Microrheology with optical tweezers†
Alison
Yao
b,
Manlio
Tassieri
a,
Miles
Padgett
b and
Jonathan
Cooper
*a
aDepartment of Electronics and Electrical Engineering, University of Glasgow, Glasgow, UK. E-mail: j.cooper@elec.gla.ac.uk; Fax: +44 (0) 141 330 6010; Tel: +44 (0) 141 330 4931
bSUPA, Department of Physics and Astronomy, University of Glasgow, Glasgow, UK. E-mail: m.padgett@physics.gla.ac.uk; Fax: +44 (0)141 330 2893; Tel: +44 (0)141 330 5389
First published on 15th June 2009
Abstract
Microrheology is the study of the flow of materials over small scales. It is of particular interest to those involved with investigations of fluid properties within Lab-on-a-Chip structures or within other micron-scale environments. The article briefly reviews existing active and passive methods used in the study of fluids. It then explores in greater detail the use of optical tweezers as an emerging method to investigate rheological phenomena, including, for example, viscosity and viscoelasticity, as well as the related topic of flow. The article also describes, briefly, potential future applications of this topic, in the fields of biological measurement, in general, and Lab-on-a-Chip, in particular.
 Alison Yao Alison Yao | Alison Yao graduated from Heriot-Watt University, Edinburgh with a BSc (Hons) in Applied Physics with Semiconductor Electronics. She did a PhD in Computational & Nonlinear Optics at the University of Strathclyde, Glasgow, modelling nonlinear optical systems. She currently works in the Optics Group at the University of Glasgow where her research focuses on hydrodynamic interactions in microfluidic systems. |
 Manlio Tassieri Manlio Tassieri | Manlio Tassieri graduated as a chemical engineer from The University of Naples, “Federico II”. He did a PhD in microrheology of semi-flexible polymers at The University of Leeds, designing a magnetic microrheometer and making new discoveries on polymeric dynamics, then spent two years working on polymer composites. He is now at the University of Glasgow where his research focus is biophysics within microfluidic systems. |
 Miles Padgett Miles Padgett | Miles Padgett is Professor of Optics at the University of Glasgow. He heads a 15-person team covering a wide spectrum of projects from blue-sky research to applied commercial development, funded by a combination of government, charity and industry. In 2001 Padgett was elected to Fellowship of the Royal Society of Edinburgh. In 2008 he was awarded the Institute of Physics Optics and Photonics Division Prize. |
 Jonathan Cooper Jonathan Cooper | Professor Jon Cooper holds the Wolfson Chair in Bioengineering. His primary focus is biological systems at the micro- and nanoscale. He has strong collaborations in the fields of cancer and cardiovascular medicine and close involvement in the commercialisation of chip based diagnostic devices (http://www.modedx.com). He became a Fellow of the Royal Society of Edinburgh in 2001 and a Fellow of the Royal Academy of Engineering in 2004. |
Introduction
Rheology
Rheology is the study of the flow of materials. The word rheology comes from rheos, the Greek word for flow, and ology, meaning study of. In practice, rheology is concerned with materials whose mechanical behaviour presents features that can be partially described by both the classical theory of elasticity and by simple (Newtonian) fluid mechanics.Fluids flow when subjected to a stress, which is defined as a force divided by its area of application. When a constant stress is applied to a generic material (a so-called creep measurement) one of two behaviours is normally expected: (I) elastic-like behaviour, i.e. the material deformation strain increases linearly with increasing applied stress, or (II) viscous-like behaviour, i.e. the material deformation rate increases linearly with increasing applied stress.
Simple fluids, such as water, honey, glycerol, are purely viscous and thus can be characterised simply by their resistance to flow, i.e. their viscosity. On the other hand, complex materials, such as mayonnaise, starch solution, chocolate, bread dough, do not have a constant viscosity, but one that can vary depending on the applied stress. For example, starch solution behaves like a fluid when stress is applied slowly, but like a solid when stress is applied in short time intervals (e.g. stress-pulses). If neither the shear strain nor its time derivative (shear rate) are linearly related to the applied stress then the material is rheologically defined as viscoelastic. These materials cannot be characterized by a single value of the viscosity and instead require to be characterized by properties which relate their behaviour to the stress and strain under different flow conditions.
Macrorheology
On a macroscopic scale, viscosity can be measured in many ways. An old fashioned example of a viscometer is one based on a falling sphere. It calculates the velocity of a sphere, of known size and density, falling through a liquid by measuring the time taken for the sphere to pass two marks on a glass tube. The viscosity is then calculated by balancing the gravitational force, ρVg, with the drag force of the sphere, 6πηav (where ρ is the density of the sphere, V is its volume, g is the acceleration due to gravity, η is the viscosity of the fluid, a is the radius of the sphere and v is its velocity). At present, the most common viscometers are rotational rheometers. These can be either stress-controlled or strain-controlled and are based on the concept that the torque required to rotate an object of known geometry in a fluid is a function of the viscosity of the fluid.Typical ranges of sensitivity in terms of torque, strain, required sample volume and temperature are of the order of: from ∼1.0e−7 to 1 Nm, ≥1 µrad, ≥1 ml and from −20° to ∼300 °C, respectively.
Microrheology
Nowadays, the study of physical phenomena at very small scales has been made possible thanks to the progressive technological developments obtained over the last few decades and to the commercialization of high-tech equipment at relatively low prices. This has encouraged scientists to develop new investigative techniques into the micro- and nano-technology research fields.Microrheology is a branch of rheology which has the same principles as conventional bulk rheology, but works on micron length scales. Microrheological techniques can be classified as either (I) passive or (II) active, depending on whether they monitor (I) the free or (II) the driven motion of tracer particles introduced into the fluid under investigation. The first technique is based on the observation of a thermally excited single (or low concentration) sphere, of order of a micron in size, suspended in the solution under investigation. The second is based on the response of a single micro-bead to an external force field. Both techniques directly relate the bead trajectories to the viscoelastic properties (e.g. complex shear modulus, G*(ω)) of the surrounding matrix (fluid).
A major advantage of this technique is the use of very small amounts of sample (∼10 µl) compared with the sample volumes required for conventional rheology. Furthermore, microrheological measurements can be performed in situ in an environment that cannot be reached by a bulk rheology experiment, for instance inside a living cell. Several microrheological techniques have been developed so far, including video particle tracking microrheology,1diffusing wave spectroscopy,2,3atomic force microscopy,4magnetic tweezers5,6 and optical tweezers.7–9 For a good overview and understanding of the historical roots of all of these the reader is referred to the reviews written by T. A. Waigh10 and C. J. Pipe & G. H. McKinley.11
Optical tweezers
Optical tweezers are based on the pioneering work of Ashkin12–14 who demonstrated that the forces exerted by a strongly focused beam of light could be used to trap and move a micron-sized object. Since then they have been extensively developed15 and have proved to be an invaluable tool throughout the biological and physical sciences. These instruments have been used to apply forces in the pN-range and to measure displacements in the nanometer range of objects ranging in size from 10 nm to over 100 mm.16,17 For reviews of optical trapping see refs. 17 and 18.Many of the early uses of optical tweezers were in biological systems, capturing and manipulating biological samples such as bacteria.19 Because most biological materials absorb only weakly in the near infra-red region of the electromagnetic spectrum, Nd:YAG (λ = 1064 nm) lasers are well-suited to optical tweezers applications in biology.20 The fact that their mechanical stiffness can be adjusted over a range that spans that of biological molecules makes them suitable to perform studies that currently challenge other techniques like atomic force microscopy (AFM).
Optical tweezers have found particular use in cell biology21,22 and have been successfully used as sensitive tools (i.e. as force transducers) for purposes such as measuring the compliance of bacterial tails,23 the forces exerted by single motor proteins,24 the stretching of single deoxyribonucleic acid (DNA) molecules25 and the mechanical properties of human red blood cells.26 The biggest impact in biology so far, however, has been the ability to make ultrahigh resolution mechanical measurements on individual biological molecules.19,27–29
Beyond trapping a single particle, multipleoptical tweezers can now be synthesized using holographic elements.30–36 In conjunction with recent advances in camera technology,37,38 this has meant that many particles can now be trapped simultaneously and their positions tracked at frame rates of kHz for indefinite periods of time.39 This ability to trap, position and track many particles has found particular use in colloidal science40–43 where the hydrodynamic interactions between particles are of great importance in understanding common colloid phenomena such as sedimentation and aggregation.
Another advantage of high-speed cameras is the ability to study hydrodynamic interactions between particles trapped in much less viscous media, such as air,44,45 allowing investigation into aerosols. It also allows the observation of a parametrically excited resonance within a Brownian oscillator44 and underdamped modes in periodic arrays of trapped particles.45
The inherent scale of optical tweezers makes them readily applicable to microfluidic systems. These systems offer many advantages over conventional methods for biological and chemical measurements. The miniaturisation of micro-analytical devices results not only in a low fabrication cost and a reduction in the volume of potentially expensive reagents used, but also in an increased speed of analysis and the ability to run multiple analytical processes in parallel. In recent years there have been a number of applications using optical tweezers with microfluidics, for example, to sort cells,46–48 or to manipulate and measure fluids within microdevices.49–51
One of the most prominent applications in microfluidics has been the work by Guck et al. and their “optical stretcher”.52–54 An object placed in a dual beam trap becomes stretched: while the total force acting on the object is zero there is a momentum transfer from the light to the surface as the light passes through the interface acting away from the beam propagation direction. Hence, a suitably elastic object will become stretched in a dual beam trap. The technique allows one to probe the viscoelastic properties of the trapped particles which is particularly useful for cellular material where the deformability provides information about the cytoskeleton. This has been shown to be useful as a cell marker for detecting cancerous cells.55 By integrating the device into a microfluidic system cells can be analysed in a high throughput manner.56,57
Optical tweezers are particularly useful in microrheology58 where techniques often involve introducing micron-sized particles to a fluid and tracking their thermal motion (passive microrheology),59–61 providing information about rheology at the micron-scale. A variant of this method involves actively applying a force to a particle (active microrheology) to gain more information about the dynamic properties of the fluid. Active microrheology has been attempted by several means including atomic force microscopes,4magnetic tweezers5,6 and optical tweezers.7–9
Instrumentation
There are many different configurations of optical tweezers systems depending on the particular application required. One particular configuration of an optical tweezers system, based upon an inverted microscope, is shown in Fig. 1. The objective lens, 100x 1.3NA, (Zeiss, Plan-Neofluor) is mounted on a piezo-controller and used to both focus the trapping beam and to image the particles. A 50 W tungsten-halogen lamp and condenser is used to illuminate the sample. Trapping is achieved using a CW Ti:sapphire laser system (M2, SolsTiS). The laser is expanded to slightly overfill the aperture of a spatial light modulator, SLM (Hamamatsu, LCOS X1046802), and then coupled into the tweezers system by imaging the SLM on to the back aperture of the microscope objective lens. By appropriate hologram design, this allows multiple optical traps to be created in the sample plane. The microscope slide and cover slip, forming the sample cell, are mounted on a motorized microscope stage (ASI, MS-2000) above the objective. | ||
| Fig. 1 Example optical tweezers setup. | ||
Example of applications of optical tweezers
Viscosity
Fluid viscosity has been measured successfully by various methods with optical tweezers using a single micron sized particle as a probe.62–66 Most of these approaches have inferred the surrounding viscosity by using a quadrant photodiode (QPD) to track the thermal motion of the particle.Assuming a particle of mass m and radius a is localized in an optical tweezers of stiffness κ, tens of radii from any surfaces, then for small displacements its equation of motion results from the balance between inertial, viscous, elastic and thermal (Brownian) forces:
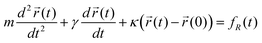 | (1) |
 is the trajectory of the particle,
is the trajectory of the particle,  is its equilibrium position, or trap centre, γ is the drag coefficient (i.e. 6πηa, where η is the viscosity of the surrounding fluid) and fR(t) describes the Brownian (thermal) forces acting on the particle.
is its equilibrium position, or trap centre, γ is the drag coefficient (i.e. 6πηa, where η is the viscosity of the surrounding fluid) and fR(t) describes the Brownian (thermal) forces acting on the particle.
The translational drag coefficient, and hence the fluid viscosity, can be calculated using two nominally equivalent methods in the time and frequency domains. In the time domain, the autocorrelation function of the position data x over time t, in one dimension, has the form:
 | (2) |
 | (3) |
In the frequency domain, a power spectrum analysis68 can be used.62 In the usual case, where the motion of the particle is massively over-damped, the inertial term can be neglected and the power spectral density, S(ω), is a Lorentzian of the form:
 | (4) |
Alternatively, it is also possible to determine the viscosity from the rotational velocity of a particle subject to a known torque.69 The ability of light to transport and transfer angular momentum as well as linear momentum70 has been widely applied to rotate microparticles in optical tweezers.71–75 In rotationally symmetric beams71,76 particles spin at a constant speed, indicating that the optical torque driving the particle is balanced by the viscous drag of the surrounding medium. Hence, by knowing the torque required to rotate a particle at a constant angular velocity, the viscosity of the surrounding liquid can be determined.
For a circularly polarized pure Gaussian beam, each photon has an angular momentum of ±ℏ about the beam axis. This is equivalent to an angular momentum flux of ±P/ω for the beam, where P is the power of an incident beam, and ω is the angular frequency of the optical field.69 The reaction torque on a transparent object that changes the degree of circular polarization of the incident light is given by77
| τR = ΔσP/ω | (5) |
By equating this torque to the drag torque on a sphere rotating in a fluid at low Reynolds number, given by78
| τD = 8πηa3Ω | (6) |
| η = ΔσP/(8πa3Ωω). | (7) |
This analysis can be implemented at the micrometer scale using a system based on optical tweezers. In this case birefringent spheres of synthetically grown vaterite are trapped in a circularly polarized optical tweezers and rotated.69 The frequency of rotation and the polarization change of light passing through the particle are measured to determine the viscosity of the surrounding fluid. This method allows highly localized measurements to be made, since the probe particle does not move in the surrounding medium. Also, since the probe particle is rotationally driven by the optical trap, the rotation rate can be readily controlled.
Effect of walls
The Stokes drag force acting on a sphere translating in a fluid is increased by the presence of a neighbouring wall by a factor given by Faxén's correction.79,80 This effect is especially important in microfluidic devices. Here it is not possible for particles to be tens of radii away from any surfaces and these have a strong influence on the fluid flow and drag forces on moving objects.A particle moving perpendicular (parallel) to the wall will experience a drag coefficient, γ⊥ (γ||), which is related to its unbounded drag coefficient, γ, by:
 | (8a) |
 | (8b) |
A similar increase in the rotational drag coefficient, β⊥ (β||), is seen for particles rotating close to a wall:82
 | (9a) |
 | (9b) |
One can see immediately that the rotational correction is required at much shorter separations than the translational correction, as has previously been noted in relation to microrheology using spinning particles.69 As a result, the influence of surfaces on translational or rotational particle dynamics occurs at different length scales. The extremely short range nature of the rotational case means that, for example, a 10% increase in drag coefficient occurs at a distance of five radii for the translation of a sphere parallel to the surface and within one radius of the surface for rotational motion. Since the effect of walls occurs at much shorter distances for rotating particles than for translating particles, rotating particles are better used to measure viscosity, while translating particles are better used to map micro-landscapes.
Hydrodynamic coupling
When more than one particle is present in the fluid the motion of any given particle will be influenced by the configuration and motion of the other particles. These hydrodynamic interactions between the particles tend to favour correlated motion, where every particle can move within the “slip-stream” of its neighbours, and hinder anti-correlated motion, where particles move in opposite directions.83 The effect of the hydrodynamic interaction, then, is to modify the damping force acting on the particles.Hydrodynamic coupling is particularly important within colloidal science. The role that colloids play in our everyday lives is huge. Typical examples include blood, milk, paint and aerosols. The forces between particles which make up colloidal suspensions are very important in determining their behaviour and optical tweezers are a convenient method to probe such forces. The multiplexing of optical tweezers using scanning or holographic techniques allows the experimenter to create arrays of particles. This gives rise to various possibilities with colloidal particles: examining the interaction between particles, creating arrays for template studies and the organisation of large arrays into complex structures.
Recent work84 has predicted that when micron sized particles are trapped in an extended periodic array the system might behave like an elastic medium which could support underdamped propagating elastic waves with uniformly negative group velocities (see figure 3 of ref. 84). This has been demonstrated experimentally45 for water droplets trapped in air.
Viscoelasticity
In all of these previous analyses we have made the assumption that the fluid is purely viscous (i.e. Newtonian). When we come to consider viscoelastic (non-Newtonian) fluids the simplest and most intuitive approach is to use the mean square displacement (MSD) of the particles' positions, whether they are free to diffuse or localized in space by optical tweezers.The majority of microrheological techniques are based on the observation of the (free or driven) motion of tracer particles introduced into the fluid under investigation. They can be classified as: (I) passive microrheology and (II) active microrheology. The first technique is based on the observation of thermally excited (free) motion of low concentration (i.e. non-interacting) spheres, of order of a micron in size, suspended in the viscoelastic system under investigation. The second is based on the response of a single (or small number of) tracer particle (e.g. a paramagnetic or ferromagnetic bead in the case of magnetic tweezers) to an external force field (generating the confining or the driving force). Both techniques directly relate the tracer trajectories to the viscoelastic properties of the matrix (fluid) as explained hereinafter.
In the case of the thermally excited motion of low concentration spheres in a purely viscous fluid (having a time independent viscosity), the d-dimensional mean square displacement  evolution of the probe particles is linear in the time interval (i.e. lag time τ = Δt), with a growth rate given by the diffusion coefficient, D:
evolution of the probe particles is linear in the time interval (i.e. lag time τ = Δt), with a growth rate given by the diffusion coefficient, D:
 | (10) |
 | (11) |
 the position of a particle. The average is taken over all the initial times and particles considered in the experiment.
the position of a particle. The average is taken over all the initial times and particles considered in the experiment.
The diffusion coefficient is related to the radius, a, of the particles by the Stokes-Einstein relationship:
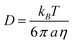 | (12) |
In the opposite limit of a purely elastic medium, the tracer particles move due to thermal motion, but without diffusing through the sample. Their motion can be considered as similar to the stretching of a spring, so the thermal energy of the system can be written as follows:
 | (13) |
In the general case of viscoelastic materials, the time evolution of the mean square displacement is intermediate between the previous limits. Thus the slope of the MSD versus time in a log-log plot representation always lies between 0 (purely elastic) and 1 (purely viscous), as shown in Fig. 2.
 | ||
| Fig. 2 Representation of the mean square displacement (MSD) behaviour of thermally excited probe particles as function of time for three different materials – purely viscous, purely elastic, and viscoelastic. | ||
In the case of a single bead trapped in stationary optical tweezers, the equation describing the tracer motion in a complex fluid can be derived by means of the generalized Langevin equation with an extra term that takes into account the trap stiffness, κ. In one dimension it is:
 | (14) |
By taking the unilateral Laplace transform of Eq. 14 one obtains:
m[s![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (s) − v(0)] = (s) − v(0)] = ![[f with combining tilde]](https://www.rsc.org/images/entities/i_char_0066_0303.gif) R(s) − R(s) − ![[small zeta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e126.gif) (s) (s)![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (s) − κ (s) − κ ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) (s) (s) | (15) |
![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) (s) can be expressed as
(s) can be expressed as ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) (s) =
(s) = ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (s)/s with
(s)/s with ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) (0) = 0, thus:
(0) = 0, thus: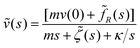 | (16) |
Based on the same statistical mechanics assumptions adopted by Mason and Weitz,85 at thermal equilibrium where 〈v(0)fR(t)〉 = 0 and m〈v(t)v(t)〉 = kBT at any time, Eq. 16 can be re-organised as:
 | (17) |
Moreover, assuming the validity of the approximation made by Mason and Weitz, that the microscopic memory function is proportional to the bulk frequency-dependent viscosity of the fluid ![[small zeta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e126.gif) (s) = 6 πa
(s) = 6 πa![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) (s), Eq. 17 can be written as:
(s), Eq. 17 can be written as:
 | (18) |
![[G with combining tilde]](https://www.rsc.org/images/entities/i_char_0047_0303.gif) (s) is the complex modulus (G*(ω)) expressed in the Laplace form and d is the dimension at which the motion is observed.
(s) is the complex modulus (G*(ω)) expressed in the Laplace form and d is the dimension at which the motion is observed.
The first term in the brackets of Eq. 18 reflects the viscoelasticity of the medium while the second term is related to the inertia of the bead due to its finite mass. However, for a bead of radius 1 µm and density 2000 kg/m3 the bead mass is of the order of 10−14kg. Thus the product ms is negligible with respect to the first term for the majority of the experimentally accessible frequencies. Indeed, in the case of water (or for any Newtonian fluid of known viscosity) where 〈Δ![[r with combining tilde]](https://www.rsc.org/images/entities/i_char_0072_0303.gif) 2(s)〉 = 2dD/s2 and D = kBT/(2dηa), the term ms is negligible for s ≪ 2dπηa/m ≈ 106.
2(s)〉 = 2dD/s2 and D = kBT/(2dηa), the term ms is negligible for s ≪ 2dπηa/m ≈ 106.
The third term takes in account the trap strength. In particular, two limiting cases can be distinguished: (I) in the limit κ/s → 0, which can be obtained either for vanishing trap strength or for measurements performed at high frequencies (but lower than 106), Eq. 18 gives the generalized Stokes-Einstein relationship derived by Mason and Weitz.85 (II) In the limit κ/s → ∞, which can be obtained either for an infinitely strong optical trap or for measurements performed at low frequencies, Eq. 18 gives the same result as if the bead were embedded in a purely elastic medium (i.e.Eq. 13). For all the intermediate cases, where 0 < κ/s < ∞, this term will only affect the real part of the complex modulus (G′) and its contribution can be directly subtracted from the observed storage modulus (G′obs): G′ = G′obs − κ/6πa.
Flow
Microfluidics is closely connected to the field of microrheology. It considers such phenomena as those involved in polymer injection micro-moulding, ink jet printing and flow in miniaturised Lab-on-a-Chip devices. The change of emphasis, which separates the two fields, is whether or not the viscoelastic properties of the fluid at micron length scale are known or need to be measured. The overlap is thus quite strong and the fluid mechanics of materials in confined micro-geometries is a common area to the two research fields. Fluid flow in microfluidic devices can be characterized by the Reynolds number, expressed as the ratio between the inertial and viscous forces. Often, where there is fast flow in small channels, the microflow is strongly influenced by viscous forces and hence the fluid behaviour is very different to that observed at the macroscale. Successful fluid manipulation is crucial in the design of any microfluidic device, and accurate measurement of the flow in such systems is both challenging and important, providing information that can be used to better understand flow fields within laboratory-on-a-chip devices and to validate computational simulations.Optical tweezers can be used to generate49,86,87 and measure88,89 fluid flow in two and three dimensions around microstructures. The generation of fluid flow relies on the optical momentum of the light driving micron-sized beads to create a fluid flow which, although only amounting to a few picolitres, may have application to the delivery of small liquid doses. The measurements are based on the observed motion of micron sized beads, either as displacements from trap centres88 or during times when the optical traps are temporarily switched off.89 The measurements are highly reproducible and minimally invasive, opening the possibility for future in-depth studies of the rheological properties of biological cells and microstructures in laboratory-on-a-chip devices.
Opportunities for tweezers based rheology in biosystems
As already stated, there has been a long established role of optical tweezers in measurements involving biological objects19,21–25 either as a tool to measure motion, or as a force transducer to measure the compliance of bacterial actuators or molecular motors. The extreme sensitivity of the optical tweezers has made it particularly well suited to making ultrahigh resolution mechanical measurements on individual biological molecules.19, 27–29 This latter topic is a subject where optical tweezers is perhaps better suited than Micro-Electro-Mechanical Systems (MEMS) or atomic force microscope (AFM) based measurements due to a number of factors, including: (I) the ability to adjust the stiffness of the trap in real time, during the measurement; (II) the extra degrees of freedom afforded by the tweezers, which can be configured readily in all three dimensions; (III) the extreme sensitivity of measurement and (IV) perhaps the most appealing thing about optical tweezers is that they represent a straightforward upgrade to a conventional, high numerical aperture, microscope and therefore can be readily combined with the full suite of imaging techniques90 and associated technologies, such as microfluidics.51,91 In the future, our ability to use the tweezers to better understand the nature of mechanical interactions between biological molecules in free solution will provide a series of exciting tools to explore both structural and functional biology, enabling the technique to provide mechanistic biological information.Recently, tweezers have been applied to the measurement of the mechanical properties of human red blood cells.26 It is in this area where it is likely that optical tweezers will have a significant impact, enabling physicists and engineers to develop new tools to explore changes in the mechanical properties of cells, whilst being stimulated (e.g. by a signal or a drug), or whilst interacting with each other. In this latter respect, it will be possible to use information on the hydrodynamic coupling between neighbouring cells together with knowledge of the changing nature of the extra-cellular environment (close to the cells membranes) to explore the detailed biophysics of cell-cell interactions. Importantly, the Lab-on-a-Chip environment will provide an arena for such measurements, where it will be possible to control the local environment, including flow, around the cells, being studied.
Acknowledgements
This work was supported by the BBSRC and RCUK.References
- S. C. Kuo, J. Gelles, E. Steuer and M. P. Sheetz, J. Cell Sci. Suppl., 1991, 14, 135 Search PubMed.
- D. J. Pine, D. A. Weitz, P. M. Chaikin and E. Herbolzheimer, Phys. Rev. Lett., 1988, 60, 1134 CrossRef CAS.
- D. A. Weitz, J. X. Zhu, D. J. Durian, H. Gang and D. J. Pine, Phys. Scr., 1993, T49, 610 CrossRef.
- T. Okajima and H. Tokumoto, Nihon Reoroji Gakkaishi, 2008, 36, 81 Search PubMed.
- C. Wilhelm, Phys. Rev. Lett., 2008, 101, 028101 CrossRef.
- A. R. Bausch, W. Moller and E. Sackmann, Biophys. J., 1999, 76, 573 CrossRef CAS.
- R. R. Brau, J. M. Ferrer, H. Lee, C. E. Castro, B. K. Tam, P. B. Tarsa, P. Matsudaira, M. C. Boyce, R. D. Kamm and M. J. Lang, J. Opt. A: Pure Appl. Opt., 2007, 9, S103 CrossRef CAS.
- M. Fischer and K. Berg-Sørensen, J. Opt. A: Pure Appl. Opt., 2007, 9, S239 CrossRef.
- M. Atakhorrami, J. I. Sulkowska, K. M. Addas, G. H. Koenderink, J. X. Tang, A. J. Levine, F. C. MacKintosh and C. F. Schmidt, Phys. Rev. E, 2006, 73, 061501 CrossRef CAS.
- T. A. Waigh, Reports on Progress in Physics, 2005, 68, 685 CrossRef.
- C. J. Pipe and G. H. McKinley, Mechanics Research Communications, 2009, 36, 110 Search PubMed.
- A. Ashkin, Phys. Rev. Lett., 1970, 24, 156 CrossRef CAS.
- A. Ashkin and J. M. Dziedzic, Appl. Phys. Lett., 1971, 19, 283 CrossRef.
- A. Ashkin, J. M. Dziedzic, J. E. Bjorkholm and S. Chu, Opt. Lett., 1986, 11, 288 Search PubMed.
- J. E. Molloy and M. J. Padgett, Contemp. Phys., 2002, 43, 241 CrossRef CAS.
- F. Gittes and C. F. Schmidt, Opt. Lett., 1998, 23, 7 Search PubMed.
- K. C. Neuman and S. M. Block, Rev. Sci. Instrum., 2004, 75, 2787 CrossRef CAS.
- D. G. Grier, Nature, 2003, 424, 810 CrossRef CAS.
- A. Ashkin and J. M. Dziedzic, Science, 1987, 235, 1517 CrossRef CAS.
- K. Svoboda and S. M. Block, Annu. Rev. Biophys. Biomolec. Struct., 1994, 23, 247 CrossRef CAS.
- W. Wright, G. J. Sonek, Y. Tadir and M. W. Berns, Special Issue, IEEE J. Quant. Elec., 1990, 26, 2148 Search PubMed.
- M. P. Sheetzed, Laser tweezers in cell biology, Methods in cell biology, ed. L. Wilson and P. Matsudaira,Vol. 55Academic Press: San Diego223 ( 1998) Search PubMed.
- S. M. Block, D. F. Blair and H. C. Berg, Nature, 1989, 338, 514 CrossRef CAS.
- J. T. Finer, R. M. Simmons and J. A. Spudich, Nature, 1996, 368, 113.
- W. D. Wang, H. Yin, R. Landick, J. Gelles and S. M. Block, Biophysics J., 1997, 72, 1335 Search PubMed.
- Y. Yoon, J. Kotar, G. Yoon and P. Cicuta, Phys. Biology, 2008, 5, 036007 Search PubMed.
- L. Tskhovrebova, J. Trinick, J. A. Sleep and R. M. Simmons, Nature, 1997, 387, 308 CrossRef CAS.
- A. D. Mehta, M. Rief, J. A. Spudich, D. A. Smith and R. M. Simmons, Science, 1999, 283, 1689 CrossRef CAS.
- S. M. Block, L. S. B. Goldstein and B. J. Schnapp, Nature, 1990, 348, 348 CrossRef CAS.
- J. M. R. Fournier, M. M. Burns and J. A. Golovchenko, in Practical Holography IX, S. A. Benton, ed. ( 1995), pp. 101–111 Search PubMed.
- E. R. Dufresne and D. G. Grier, Rev. Sci. Instrum., 1998, 69, 1974 CrossRef CAS.
- Y. Hayasaki, M. Itoh, T. Yatagai and N. Nishida, Opt. Rev., 1999, 6, 24 Search PubMed.
- M. Reicherter, T. Haist, E. U. Wagemann and H. J. Tiziani, Opt. Lett., 1999, 24, 608 Search PubMed.
- J. Liesener, M. Reicherter, T. Haist and H. J. Tiziani, Opt. Commun., 2000, 185, 77 CrossRef CAS.
- E. R. Dufresne, G. C. Spalding, M. T. Dearing, S. A. Sheets and D. G. Grier, Rev. Sci. Instrum., 2001, 72, 1810 CrossRef CAS.
- J. E. Curtis, B. A. Koss and D. G. Grier, Opt. Commun., 2002, 207, 169 CrossRef CAS.
- S. Keen, J. Leach, G. Gibson and M. J. Padgett, J. Opt. A: Pure Appl. Opt., 2007, 9, 264.
- G. M. Gibson, J. Leach, S. Keen, A. J. Wright and M. J. Padgett, Opt. Express, 2008, 16, 14561 CrossRef.
- R. Di Leonardo, G. Ruocco, J. Leach, M. J. Padgett, A. J. Wright, J. M. Girkin, D. R. Burnham and D. McGloin, Phys. Rev. Lett., 2007, 99, 010601 CrossRef CAS.
- J. C. Crocker and D. G. Grier, Phys. Rev. Lett., 1994, 73, 352 CrossRef CAS.
- A. D. Dinsmore, A. G. Yodh and D. J. Pine, Nature, 1996, 383, 239 CrossRef CAS.
- J. C. Crocker, J. Chem. Phys., 1997, 106, 2837 CrossRef CAS.
- J.-C. Meiners and S. R. Quake, Phys. Rev. Lett., 1999, 82, 2211 CrossRef CAS.
- R. Di Leonardo, S. Keen, J. Leach, C. D. Saunter, G. D. Love, G. Ruocco and M. J. Padgett, Phys. Rev. E, 2007, 76, 061402 CrossRef CAS.
- A. M. Yao, S. A. J. Keen, D. R. Burnham, J. Leach, R. Di Leonardo, D. McGloin and M. J. Padgett, New J. Phys., 2009, 11, 053007 CrossRef.
- M. P. MacDonald, G. C. Spalding and K. Dholakia, Nature, 2003, 426, 421 CrossRef CAS.
- G. Milne, D. Rhodes, M. MacDonald and K. Dholakia, Opt. Lett., 2007, 32, 1144 CrossRef.
- R. L. Smith, G. C. Spalding, K. Dholakia and M. P. MacDonald, J. Opt. A-Pure Appl. Opt., 2007, 9, S134 CrossRef.
- J. Leach, H. Mushfique, R. Di Leonardo, M. Padgett and J. Cooper, Lab Chip, 2006, 6, 735 RSC.
- H. Mushfique, J. Leach, H. Yin, R. Di Leonardo, M. J. Padgett and J. M. Cooper, Anal. Chem., 2008, 80, 4237 CrossRef CAS.
- H. Mushfique, J. Leach, R. Di Leonardo, M. J. Padgett and J. M. Cooper, Proc. IMechE Vol. 222 Part C: J. Mechanical Engineering Science, 2008, 829, 37 Search PubMed.
- J. Guck, R. Ananthakrishnan, T. J. Moon, C. C. Cunningham and J. Kas, Phys. Rev. Lett., 2000, 84, 5451 CrossRef CAS.
- J. Guck, R. Ananthakrishnan, H. Mahmood, T. J. Moon, C. C. Cunningham and J. Kas, Biophys. J., 2001, 81, 767 CrossRef CAS.
- J. Guck, R. Ananthakrishnan, C. C. Cunningham and J. Kas, Phys.-Condes. Matter., 2002, 14, 4843 Search PubMed.
- J. Guck, S. Schinkinger, B. Lincoln, F. Wottawah, S. Ebert, M. Romeyke, D. Lenz, H. M. Erickson, R. Ananthakrishnan, D. Mitchell, J. Kas, S. Ulvick and C. Bilby, Biophys. J., 2005, 88, 3689 CrossRef CAS.
- B. Lincoln, S. Schinkinger, K. Travis, F. Wottawah, S. Ebert, F. Sauer and J. Guck, Biomed. Microdevices, 2007, 9, 703 CrossRef.
- B. Lincoln, F. Wottawah, S. Schinkinger, S. Ebert and J. Guck, Cell Mechanics, 2007, 83, 397 Search PubMed.
- P. Cicuta and A. M. Donald, Soft Matter, 2007, 3, 1449 RSC.
- M. A. K. Williams, R. R. Vincent, D. N. Pinder and Y. Hemar, J. Non-Newton. Fluid Mech., 2008, 149, 63 CrossRef CAS.
- M. Atakhorrami, K. M. Addas and C. F. Schmidt, Review of Scientific Instruments, 2008, 79, 043103 CrossRef CAS.
- M. Atakhorrami, D. Mizuno, G. H. Koenderink, T. B. Liverpool, F. C. MacKintosh and C. F. Schmidt, Phys. Rev. E, 2008, 77, 061508 CrossRef CAS.
- G. Pesce, A. Sasso and S. Fusco, Rev. Sci. Instrum., 2005, 76, 115105 CrossRef.
- A. Buosciolo, G. Pesce and A. Sasso, Opt. Commun., 2004, 230, 357 CrossRef CAS.
- A. Rohrbach, C. Tischer, D. Neumayer, E.-L. Florin and E. H. K. Stelzer, Rev. Sci. Instrum., 2004, 75, 2197 CrossRef CAS.
- B. A. Nemet, Y. Shabtai and M. Cronin-Golomb, Opt. Lett., 2002, 27, 264 Search PubMed.
- R. Lugowski, B. Kolodziejczyk and Y. Kawata, Opt. Commun., 2002, 202, 1 CrossRef CAS.
- A. Pralle, E. L. Florin, E. H. K. Stelzer and J. K. H. Horber, Appl. Phys. A: Mate.r, 1998, 66, S71 CrossRef CAS.
- K. Berg-Sørensen and H. Flyvbjerg, Rev. Sci. Instrum., 2004, 75, 594 CrossRef CAS.
- A. I. Bishop, T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, Phys. Rev. Lett., 2004, 92, 198104 CrossRef.
- R. A. Beth, Phys. Rev., 1936, 50, 115 CrossRef.
- M. E. J. Friese, J. Enger, H. Rubinsztein-Dunlop and N. R. Heckenberg, Phys. Rev. A, 1996, 54, 1593 CrossRef CAS.
- M. E. J. Friese, T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, Nature (London), 1998, 394, 348 CrossRef CAS; M. E. J. Friese, T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, Nature (London), 1998, 395, 621 CrossRef CAS (E).
- E. Higurashi, H. Ukita, H. Tanaka and O. Ohguchi, Appl. Phys. Lett., 1994, 64, 2209 CrossRef CAS.
- S. Bayoudh, T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, J. Mod. Opt., 2003, 50, 1581.
- L. Paterson, M. P. MacDonald, J. Arlt, W. Sibbett, P. E. Bryant and K. Dholakia, Science, 2001, 292, 912 CrossRef CAS.
- A. I. Bishop, T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, Phys. Rev. A, 2003, 68, 033802 CrossRef.
- T. A. Nieminen, N. R. Heckenberg and H. Rubinsztein-Dunlop, J. Mod. Opt., 2001, 48, 405.
- L. D. Landau and E. M. Lifshitz, Fluid Mechanics, Course of Theoretical PhysicsVol. 6 (Butterworth-Heinemann, Oxford, 1987), 2nd ed Search PubMed.
- H. Faxen, Annalen der Physik, 1922, 4, 89 CrossRef.
- J. Happel and H. Brenner, Low Reynolds number hydrodynamics, Kluwer Academic Publishers, 1983 Search PubMed.
- S. Keen, A. Yao, J. Leach, R. Di Leonardo, C. Saunter, G. Love, J. Cooper and M. Padgett, Lab Chip, 2009 10.1039/b900934e.
- J. Leach, H. Mushfique, S. Keen, R. Di Leonardo, G. Ruocco, J. M. Cooper and M. J. Padgett, Phys. Rev. E, 2009, 79, 026301 CrossRef CAS.
- E. R. Dufresne, T. M. Squires, M. P. Brenner and D. G. Grier, Phys. Rev. Lett., 2000, 85, 3317 CrossRef CAS.
- M. Polin, D. G. Grier and S. R. Quake, Phys. Rev. Lett., 2006, 96, 088101 CrossRef.
- T. G. Mason and D. A. Weitz, Phys. Rev. Lett., 1995, 74, 1250 CrossRef CAS.
- A. Terray, J. Oakey and D. W. M. Marr, Science, 2002, 296, 1841 CrossRef CAS.
- K. Ladavac and D. G. Grier, Opt. Express, 2004, 12, 1144 CrossRef.
- G. Knöner, S. Parkin, N. R. Heckenberg and H. Rubinsztein-Dunlop, Phys. Rev. E, 2005, 72, 031507 CrossRef.
- R. Di Leonardo, J. Leach, H. Mushfique, J. M. Cooper, G. Ruocco and M. J. Padgett, Phys. Rev. Lett., 2006, 96, 134502 CrossRef.
- D. L. J. Vossen, A. van der Horst, M. Dogterom and A. van Blaaderen, Rev. Sci. Instrum., 2004, 75, 2960 CrossRef CAS.
- K. Sott, E. Eriksson, E. Petelenz and M. Goksör, Expert Opinion on Drug Discovery, 2008, 3, 1323 CrossRef CAS.
Footnote |
| † Part of a Lab on a Chip themed issue on fundamental principles and techniques in microfluidics, edited by Juan Santiago and Chuan-Hua Chen. |
| This journal is © The Royal Society of Chemistry 2009 |
