Themed issue: layered materials: structure and properties
Layered materials in general, and in particular those that do not contain silicon and/or aluminium, are the products of synthetic chemistry, and represent a large class of compounds which is constantly growing in terms of chemical compositions and structures. They include, among others, sulfides, metal dichalcogenides, graphite and graphene, and clay minerals such as aluminosilicates and layered double hydroxides (LDHs), as well as hybrid inorganic–organic compounds. Ingenious procedures at high temperatures and/or high pressures are used to obtain layered solids that display structures similar to clay minerals. Soft chemical routes began to appear more recently, again very much based on the procedures known for clays and silicates/aluminosilicates. A wide range of uses of layered materials, especially in the field of nanotechnology, is due to their capacity to: i) intercalate a large variety of inorganic and organic species and ii) exfoliate under controlled experimental conditions. The latter procedure leads to lamellar nanosized two-dimensional single crystals, named nanosheets, which are used as building blocks for the development of a wide range of novel materials with long controlled structure periodicity. 2D nanosheets very often show unique anisotropic properties which are exploited for the fabrication of sophisticated nanodevices.The themed issue is based on four main topics related to properties of layered materials: Optical, Electronic, Magnetic Properties and ApplicationsforEnergy and Environmental Issues. Theoretical studies and their advances in predicting/modeling structure and properties of layered materials are contained in another topic of the theme issue, which includes also Structural studies of novel materials. The issue contains (i) full papers reporting original research and (ii) review-type articles (Feature, Highlights and Applications).
Several fascinating reviews show in very effective and elegant fashion the relevance of layered materials in offering new opportunities and strategies for applications in several fields such as electronics, optics, spin-electronics, energy production and storage, environmental solutions, catalysis and photocatalysis, biomedical applications, and so forth. This is especially true in the realm of nanoscience and nanotechnology, e.g. when dealing with nanostructures or nanosystems.
The Highlight contribution by Rao et al., for instance, provides a nice and adequate path from materials with specific properties to materials that can be modulated/assembled/modified to show a plethora of different properties. Fig. 1 shows how graphene is the parent of all the other graphitic forms of carbon. The authors indicate that many aspects of graphene chemistry are still either to be unveiled or to be developed. It starts with the preparation itself: synthesis methods which are able to afford large quantities of graphene with controlled number of layers are still missing. The way in which electronic, magnetic and electrochemical properties appear in these materials, and the means to control them, are also of interest; these studies seem to be dependent on the possibility of preparing the materials by different methods. The interesting effects that graphene induces in polymers are also important targets of research. These materials also exhibit properties as gas adsorbers that could be modulated to use for environmental purposes. The synthesis, characterization and properties of single-, bi- and multi-layer graphenes, and their capability for electrochemical doping and for molecular charge transfer, are presented in some detail.
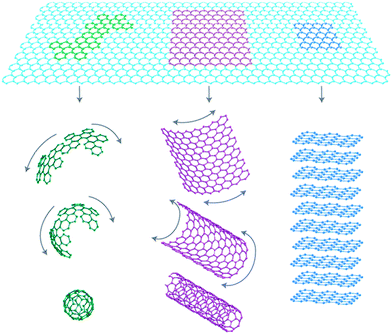 | ||
| Fig. 1 Graphene as parent to all forms of graphitic carbons (reproduced with permission from ref. 1 of the Highlight paper). | ||
Exfoliation of layered metal oxides is a method of choice to obtain layers that can then be re-assembled into nanodevices with sophisticated functionalities. Osada and Sasaki review the status of materials where electron-transport phenomena can be modulated due to 2D confinement. These materials can be used as photocatalysts, as photoconductors, they exhibit photoluminescence, they can be used as low-k dielectrics, as supercapacitors, can exhibit electrochromic behaviour and can be used in photoconducting cells. Magneto-optic and magneto-electronic devices can also be constructed from layered oxides, as shown by the interest in room-temperature ferromagnetic semiconductors and low-dimensional magnetic nanostructures.
A few papers in this issue report on the adequacy of layered materials as sources of nanobuilding blocks for the construction of complex advanced multifunctional materials. Constantino et al. show how this path is followed by layered niobates. Niobate nanoparticles can be prepared by soft chemical routes and present unique features: flexibility and transparency, net electric charge, they are semiconducting, photosensitive, they exhibit surface acidic sites and chemical stability in a large range of pH, to name a few. These characteristics prompt their use as thin films for coatings, conductive membranes or sensors, porous materials for (photo-)catalysis and adsorption, magnetic materials for liquid crystal displays, nanocomposites with organic polymers, phosphors and photovoltaic devices. These possibilities are nicely represented in Scheme 1.
 | ||
| Scheme 1 Niobate nanosheets as building blocks for materials assembly (reproduced from the Feature Article of Bizeto, Shiguihara and Constantino). | ||
Optical properties of rare earth metals in layered materials and their luminescent behaviour are presented by Brunet et al. and Rocha et al. In the first case, a layered zirconium phosphate was covalently pillared with polyoxygenated chains and further interacted with the lanthanide ions. The authors found that the emission from these systems was approximately double that found in non-intercalated zirconium phosphate. A type of antenna effect was invoked to explain this observation. The same oxygenated chains are supposed to be particularly organized in the presence of a chiral molecule and to retain the memory of this arrangement after the molecule is withdrawn. Further developments in the direction of its use in display devices, optical storage devices, and in asymmetric photochemical synthesis are referenced as well as the possibility of using the system for production of circularly polarized luminescence exceeding the usual limit of 0.1%. Rocha et al. discussed photoluminescence of a large series of rare earth metals (and one mixed lanthanide) that are part of the layers in hybrid layered networks. Efficient Gd3+-to-Eu3+ energy transfer was observed in a mixed lanthanide material, and this prompted the authors to suggest that optical devices could be engineered by properly tuning the ratios of different lanthanides in the layered frameworks. The relevance of this study, however, stands in the discovery of a bi-functional property of these materials, which are also catalytically active in the cyclodehydration of xylose to furfural. These represent the first examples of photoluminescent and catalytic rare-earth-based layered materials.
Besides the already mentioned Feature Article from Osada and Sasaki, a range of electronic properties were examined by several contributions. Huang, Kaner et al. reported on thermoelectric materials produced from alloys of bismuth telluride and selenide, Bi2Te3 and Bi2Se3, by the use of the reduction power of solvated electrons prepared using Li/NH3 and further exfoliation. Highly oriented films of the materials were obtained by annealing at reasonably low temperatures affording a means to produce bulk quantities of nanostructured thermoelectric materials that can also be used fruitfully in waste heat recovery and electronic refrigeration. Yamanaka et al. demonstrated that the intercalation of pyridine and Li, Na, K and Rb into TiNCl layered materials (Fig. 2) afforded superconductors with transition temperature (Tc) of 8.6 K and 16.3 respectively. The appearance of different kinds of polytypes with centered cells depending on the type of intercalants suggests the possibility of different density of states profiles that would allow different characters in superconductivity. The pyridine intercalation compound can be classified as an organic–inorganic superconductor which will open a new research field of superconductors.
 | ||
| Fig. 2 The arrangements of Py molecules and K ions in TiNCl layered compounds (reproduced from the article of Yamanaka et al.). | ||
Organic–inorganic layered materials may display interesting magnetic properties: Cheetham et al. present a novel hybrid cobalt hydroxide pillared by ethanedisulfonate ligands, which exhibits a field-induced magnetic transition. The authors report evidence that the magnetic ordering is only two dimensional, e.g. exclusively within the inorganic layers. This study proposes novel routes for the preparation of dense hybrid materials with metal oxide-like properties, such as magnetism and ferroelectricity, with improved hardness and thermal stability.
A few articles cover the topic of energy and environmental issues. Examples are reported in the reviews mentioned above, especially in the context of the use of layered materials for photocatalysis or photovoltaic applications. Work on the use of layered oxides, and some phosphates, of vanadium and molybdenum in Li batteries and on electrochromic materials is reported by Whittingham et al. The advantages and drawbacks of both materials are revised with indications of innovative applications for each of them. The points still to be addressed in the studies aiming at removing the disadvantages of these materials are highlighted and discussed. One important point is the need to resolve the stability concerns of vanadium dissolution and the tendency of lithium and vanadium to mix changing the crystal structure on cycling the lithium in and out, during the use of pacemakers. In electrochromic displays, higher reactivities of both vanadium and molybdenum oxides provided by nanomorphologies have to be explored.
The perspectives offered by 2D solids as new bio-inorganic green materials were explored by Choy et al. Layered double hydroxides (LDH), hydroxyl double salts and cationic clays were demonstrated to be useful solids acting as biomolecule reservoirs, in pharmaceutical applications of anticancer, antibiotics, antinflammatory and anticoagulation drugs, as well as hypertension drugs. In general, the inclusion of drugs into these particular solids increases their resistance to decomposition. The properties of layered materials provide not only a methodology for safe storage of unstable biomolecules but also a design strategy for delivery vehicles and advanced functional materials.
The capacity of controlled displacement of layers to afford a material displaying larger pores can be performed by simple chemical procedures. Clearfield et al. showed it elegantly by preparing tin(IV) phosphonates as porous spherical aggregates and as three-dimensional structures depending on the synthesis conditions used. The control of porosity can be performed by careful choice of P-substituting organic groups. The pores can be further modified by reaction with the organic groups attached to the layers thus providing interesting reactivity.
Theoretical and structural studies are the last topics of the theme issue. Clays and clay minerals, other layered model materials and some real layered silicates are addressed by Cygan et al. in their contribution. The authors review the computational chemistry approaches based on classical force fields and quantum-chemical methods of electronic structure methods calculations and discuss their application to evaluate structures and dynamics at the atomic scale of inorganic–organic and clay–polymer nanocomposite phases and layered double hydroxides. The potential and the utility of modeling and molecular dynamics (MD) simulations to understand the properties of interfaces in layered materials are illustrated by elegant examples (Fig. 3).
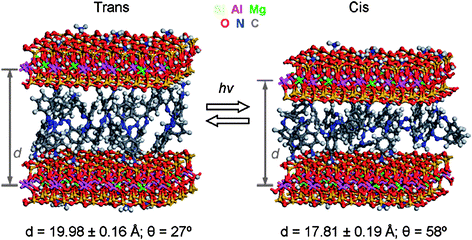 | ||
| Fig. 3 Schematic representation of light-induced trans/cis conversion of phenylazophenylammonium ions intercalated in montmorillonite, which can be exploited for the design of nanoscale actuators (Fig. 4 of the Application article from Cygan et al). | ||
Large-scale atomistic and coarse-grained molecular dynamics simulations are discussed by Coveney et al. Large scale models of clay minerals, increasingly close to the real materials, allow mesoscopic simulations and the study of complex phenomena such as structural and mechanical properties, so decisive in the applications of these materials.
Two contributions address the events occurring in the interlayer spaces. The energies involved in the maintenance of the layered features of a given material were discussed by Ugliengo et al. The effects of dispersive interactions on the structure, energetic and vibrational features of brucite, portlandite and kaolinite were examined by the authors. In the case of kaolinite, the interlayer interaction energy is increased by the dispersion contribution which justifies the need for strong Lewis basic molecules to effectively intercalate into the material.
A combined experimental and theoretical approach, which is based on DFT calculations, is used by Cossi et al. to follow the structural modifications of a layered silicate, Na-RUB-18, observed by removal of hydration water molecules. Structure and vibrational properties of computational models are used to describe the removal of specific water molecules and the migration of half-bare sodium atoms towards the framework oxygen atoms. Silanol/silanolate bridges, which are responsible for proton conduction in this material, are described by a combined computational and spectroscopic approach, which is proposed to be of general utility to refine structures which are not fully accessible to standard X-ray structure analysis.
Alekseev et al. show three novel layered uranyl arsenates prepared by high temperature solid-state reactions. Also a new building block, a zigzag-like tetramer of arsenium, [As4O13]6−, appears in Ag6[(UO2)2(AsO4)2(As2O7)] and Na6[(UO2)2(AsO4)2(As2O7)]. In Ag6[(UO2)2(As2O7)(As4O13)] the polymerization between K2[(UO2)As2O7] and [As4O13]6− is different and the alternating chain is not observed.
This Themed Issue was intended to provide an overview of the general topics of research in the area of Layered Materials; it has never been intended to be comprehensive. The editors are aware that many important applications/systems/groups were not focused upon here, which can only indicate the need for future efforts to involve them in another issue. The Guest Editors are profoundly grateful to all authors that contributed to assemble such a high-quality material, especially devoted to one specific type of material. The staff of Journal of Materials Chemistry cannot be forgotten. Their high-quality and professional work and their constant help, the updates and the suggestions were very much appreciated. We particularly acknowledge The Royal Society of Chemistry for their support for the idea of a Themed Issue on Layered Materials: Structure and Properties, and for providing us with the background that made it all possible.
 | ||
| Plate1 Heloise O. Pastore, Brazil | ||
 | ||
| Plate2 Leonardo Marchese, Italy | ||
| This journal is © The Royal Society of Chemistry 2009 |
