of these preliminary investigations, MLV (multilamellar vesicles) and SV-NFT (extruded vesicles not subjected to freeze-thaw cycles) were chosen as model membranes, together with the two other dispersions derived from protocol E and F. Physico-chemical and biological characterization of Gα subunit, reconstituted in vesicles characterized by different extent of lamellarity and different sizes, were performed on filtered (in order to remove the unbound protein) and not filtered systems.
Circular dichroism
Circular Dichroism (CD) spectroscopy provides indication of protein folding, as it allows the characterization of secondary and tertiary structures of proteins in native, unfolded, and partially folded states. The ellipticity at 222 nm is usually correlated with the α-helical content of a protein. The thermal stability of the Gα-mir subunit and protein folding reversibility were investigated by means of circular dichroism.At room temperature, the far-UV CD spectrum of Gα-mir in TRISbuffer (50 mM TRIS, pH 8.0) shows an ellipticity pattern typical of a high content of α-helical portions (double minima at 222 and 208 nm). Upon heating, a progressive and expected loss of the α-helical conformation may be observed and a typical melting profile, reported in Fig. 2, was obtained. The solution at 60 °C was then cooled down to 20 °C, and the upscan repeated. The 222 nm molar ellipticity was not recovered upon cooling, revealing a thermal sensitivity. CD spectra of the myristoylated Gαi subunit in buffer (full spectra acquired during melting are available in the ESI† ) are analogous to those previously obtained20 on unmodified species; this similar behavior rules out any possible effect of the myristoyl graft on the protein secondary structure.
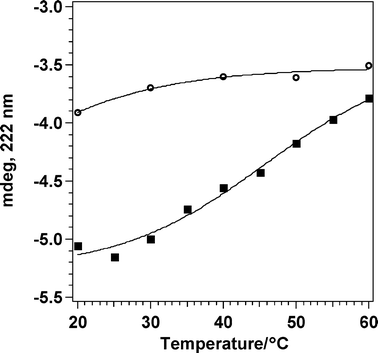 | ||
| Fig. 2 Melting profile of Gα subunit in TRISbuffer (ref. 35 = 222 nm). Square: first upscan, from 20 °C to 60 °C; empty circle: second upscan, from 20 °C to 60 °C on the same solution as the first upscan. | ||
Dynamic light scattering
Dynamic Light Scattering measurements were performed on: (a) different protein/liposomes dispersions in order to assess the effect of the preparation procedure on the size and on the polydispersity of the vesicles; (b) different BC5/DPPC mixed liposomes in order to evaluate the effect of BC5 on the structural properties of vesicles.Fig. 3 shows the diameters and the polydispersity of the SV-NFT and the SUV-upt (see Fig. 1) dispersions. In both cases lamellarity is reduced by extrusion, and slight differences between filtrated (panel A) and not filtrated liposomes (panel B) can be appreciated. Preformed SUV loaded with Gα are generally larger than liposomes hydrated with Gα-containing buffer. The same trend is reflected by the polydispersity (data not shown). For MLV/Gα, a consistent change in the average size can be detected before and after filtration (data not shown); moreover, different protein reconstitution strategies in multilamellar vesicles (between liposomes obtained by hydrating the phospholipidic film with Gα-containing buffer and Gα-loaded preformed liposomes) promote changes in size as well.
 | ||
| Fig. 3 Particle size distributions of small unilamellar vesicles, as obtained by fitting the experimental autocorrelation functions with the CONTIN algorithm. Panel A: distributions obtained before liposome filtration on Vivaspin2 columns; Panel B: distributions obtained after liposome filtration. Black shadowed bars: SUV obtained by film hydration by protein-containing buffer (SV-NFT); black empty bars: preformed SUV incubated with a protein-containing solution (SUV-upt). | ||
The stability of the different BC5/DPPC mixed liposomes with respect to lysis or aggregation was tested over one week. Fig. 4A reports the normalized autocorrelation functions (ACFs), collected for increasing amounts of BC5 inserted in the membranes and prepared according to Table 2 (see Experimental). The autocorrelation functions of the DPPC/BC5 formulations can be fitted through the cumulant method, yielding a single relaxation time, ascribable to the Brownian motion of the mixed liposomes. Very slight differences in size and polydispersity among liposomal preparations containing increasing amount of BC5 can be appreciated (Table 3), until there is a clear shift of the relaxation time towards lower values, marking a decrease of liposomal size. This trend is compatible with the bilayer-to-micelle transition proposed by Lichtenberg et al.27 and more recently by Lopez et al.28
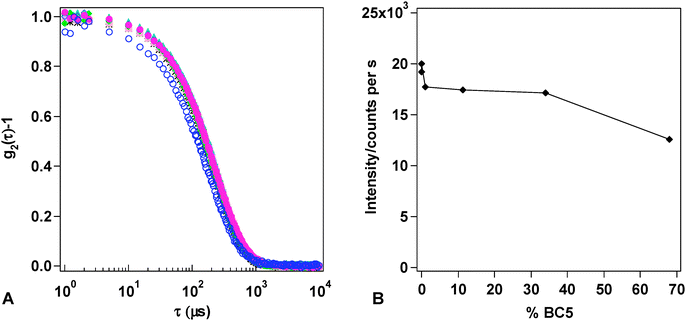 | ||
Fig. 4 Panel A: Autocorrelation function of the different liposome formulations prepared:  -DMPC liposomes; -DMPC liposomes;  -DPPC liposomes; DPPC/BC5 liposomes: -DPPC liposomes; DPPC/BC5 liposomes:  -formulation 2; -formulation 2;  -formulation 3; -formulation 3;  -formulation 4; -formulation 4;  -formulation 5; -formulation 5;  -formulation 6. In formulations 5 and 6 BC5 is above its nominal cmc. Panel B: Scattered intensities of DPPC/BC5 liposomes as a function of the percentage of the surface active drugBC5 in DPPC bilayer. -formulation 6. In formulations 5 and 6 BC5 is above its nominal cmc. Panel B: Scattered intensities of DPPC/BC5 liposomes as a function of the percentage of the surface active drugBC5 in DPPC bilayer. | ||
| Formulation | [DPPC]/mM | [BC5]/mM |
|---|---|---|
| 1 | 0.4400 | / |
| 2 | 0.4396 | 0.0004 |
| 3 | 0.4352 | 0.0048 |
| 4 | 0.3989 | 0.0502 |
| 5 | 0.2904 | 0.1496 |
| 6 | 0.1408 | 0.2992 |
| DPPC liposomes | 〈Dh〉/nm |
|---|---|
| Formulation 1 | 127.9 ± 5.1 |
| Formulation 2 | 130.8 ± 5.2 |
| Formulation 3 | 123.8 ± 4.9 |
| Formulation 4 | 120.5 ± 4.8 |
| Formulation 5 | 124.4 ± 5.0 |
| Formulation 6 | 95.1 ± 3.8 |
The trend of the average intensity of the light scattered by each sample can provide interesting information: for the lowest quantity of BC5 added there is a slight increase (accompanied to an increase of average radius); then the intensity levels to a plateau, until there is an abrupt decrease of the light scattered, that corresponds to the decrease of liposomal size (Fig. 4B).
A Laplace inversion of the ACFs, performed with the CONTIN algorithm and reported in Fig. 5, allows appreciating further details of the size distributions. If DPPC liposomes (5A) are compared to DPPC/BC5 liposomes (formulation 6, BC5 68% of the total lipid concentration, 5B), smaller aggregates and a considerably higher polydispersity can be easily appreciated.
 | ||
| Fig. 5 Size distributions as obtained by fitting the corresponding function according to the CONTIN analysis. (A): DPPC liposomes; (B): DPPC/BC5 liposomes (formulation 6); (C): DMPC liposomes + formulation 6 (1st day); (D): DMPC liposomes + formulation 6 (5th day). | ||
If pure DMPC liposomes (i.e. the host system for Gα-mir) are mixed with formulation 6, mimicking the interaction of the drug loaded vehicle with the structural skeleton of a cell membrane, a fusogenic effect of BC5 can be monitored immediately, as suggested by the appearance of a large-sized population. This effect persists after 5 days, without any further evolutions. The presence of the surface-active drug in solution as a monomer or in mixed micelles-not monitored by LS-probably induces fusion of micelles and DMPC bilayers in bigger aggregates. Further investigations about the effect of BC5 on the structure of the membrane were assessed by means of differential scanning calorimetry.
Differential scanning calorimetry
Inclusion of additives in liposomal formulations may affect the packing of the acyl chain inside the bilayer, leading to a shift in the main transition temperature, or may modulate the cooperativity of the systems, leading to a broadening of the transition peak.In order to evaluate the fusogenic effect of BC5 towards lipidic membranes, and to detect a possible dependence on the acyl chain length of the host phospholipid, the surface active drug was incorporated in DPPC and DMPC liposomes in increasing amounts, keeping constant the total lipid concentration. The total concentration required for the sensitivity of DSC is considerably higher than that necessary for DLS. Therefore, while keeping the molar ratio equivalent in the two cases, the overall concentration investigated with DSC is more than 100 times higher, i.e. BC5 is always (except for formulation 2) above its nominal cmc; the data obtained with this technique should be therefore compared with due caution to the DLS results.
The inclusion of the surface-active drug in the formulation induces a broadening of the DPPC transition peak and a shift of the transition toward higher temperatures until a molar percentage of BC5 in the mixed film equals to 11.4% (thermograms available in the ESI section† ). Above this content, no main transition is distinctly observable, indicating dissolution of the bilayer (also clearly observed by visual inspection of the samples, that become optically clear). The effect of BC5 on the phospholipid phase transition was not dependent on the chain length of the lipids: the changes on main transition temperature of the two lecithins were in fact similar (DMPC data not shown). In Fig. 6 (panel A) the temperature shift ΔT is reported as the difference between Tm,0 melting temperature of pure phospholipid and Tm of the phospholipid in the mixed system. Changes in ΔH are reported in Fig. 6B (black squares). Enthalpy increases when an extremely low percentage of the surfactant is present in the bilayer; then it slowly decreases till flattening of the ΔH values when a percentage of BC5 higher than cmc is used. The onset of the drastic ΔH change was interpreted as the beginning of the coexistence of mixed micelles with vesicles as hypothesized in the model of Lichtenberg et al.27,29
 | ||
| Fig. 6 Squares: DPPC in DPPC/BC5 liposome (formulations 1–4 panel A, 1–6 panel B); circles: DPPC in DPPC/BC5 liposomes (formulations 1–4 panel A, 1–6 panel B) mixed to DMPC liposomes; triangles: DMPC in DMPC liposomes mixed to DPPC/BC5 liposomes (formulations 1–4 panel A, 1–6 panel B). | ||
Fluorescence
Differences in environment polarity can be appreciated thanks to distinct maxima in the emission spectra and differences in the intensity of emission of ANS.30,31 According to the observed changes in the features of the emission spectra, some speculations upon the chemical environment of the probe can be made. In this paper we investigated ANSemission spectra as (a) function of Gα-mir subunit insertion in DMPC bilayer and as (b) function of BC5 percentage in DPPC bilayer.In Fig. 7A, a comparison between the spectra relative to differently prepared samples are reported. For each sample, ANS is added to the liposomal suspension and the spectrum is acquired ten minutes later, therefore it probes the most external leaflet of the liposomal suspension.
 | ||
| Fig. 7 Panel A: Fluorescence emission spectra of ANS added to differently prepared dispersions. Solid blue line—Gα subunit in buffer; black solid line—DMPC unilamellar vesicles; green solid line—DMPC MLV/Gα prepared hydrating the lipidic film with Gα-containing buffer; red dotted line—DMPC MLV/Gα prepared adding Gα to preformed DMPC MLV; cyan dashed line—SUV prepared adding Gα to preformed DMPC SUV. Panel B: Fluorescence emission spectra relative to DPPC/BC5 liposome formulations. Solid black line-DMPC SUV; red solid line- DPPC SUV; DPPC/BC5 formulations: blue dotted line-formulation 2; long dash green line-formulation 3; dash-dot pink line-formulation 4; dash-dot-dot light blue line-formulation 5; short dash purple line-formulation 6. | ||
ANS has scarce binding affinity for Gα-mir: the probe spectrum does not show appreciable deviation from emission in buffer; therefore any possible observed shift for liposomal/protein systems has to be ascribed to membrane binding and any differences among different preparation protocols have to be attributed to a modulation of membrane polarity operated by the protein.
The emission obtained for DMPC liposomes without Gα-mir shows a maximum at 481 nm; for MLV obtained by hydration with a Gα-containing buffer, there is a slight increase in quantum yield and no spectral shift; marked blue shift is instead observed (473 nm and 475 nm) when Gα is added to preformed multilamellar or unilamellar DMPC liposomes respectively. In these two last formulations, a late addition of the protein to the bilayer seems to affect the interfacial polarity.32
The emission spectra of ANS in DMPC liposomes compared to those obtained in DPPC liposomes (black solid line and red solid line in Fig. 7B) indicate clearly how the fluidity of the membrane affects the probe permeability to the bilayer. ANS is added to preformed liposomes and the experiments are performed after 10 min incubation at 30 °C, that is above the DMPC melting temperature but below DPPC melting temperature. If the lipid bilayer is in the gel phase, ANS insertion between the acyl chains is hindered and, as a consequence, its quantum yield is lower and comparable to the spectrum in buffer. The presence of BC5 in the DPPC formulation does not seem at first to change the microenvironment of the fluorescent probe in terms of polarity, nor it affects the bilayer permeability. However, when the molar concentration of the surface-active drug exceeds 11.4%, the quantum yield start to increase. This can be due to the presence of the BC5, which is positively charged, on the membrane that might favor the binding of the fluorescent probe to the bilayer, or can be due to an effective increase of the membrane fluidity. This trend is confirmed for higher values of BC5 concentration. However there is an inversion for the quantum yield values for the highest BC5 concentration, as shown in Fig. 8. With increasing concentration of BC5 in the bilayer, fluorescence intensity of ANS increases until it reaches a maximum, after which the intensity decreases; this effect can be interpreted with the formation of mixed BC5/DPPC micelles.33
Surface tension
A precise knowledge of the aggregation behavior of BC5 is necessary to understand the interactions of this amphiphilic drug with plasma membrane, that are possibly connected with undesired side effects. Self-aggregation can be easily monitored by surface tension. Surface tension vs.BC5 concentration plots are reported in Fig. 9: the semilogarithmic plot of air/water surface tension against BC5 concentration, where a characteristic discontinuity, corresponding to the onset of aggregation, is reported (BC5 cmc: 1 × 10−4 M). Surface tension measurements of the binary system were performed in order to understand if the presence of the protein in solution may interfere with the aggregation properties of the surface-active drug. Gα subunit concentration was kept constant in each set of measurements (30 and 150 nM): these two values correspond to typical protein concentration in in vitro tests. When Gα concentration is 30 nM, no appreciable change apart from different slopes of the premicellar region, can be observed in the surface tension curve. When Gα concentration in solution is raised to 150 nM, the cmc value increases: micelle formation needs a higher amount of bulk BC5 concentration, probably because part of the drug is involved in binding to the protein and is no longer surface-active. An extrapolation of the amount of Gα-bound/BC5 starting from surface tension plots has been performed. The resulting curve is reported in Fig. 10.![Surface tension vs. [BC5] plots relative to pure BC5 solution (black squares); BC5 in a 30 nM Gα solution (empty circles); BC5 in a 150 nM Gα solution (empty triangles).](/image/article/2009/MB/b815042g/b815042g-f9.gif) | ||
| Fig. 9 Surface tension vs. [BC5] plots relative to pure BC5 solution (black squares); BC5 in a 30 nM Gα solution (empty circles); BC5 in a 150 nM Gα solution (empty triangles). | ||
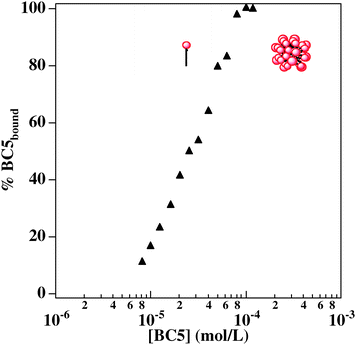 | ||
| Fig. 10 Concentration of bound BC5 as a function of increasing amount of BC5 in solution. The plateau corresponds to micelle formation. | ||
Binding assay
Table 4 reports the results of a functional evaluation of the α subunit inserted in DMPC bilayers subjected to the action of BC5 in DPPC bilayer, performed by GTPγS binding assay.| DPPC/BC5 liposomes | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 (basal activity) | 3 [BC5] = 1 × 10−5 M | 4 [BC5] = 1 × 10−7 M | 5 [BC5] = 1 × 10−6 M | 6 [BC5] = 1 × 10−5 M | 7 [BC5] = 3 × 10−5 M | |
| Buffer | 29.16 ± 1.18 | 0 | 0 | 13.20 ± 1.08 | 43.25 ± 3.05 | ||
| αi1-mir | 9.16 ± 0.89 | 62.36 ± 2.46 | 13.69 ± 0.69 | 20.20 ± 1.08 | 57.99 ± 2.65 | 102.86 ± 5.71 | |
| MLV | 6.31 ± 0.55 | 46.08 ± 10,41 | 5.91 ± 1.08 | 15.37 ± 0.89 | 32.71 ± 3.55 | 62.56 ± 27.88 | |
| * | MLV | 4.73 ± 0.85 | 50.44 ± 4.36 | 6.11 ± 1.58 | 12.41 ± 3.15 | 29.36 ± 4.73 | 77.93 ± 43.84 |
| MLV-upt | 12.81 ± 0.49 | 62.27 ± 2.66 | 16.65 ± 0.89 | 21.67 ± 0.98 | 56.85 ± 1.67 | 90.74 ± 4.33 | |
| * | MLV-upt | 11.82 ± 0.29 | 59.70 ± 1.77 | 15.57 ± 1.18 | 25.91 ± 2.17 | 49.46 ± 1.87 | 88.57 ± 2.56 |
| SUV-upt | 15.86 ± 0.98 | 64.93 ± 2.17 | 18.72 ± 1.18 | 24.93 ± 0.89 | 59.60 ± 1.67 | 98.72 ± 3.84 | |
| * | SUV-upt | 14.38 ± 0.79 | 61.97 ± 2.07 | 17.83 ± 0.59 | 23.74 ± 0.59 | 57.14 ± 1.48 | 93.69 ± 2.46 |
| SUV-NFT | 13.78 ± 0.50 | 58.91 ± 1.85 | 16.26 ± 0.79 | 22.86 ± 1.77 | 55.17 ± 2.36 | 91.53 ± 5.12 | |
| * | SUV-NFT | 11.21 ± 0.34 | 56.99 ± 2.03 | 17.14 ± 1.18 | 25.12 ± 2.09 | 48.18 ± 2.09 | 86.70 ± 5.02 |
It is worthwhile to recall that in this case BC5 is delivered to the protein in different liposomal formulations, while for the results shown in Table 1 the surface-active drug was simply added to the solution.
As detailed in the Materials and methods, increasing concentrations of BC5 were embedded in a DPPC bilayer by preparing mixed lipidic films (DPPC/BC5: formulations 2–6, Table 2). For comparison, a sample of BC5 1 × 10−5 M not incorporated in DPPC liposomes was tested. Row 1, termed “Buffer”, refers to the aspecific binding (i.e. without protein), while Row 2 reports the results for Gα protein in buffer, i.e. when it is not hosted in liposomal membranes. The following rows refer to different reconstitution procedures of the protein in bilayers, as specified in the experimental part. GTPγS binding was performed on Gα-mir/DMPC system both before and after (asterisked rows) Vivaspin 2 filtration, but no significative change was observed in terms of G protein activity.
As reported in Table 1 and 4, there is a dependence of Gα activity upon the reconstitution strategy in liposomes. Providing a lipid environment to the Gα subunit results in an improved basal activity (column 2), except for the preparation MLV and MLV-NFT.
For a given concentration (BC5 = 1 × 10−5 M, column 6 and 3) there is a significant reduction of aspecific GTPγS binding for DPPC/BC5 formulation (i.e. monitored without the presence of G protein, 13.20 ± 1.08 fmol) with respect to the same BC5 concentration in the absence of liposomes (29.16 ± 1.18 fmol).
From the experimental results reported in Table 4, it is possible to extract the more informative parameter r, defined as for Table 1, whose values for Column 3 and Column 6 (same BC5 concentration in solution and in liposomal formulation, respectively) are shown in Table 5.
| r (BC5 1 × 10−5 M + DPPC) | r (BC5 1 × 10−5 M alone) | EC50/μM (BC5 + DPPC) | |
|---|---|---|---|
| a P < 0.01 is considered as significantly different from r obtained in the absence of liposomes. | |||
| αi1-mir | 4.89 ± 0.17a | 3.62 ± 0.14 | 12.02 ± 2.19 |
| MLV | 3.42 ± 0.21a | 4.50 ± 0.21 | 17.61 ± 1.79 |
| MLV-upt | 3.07 ± 0.12a | 2.58 ± 0.08 | 10.81 ± 2.46 |
| SUV-upt | 3.06 ± 0.08a | 2.28 ± 0.08 | 11.64 ± 3.83 |
| SV-NFT | 3.12 ± 0.18a | 2.48 ± 0.04 | 12.93 ± 2.22 |
When the Gα subunit is dissolved in buffer, the formulation DPPC/BC5 (BC5 = 1 × 10−5 M) is able to stimulate Gα-mir activity up to r = 4.89 ± 0.17 (liposomal activation), a ratio significantly higher than that obtained in the absence of DPPC (r = 3.62 ± 0.14, non-liposomal activation). However this increase should be regarded with some caution, because the incorporation of the protein in the BC5 containing liposomes cannot be ruled out. When Gα-mir was reconstituted in DMPC MLV, the basal activity is decreased as reported in Table 4; the stimulation ratio in liposomal activation is decreased up to 3.42 ± 0.21 while is increased up to 4.50 ± 0.21 in the non liposomal one. Nonetheless, MLV dispersion shows the best stimulation ratio r in a lipidic environment. SUV dispersions allows to obtain a better basal activity than G protein in the absence of liposomes and a good r both in liposomal and non liposomal activation when G protein is not subjected to freeze–thaw cycles (3.06 ± 0.08 and 2.28 ± 0.08 for SUV-upt—liposomal and non liposomal activation respectively; 3.12 ± 0.18 and 2.48 ± 0.04 for SV-NFT—liposomal and non liposomal activation respectively). When G protein is reconstituted in preformed liposomes (MLV-upt and SUV-upt) its direct activation is more effective when BC5 is embedded in DPPC liposomes. The same result is obtained when the surface active drug is loaded in liposomes and tested on SV-NFT liposomes.
In all the above cases, except for MLV, incorporation of the amphiphilic drug in a DPPC bilayer induces a remarkable improvement of G protein direct activation.
The Effective Concentration 50 (EC50, reported in Table 5) was not significantly altered by Gαi1-mir reconstitution in liposomes.
Discussion
In this paper, Gα-mir subunits have been reconstituted in lipid bilayers, in order to mimic their in vivo microenviroment, possibly promoting their correct functional folding. This should result in an increased basal activity, measured in terms of GTPγS binding, with respect to the solution value.Several procedures are commonly used to incorporate lipophilic guests in bilayer, starting from hydration of dry lipid stacks with buffer containing the guest molecule, to addition of the molecule to preformed liposomes. It is important to consider however that several protocols used to reduce the lamellarity and the size of the liposomes might compromise the conformational or chemical integrity of the protein. In our case the basal activity is a measure of the protein functional integrity and of GTPγS access to the protein anchoring sites.
We have found that the basal activity follows the trend SUV > SUV-upt > MLV-upt > SV-NFT > TRIS > MLV-FT > MLV. The highest basal activity was obtained for SUV (protocol D) and for the formulations obtained by adding the G protein-containing buffer to preformed liposomes, both multilamellar and unilamellar. The lowest activity observed for Gα-mir hosted in MLV can be explained with the homogeneous distribution of the protein in the lamellar stacks of the onion-like structure. The highly charged probe molecules cannot presumably cross the multiple bilayers and therefore its binding cross section with the target is reduced.
The same conclusions can be drawn from ANS emission: there is a blue shift accompanied to a decrease of the quantum yield when Gα-mir is added to preformed liposomes, that is when it is concentrated in the external leaflet of the outer bilayer. If the negatively charged fluorophore is then added and the fluorescence emission is monitored 10 min later, the probe localization will be limited to the most external lipid leaflet. Therefore the most meaningful deviation will correspond to the highest protein concentration in the accessible bilayer.
Therefore, we can conclude that, even if the liposomal milieu is a good mimic of Gα-mir physiological environments, as proved by the generally increased basal activity, great care should be paid to the preparation protocol: a multiple lamellarity can severely affect the liability of the binding tests in vitro.
The BC5-induced activation is directly connected to the accessibility of the bilayer-hosted protein for an amphiphilic molecule, in this case the pharmacological agent. Moreover, since this amphiphilic drug is cationic, one has to consider that aspecific binding of the negatively charged GTPγS in the absence of protein is a strict limitation to in vitro functionality tests to assess the real therapeutic efficacy.
If BC5 is delivered as liposomal formulation, it is essential to determine the physical property of the aggregates, since cationic single-chain amphiphile can highly affect these self-assemblies, eventually destroying the double layer and causing the formation of mixed micelles.
These properties can be monitored through DLS, DSC and through ANS fluorescence emission. This latter method has been used for the first time in this paper to follow the vesicle to micelle transition, as BC5 percentage in the lipid film is increased. The quantum yield is strongly affected by BC5 relative abundance.
DLS, on the other side, provides a direct indication of the hydrodynamic radius of the aggregates, which is expected to decrease after an initial saturation of the bilayer. The scattering static intensity is an indication of the number density of the scattering objects, weighted for their squared volume (i.e. the 6th power of the radius). In other words, the observed radius refers in our case only to the larger scattering objects (i.e. the mixed BC5/DPPC liposomes) and possible smaller aggregates (i.e. mixed micelles) can be considered as invisible, standing the great difference in size. However, the trend of the total scattered intensity as a function of BC5 concentration is a direct indication of the presence of liposomes. This is particularly interesting for the sample BC5/5, where the complete disappearance of vesicles can be detected.
ANS data suggest that upon BC5 addition the affinity of the bilayer for the probe is increased, fluorescence intensity is enhanced and more probe molecules, presumably, are bound. Higher concentrations of the drug induce the bilayer-micellar phase transition, as seen by the scattering intensity, and the fluidizing effect of BC5 is marked by the decrease in fluorescence intensity that takes place when the fluorescent probes in the bilayer are transferred to a more polar environment like the mixed micelles.
Increasing BC5 concentration gives rise to the incorporation of most phospholipids into mixed phospholipids-surface active drug micelles, and this is supported by the decreased enthalpy of transition obtained by DSC data as well.
The DPPC/BC5 formulation is able to decrease the aspecific GTPγS binding that allows to perform a more accurate measurement of activity. On the other hand the incorporation of BC5 in the lipid bilayer induces a better stimulation. The systems SUV-upt and SV-NFT (i.e. when freeze-thaw cycles were not carried out in the presence of the protein) offers an optimal compromise solution between G protein basal activity and BC5 activation both in the liposomal and non-liposomal state.
Conclusions
The reconstitution of Gα-mir protein in a liposomal structure represents a more physiological system that improves the protein basal activity and represents a better and more reliable model for in vitro pharmaceutical testing. To the best of our knowledge, for the first time a complete characterization of a Gα subunit reconstitution in model membranes, in terms of protein activity as a function of the reconstitution protocol, has been reported. Moreover, an optimal carrier able to lower the side effects of the surface-active drugBC5 has been found: the DPPC/BC5 formulation, in fact, is a biocompatible tool useful to decrease the aspecific GTP-γ-S binding. When Gα subunit is dissolved in buffer, the formulation DPPC/BC5 (BC5 = 1 × 10−5 M) is able to stimulate Gα-mir activity up to r = 4.89 ± 0.17, a ratio significantly higher than that obtained in the absence of DPPC (r = 3.62 ± 0.14).Improved liposomal formulations will be functional for further Gαiprotein reconstitution and for a future characterization of direct activators. A pharmaceutical form for the drug formulation and an in vivo study will be also pursued.
Experimental
Chemicals
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-palmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids (Alabaster, AL), and their purity was checked by thin-layer chromatography. TRIS-HCl was purchased from Sigma. BC5 was synthesized according to the synthetic pathway proposed in ref. 19.Expression and purification of Gαi1protein
The method was described previously.21 Briefly, to ensure synthesis of myristoylated G protein human Gαi1 subunit and yeast N-myristoyltransferase were coexpressed in a single plasmid using the vector ligation-independent cloning system (LIC; Novagen, San Diego, CA). Further information is available as Supplementary Data.Liposome preparation
Dimiristoyl-sn-glycerophosphocoline (DMPC) was chosen as the lipid component of the membrane model bilayer. Four different kinds of liposomes were prepared: (A) multilamellar vesicles (MLV); (B) multilamellar vesicles subjected to freeze-thaw cycles (MLV-FT); (C) small vesicles not subjected to freeze-thaw cycles (SV-NFT); (D) small unilamellar vesicles (SUV). Two other additional protocols were carried out: G protein was loaded on preformed DMPC MLV (MLV-upt) and on DMPC SUV for 24 h at 30 °C (protocol E and F respectively). Dipalmytoyl-s,n-glycerophosphocoline (DPPC) was chosen as the lipid component of the delivery system bilayer. Mixed lipid film , formed by DPPC and BC5 (formulations 2 to 6, reported in Table 1), maintained at constant lipid concentration (0.44 mM), were hydrated with 50 mM TRISbuffer, pH 8.0. Freeze-thaw cycles and extrusion with 100 nm-sized pores membranes were then performed, with an apparatus described elsewhere;34,35 homogeneously dispersed small unilamellar vesicles were obtained, as confirmed by dynamic light scattering measurements.Gα-mir liposome entrapment
The dispersions were transferred to a VivaSpin2 column (50KDa MW cut-off—Sartorius AG, Germany) in order to remove any free Gα-mir subunit present in the solution. The VivaSpin2 was centrifuged at 6000 rpm for 45 min. The mixture in the concentrator tube was then recovered and the tube was washed several times up to reach a final volume equals to the initial loaded volume.Circular dichroism
Circular dichroism (CD) spectra were recorded on a J-715 spectropolarimeter (JASCO, Tokyo, Japan) using a 1 mm path length Hellma quartz cuvette after allowing equilibration to take place for 20 min at the chosen temperature. For each sample, four scans were accumulated at 50 nm min−1 and measurements were repeated at least twice using freshly prepared solutions to test reproducibility. Background from buffer solution and empty cell contribution was subtracted from each spectrum.Fluorescence
Fluorescence measurements were recorded on a Jasco spectrofluorimeter. The excitation wavelengths were set at 350 nm. 500 ml of vesicle solution were placed into a magnetically stirred four-window quartz cuvette held in the sample compartment thermostated at 30 °C by a circulating water bath. The spectra were registered 3 min and after 10 min after the addition of ANS to the dispersions in order to detect differences depending on the incubation time. Aliquots of ANS in water solution were added to the solution in order to have lipid: probe molar ratios of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The extrinsic fluorescence of ANS was excited at 360 nm and the emission intensity was observed at 460 nm.
1. The extrinsic fluorescence of ANS was excited at 360 nm and the emission intensity was observed at 460 nm.
Surface tension measurements
Surface tension of BC5 solutions was determined with the duNouy ring method using a KSV Sigma 70 digital tensiometer (accuracy 0.1 mN/m) (Helsinki, Finland) equipped with a Metrohm 665 Dosimat (Buckingham, UK) allowing for an automatic surfactant concentration scan. The measurements were performed at 25.0 ± 0.5 °C diluting a concentrated detergent solution contained in the thermostated vessel. For BC5/Gαi1 solutions a drop-shape tensiometer, The Tracker, from ITC (France) was used.Differential scanning calorimetry
Differential scanning calorimetry measurements were performed using a DSC Q1000 from TA Instruments and the data were elaborated with Q Series software, version 3.0.3. Each sample was maintained in a thermostatic bath at constant temperature at 30 °C for 30 min prior loading in order to homogenize the dispersions. About 10 mL of liposomes were transferred in an aluminium pan (diameter 7.4 mm, capacity 30 ml) and hermetically sealed with the appropriate cover to avoid water leaking. The measurements were carried out with the following temperature program: equilibrate to 5 °C, isothermal for 1 min, from 5 °C to 60 °C at 20 °C min−1 upscan and downscan for two times, from 5 °C to 60 °C at 5 °C min−1.Dynamic light scattering
Light scattering experiments were carried out on a Brookhaven Instruments apparatus (BI 9000AT correlator card and BI200SMgoniometer). The light source was the second harmonic of a diode-pumped Coherent Innova Nd:YAG laser (λ = 532 nm), linearly polarized in the vertical direction and impinging on the sample with 60 mW power. The signal was detected by an EMI 9863B/350 photomultiplier. The laser long-term power stability was ±0.5%. The light scattered from the sample was collected at 90° with respect to the impinging laser light radiation. To obtain the size distribution of the scattering objects, the autocorrelation functions were Laplace inverted using the CONTIN routine.36GTPγS binding
Binding of GTPγS (a non hydrolysable GTP analogue) to recombinant Giprotein was measured according to a standard method.37 3 pmol of a subunit in native conditions or reconstituted into lipidic vesicles were incubated at 30 °C for 30 min in 100 mL of a reaction mixture containing 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1 mM DTT, 1.1 mM MgSO4, and 0.1 mM [35S]GTPγS (0.1 mCi/tube; Perkin Elmer). Reaction was stopped by the addition of 1 mL of an ice-cold stop buffer (consisting of 10 mM Tris-HCl pH 8.0, 25 mM MgCl2, 100 mM NaCl). The diluted samples were filtered through cellulose nitrate membranes (0.45 mm pore size) under weak vacuum. The filters were washed with 12 mL of the same buffer and dried. The retained radioactivity was quantified by scintillation spectroscopy. Specific binding was calculated by subtracting the GTPγS bound in presence of 100 mM cold GTPγS from total binding.Acknowledgements
Consorzio Interuniversitario per lo Sviluppo dei Sistemi a Grande Interfase (CSGI) is acknowledged for financial support. Thanks are due to Dr. Francesca Ridi for the helpful discussions in DSC measurements.References
- A. G. Gilman, Annu. Rev. Biochem., 1987, 56, 615 CrossRef CAS.
- S. R. Neves, P. T. Ram and R. Iyengar, Science, 2002, 296, 1636 CrossRef CAS.
- Y. Landry and J. P. Gies, Mini-Rev. Med. Chem., 2002, 2, 361 CrossRef CAS.
- J. Lyons, C. A. Landis, G. Harsh, L. Vallar, K. Grunewald, H. Feichtinger, Q. Y. Duh, O. H. Clark, E. Kawasaki, H. R. Bourne and F. McCormick, Science, 1990, 249, 655 CrossRef CAS.
- E. A. Williamson, P. G. Ince, D. Harrison, P. Kendall Taylor and P. E. Harris, Eur. J. Clin. Invest., 1995, 25, 128 CrossRef CAS.
- A. M. Spiegel and L. S. Weinstein, Annu. Rev. Med., 2004, 55, 27 CrossRef CAS.
- B. B. Lerman, B. Dong, K. M. Stein, S. M. Markowitz, J. Linden and D. F. Catanzaro, J. Clin. Invest., 1998, 101, 2862 CrossRef CAS.
- O. Melien, Methods Mol. Biol., 2006, 367, 119 Search PubMed.
- N. Galeotti, C. Ghelardini, M. Zoppi, E. Del Bene, L. Raimondi, E. Beneforti and A. Bartolini, Cephalalgia, 2001, 21, 38 CrossRef CAS.
- N. Galeotti, C. Ghelardini, M. Zoppi, E. Del Bene, L. Raimondi, E. Beneforti and A. Bartolini, J. Rheumatol., 2001, 28, 2298 Search PubMed.
- C. Leschke, R. Storm, E. Breitweg-Lehmann, T. Exner, B. Nurnberg and W. Schunack, J. Med. Chem., 1997, 40, 3130 CrossRef CAS.
- E. Breitweg-Lehmann, C. Czupalla, R. Storm, O. Kudlacek, W. Schunack, M. Freissmuth and B. Nurnberg, Mol. Pharmacol., 2002, 61, 628 CrossRef CAS.
- K. -L. Laugwitz, A. Allgeier, S. Offermanns, K. Spicher, J. Van Sande, J. E. Dumont and G. Schultz, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 116 CrossRef CAS.
- S. A. Akhter, L. M. Luttrell, H. A. Rockman, G. Iaccarino, R. J. Lefkowitz and W. J. Koch, Science, 1998, 280, 574 CrossRef CAS.
- Y. Odagaki, N. Nishi and T. Koyama, Life Sci., 1998, 62, 1537 CrossRef CAS.
- C. Höller, M. Freissmuth and C. Nanoff, Cell. Mol. Life Sci., 1999, 55, 257 CrossRef CAS.
- B. Nurnberg, W. Togel, G. Krause, R. Storm, E. Breitweg-Lehmann and W. Schunack, Eur. J. Med. Chem., 1999, 34, 5 CrossRef CAS.
- W. W. Ja and R. W. Roberts, Trends Biochem. Sci., 2005, 30, 318 CrossRef CAS.
- D. Manetti, L. Di Cesare Mannelli, S. Dei, N. Galeotti, C. Ghelardini, M. N. Romanelli, S. Scapecchi, E. Teodori, A. Pacini, A. Bartolini and F. Gualtieri, J. Med. Chem., 2005, 48, 6491 CrossRef CAS.
- L. Di Cesare Mannelli, A. Pacini, A. Toscano, M. Fortini, D. Berti, C. Ghelardini, N. Galeotti, P. Baglioni and A. Bartolini, Protein Expression Purif., 2006, 47, 303 CrossRef CAS.
- L. Di Cesare Mannelli, A. Pacini, A. Toscano, C. Ghelardini, D. Manetti, F. Gualtieri, T. B. Patel and A. Bartolini, Arch. Biochem. Biophys., 2006, 453, 151 CrossRef CAS.
- S. Schreier, S. V. P. Malheiros and E. de Paula, Biochim. Biophys. Acta, 2000, 1508, 210 CAS.
- T. Lian and R. J. Y. Ho, J. Pharm. Sci., 2001, 90, 667 CrossRef CAS.
- W. M. Oldham and H. E. Hamm, Q. Rev. Biophys., 2006, 39, 117 CrossRef CAS.
- S. Shahinian and J. R. Silvius, Biochemistry, 1995, 34, 3813 CrossRef CAS.
- N. Anderson and J. Borlak, FEBS Lett., 2006, 580, 5533 CrossRef CAS.
- D. Lichtenberg, R. J. Robson and E. A. Dennis, Biochim. Biophys. Acta, 1983, 737, 285 CrossRef CAS.
- O. Lopez, A. de la Maza, L. Coderch, C. Lopez-Iglesias, E. Wehrli and J. L. Parra, FEBS Lett., 1998, 426, 314 CrossRef CAS.
- T. M. Bayer, G. -D. Werner and E. Sackmann, Biochim. Biophys. Acta, 1989, 984, 214 CAS.
- J. R. Lakowicz, ‘Principles of Fluorescence Spectroscopy’, Plenum, 1983 Search PubMed.
- J. Slavik, Biochim. Biophys. Acta, 1982, 694, 1 CAS.
- C. Schmitt, C. Bovay, M. Rouvet, S. Shojaei-Rami and E. Kolodziejczyk, Langmuir, 2007, 23, 4155 CrossRef CAS.
- A. Alonso, R. Sáez and F. M. Goñi, FEBS Lett., 1982, 137, 141 CrossRef CAS.
- D. Berti, P. L. Luisi and P. Baglioni, Colloids Surf., A, 2000, 167, 95 CrossRef CAS.
- D. Berti, P. Baglioni, S. Bonaccio, G. Barsacchi-Bo and P. L. Luisi, J. Phys. Chem. B, 1998, 102, 303 CrossRef CAS.
- F. B. Bombelli, D. Berti, F. Pini, U. Keiderling and P. Baglioni, J. Phys. Chem. B, 2004, 108, 16427 CrossRef.
- C. Oppi, T. Wagner, A. Crisari, B. Camerini and G. P. T. Valentini, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 8268 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary figures and experimental details. See DOI: 10.1039/b815042g |
| ‡ DMPC was chosen as the phospholipid in membrane model liposomes for two main reasons: the chain length of the lecithin is the same as the alkyl residue length linked to the Ga subunit, and this could help to achieve a better insertion of the protein into the bilayer; moreover, the gel to liquid crystalline phase transition temperature (Tm) is just below physiological temperature: this means that by using DMPC it becomes possible studying the protein behaviour as inserted both in a gel phase and in a liquid-crystalline phase. |
| This journal is © The Royal Society of Chemistry 2009 |

