Thermally controlled synthesis of single-wall carbon nanotubes with selective diameters
Enkeleda
Dervishi
*ab,
Zhongrui
Li
b,
Fumiya
Watanabe
b,
Yang
Xu
b,
Viney
Saini
ab,
Alexandru R.
Biris
c and
Alexandru S.
Biris
*ab
aApplied Science Department, University of Arkansas at Little Rock, 2801 S. University Ave, Little Rock, AR 72204, USA. E-mail: exdervishi@ualr.edu; asbiris@ualr.edu; Tel: (+501) 749-9148 Tel: (+501) 569-3203
bUALR Nanotechnology Center, University of Arkansas at Little Rock, 2801 S. University Ave, Little Rock, AR 72204, USA
cNational Institute for Research and Development of Isotopic and Molecular Technologies, P.O. Box 700, R-400293, Cluj-Napoca, Romania
First published on 17th March 2009
Abstract
High quality single-wall carbon nanotubes were synthesized on a MgO supported Fe-Co catalyst system using an inductive radio frequency catalytic chemical vapor deposition method with methane utilized as the hydrocarbon source. The synthesis temperature was varied between 700–1000 °C, and major differences when it comes to the size controllability of the catalyst nano-particles and the nanotube morphology were noticed when the reaction temperature was set at 800 and at 1000 °C. The structural and morphological properties of the catalyst system were analyzed by microscopy, X-ray diffraction and surface area analysis. The electron microscopy analyses of the catalyst system showed that the Fe/Co active metal nano-clusters have a very narrow size distribution when the catalyst system is thermally treated at 800 °C. As a result, highly crystalline single-wall carbon nanotubes with a narrow diameter distribution were successfully synthesized. It was found that by increasing the reaction temperature from 800 to 1000 °C, the diameter distribution of Fe/Co nano-particles broadens and hence affects the nanotube diameters. At 1000 °C the nanotubes presented a wider diameter distribution when compared to the ones grown at lower temperatures, a fact correlated to the changes in the catalyst structural morphology. Furthermore, the nanotubes synthesized at different reaction temperatures were analyzed using several techniques such as electron microscopy, thermogravimetric analysis, Raman and UV-Vis-NIR spectroscopy.
Introduction
Carbon nanotubes (CNTs) have exceptional electrical, optical and mechanical properties making them attractive for numerous applications.1,2,3 Single-wall carbon nanotubes (SWCNTs) with narrow diameter distributions are especially excellent candidates for nano-electronic applications, such as advanced nano-electronic switches and field-effect transistors.4,5 Various methods such as electric arc discharge,6laser ablation7 and chemical vapor deposition (CVD)8 are utilized for carbon nanotube synthesis. However, the most widely used method for nanotube growth is the CVD process. Utilizing this method, the morphological properties of CNTs can be controlled to a certain extent by varying the reaction parameters. Another advantage of the CVD process is that it can be scaled up for large-scale production of high-quality CNTs at a relatively low cost. This method is based on the pyrolysis of different hydrocarbon gases, carbon monoxide (CO), or alcohol at high temperatures (typically between 700–1000 °C) in the presence of a catalyst system.9,10Since the discovery of CNTs a lot of work has been done trying to understand the kinetics of nanotube formation.11 Synthesis conditions such as reaction temperature, flow rates of the carrier and hydrocarbon gas, hydrocarbon type, catalyst composition and many others have enormous effects on nanotube growth, morphology, and properties. Since the ability to reliably produce CNTs with homogenous diameters is very desirable for many applications, it is crucial to fully understand the influence of the reaction conditions on the morphology of the nanotubes. The diameter, chirality and yield of CNTs are very sensitive to the chemical composition of the catalyst system utilized in the growth process.12 Different transition metals such as Fe, Co, Ni and others are employed as active catalyst materials to decompose the hydrocarbon sources and instigate nanotube growth.12 Although many monometallic catalysts have been successfully used to grow different types of CNTs, bimetallic alloys formed by the transition metals generally provide a more efficient CNT synthesis.13 By comparing the alloys of different transition metals, Flahaut et al. found that SWCNTs with the highest yield were obtained when Fe/Co alloy nano-particles were employed.14 In our previous work, we systematically investigated the effects of the Fe-Co interaction on the CNT crystallinity and production efficiency.15,16 It was found that the Co impurity in Fe enhances the bond rearrangement of both carbon and transition metal atoms for easier carbon absorption, diffusion and orientation of tubular C6 rings into the nanotube structures.17,18
For controlled growth of SWCNTs with a very narrow diameter distribution, it is of great importance to understand how the reaction parameters affect the size of metal nano-particles. It has been experimentally and theoretically proven that the diameter of the SWCNTs is directly related to the size of the metal nano-particles present on the catalyst support.19 It is also worth mentioning that the support materials utilized in the catalyst system also play an important role in the nanotube growth. Different inorganic supports such as MgO, Al2O3, SiO2, etc. are employed to stabilize the fine nano-particles and prevent them from coalescing or agglomerating into large clusters at high temperatures.20,21
It is commonly believed that CNT growth is initiated once a carbon monolayer covering a catalyst particle becomes unstable due to the incorporation of additional carbon atoms or thermal vibrations.22,23 Obviously, the thermal treatment plays an important role in the nucleation and the carbon nanotube growth. By cautiously selecting the proper synthesis temperature one can control the nanotube diameter distribution. Ago et al. studied the effects of the reaction conditions on Fe nano-particles supported on porous MgO,21,24 but so far no thorough studies have been found on the Fe-Co bimetallic catalyst system. The aim of this research is to study the influence of the synthesis temperature on the Fe-Co/MgO catalyst system and the CNT growth properties. Furthermore, at proper reaction conditions this bimetallic catalyst system produces high yield and high quality SWCNTs. We have utilized a porous MgO support because it can be removed by a relatively mild acid (HCl), while other supports such as silica require highly toxic treatment. Spectroscopy, thermal gravimetric analysis, microscopy and other techniques were used to analyze the morphological properties of the CNTs and the catalyst system.
Results and discussion
Analysis of the catalyst system
The catalyst system Fe-Co/MgO with a stoichiometric composition of 2.5:2.5:95 wt% was utilized to grow single-wall and double-wall carbon nanotubes at different reaction temperatures. The synthesis temperature was varied between 700–1000 °C, and major differences when it comes to the size controllability of the catalyst nano-particles were noticed when the temperature was set at 800 and 1000 °C. To understand the effect of the reaction temperature on the catalyst morphology, the catalyst systems thermally treated at two different temperatures (800 and 1000 °C) were characterized by microscopy and other techniques. The catalyst system calcined at 500 °C will be denoted as cat_500, whereas the catalysts thermally treated at 800 and 1000 °C will be referred to as cat_800 and cat_1000 respectively. The scanning transmission electron microscopy (STEM) image of cat_800, in Fig. 1(a), shows a large number of metal nano-particles distributed on the MgO support with very similar dimensions. This is supported by the histogram in Fig. 1(b) which shows a very narrow diameter distribution of metal nano-clusters for cat_800. The diameters of the Fe/Co nano-particles present on the MgO support vary between 1–2.5 nm and a vast number of them have diameters between 1.5–2 nm. The SEM image of cat_800 in Fig. 1(c) shows the morphological structure of the catalyst when heated at a high temperature. SEM analyses indicate that both the structure and the morphology of the MgO substrate are affected by the reaction temperature. | ||
| Fig. 1 (a) STEM image of Fe-Co/MgO thermally treated at 800 °C, (b) the diameter distribution histogram of the metal nano-clusters present on the MgO support with a Gaussian fitting curve, (c) SEM image of cat_800, (d) STEM image of the Fe/Co nano-particles present on the MgO surface for cat_1000, (e) corresponding diameter distribution histogram of the Fe/Co metal nano-clusters with a Gaussian fitting curve, (f) SEM image of the Fe-Co/MgO catalyst system thermally treated at 1000 °C. | ||
Fig. 1(d) shows the STEM image of cat_1000 in which the Fe/Co nano-clusters with many different sizes are present onto the surface of the MgO support. The histogram in Fig. 1(e) indicates a wide diameter distribution of the Fe/Co nano-particles for the catalyst system thermally treated at 1000 °C. The diameters of the metal nano-clusters vary between 1.5 to 6.5 nm and approximately 27% of the nano-particles have diameters from 3 to 3.5 nm. As observed from Fig. 1, the Fe/Co nano-clusters have a much wider diameter distribution when the catalyst system Fe-Co/MgO (2.5:2.5:95 wt.%) is heated at 1000 °C compared to the one thermally treated at 800 °C. These diameter distribution changes (of the metal nano-particles heated at 1000 °C) could be explained by thermally activated phenomena such as surface diffusion or Ostwald ripening.25 The SEM image in Fig. 1(f) demonstrates the morphological structure of cat_1000. Due to the resolution limitations, no significant differences were detected between the catalysts cat_800 and cat_1000 based on the SEM analysis. However, at high temperatures the structure of the catalyst system particularly of the support (MgO) changed and various MgO particles agglomerated into larger clusters.
Surface area analyses of the catalyst systems thermally treated at 500, 800 and 1000 °C are presented in Table 1. The Langmuir surface area of Fe-Co/MgO catalyst calcined at 500 °C was measured to be 48.84 m2/g. As the temperature increases from 500 to 800 °C, the BET and Langmuir surface areas of the catalyst decrease since at high temperatures the MgO particles agglomerate into larger nano-clusters yielding a lower catalyst surface area. At 800 °C, the MgO support has a relatively high surface area and small nano-pores which provide a better localization for the active metal nano-particles confining them into small nano-clusters. In addition, the strong metal-support interaction between the Fe/Co nano-particles and the MgO support assists into the formation of fine metal nano-particles with very narrow diameter distribution.24 As shown in Table 1, the surface area of cat_1000 decreases whereas the adsorption pore width increases compared to cat_800. This increase in the pore width of cat_1000 might indicate a broadening of the MgO pore size aiding the formation of nano-clusters with a wider diameter distribution. This is also supported by the STEM analysis (Fig. 1(d)) where some of the particles have diameters between 4–6.5 nm. On the other hand, the surface area of the catalyst system as well as the carbon nanotube synthesis efficiency decreases as the temperature increases from 800 to 1000 °C. The catalyst system Fe-Co/MgO (2.5:2.5:95 wt.%) lost approximately 25% weight after calcination at 500 °C for 2 hours. The TGA analyses (not shown here) demonstrate that the Fe and Co nitrates decompose at lower temperatures than 500 °C forming Fe and Co nano-clusters. Therefore, the weight loss of the catalyst at 500 °C is correlated with the complete removal of Fe and Co nitrates as well as the water groups from the metal salts present in the catalyst system.
| Sample | SA BET(m2/g) | SA Langmuir(m2/g) | Pore Size(nm) |
|---|---|---|---|
| cat_500 | 35.45 | 48.84 | 25.24 |
| cat_800 | 25.45 | 35.18 | 16.45 |
| cat_1000 | 16.21 | 22.26 | 21.50 |
The X-ray diffraction (XRD) technique was used for phase identification, quantitative phase analysis and crystallite size estimation of thermally treated catalysts at different temperatures. Fig. 2 displays the power diffraction profiles and the Rietveld refinement pattern fitting for cat_500, cat_800 and cat_1000. The XRD patterns of cat_500 and cat_800 indicate the presence of phases characteristic of MgO and the oxides of Fe or Co, but not to Fe-Co alloys. However, the XRD profile of the catalyst thermally treated at 1000 °C shows an additional peak at 2θ = 44.8 degrees, which is attributed to the Fe-Co alloy (indicated by the arrow in Fig. 2). This could be due to the catalyst exposure to high temperature, when it is possible that the Fe/Co nano-particles form alloys when the diffusion coefficient increases sufficiently.
 | ||
| Fig. 2 X-Ray powder diffraction patterns for the calcined catalyst at 500 °C and the thermally treated catalyst systems at 800 and 1000 °C. | ||
The crystal percentages of the compositions in cat_1000 were found to be the following: MgO (92.5%), FeCo (3.7%), (Mg0.18Co0.82)(Mg0.20Co1.80)O4 (2.2%), (MgO)0.91(FeO)0.09 (1.6%). The XRD profile of cat_1000 contains an additional weak peak corresponding to the Fe-Co alloy when compared to the other catalysts. The crystal size of MgO was estimated from the diffraction peak widths (using the Scherrer equation) to be approximately 35 nm. Crystalline MgO contains few nano-pores and has narrower peak width compared with porous MgO.24 The XRD profile in Fig. 2 shows relatively broad MgO diffraction peaks, indicating the small crystallite size of the support. The size of the FeCo alloy nano-particles could not be calculated using the Scherrer equation since they are smaller than 10 nm.
Carbon nanotube analysis
The TEM image in Fig. 3(a) of the CNTs grown at 800 °C shows a bundle of SWCNTs with very uniform diameters of 3 nm. At 800 °C the nano-particles present in the MgO support have a very narrow size distribution hence yielding SWCNT growth with small and uniform diameters. The nanotubes grown at 800 and 1000 °C will be referred to as CNT_800 and CNT_1000 respectively. The TEM images of CNT_800 (not shown here) also revealed that besides SWCNTs a small amount of double-wall carbon nanotubes (DWCNTs) (approximately 5–10%) were grown. Fig. 3(b) shows the SEM image of the CNTs grown from cat_800 utilizing methane as a hydrocarbon source. There is a very high density of weblike CNTs synthesized on the Fe-Co/MgO catalyst system. | ||
| Fig. 3 (a) TEM image of the CNTs synthesized at 800 °C, (b) SEM image of CNT_800, (c) TEM image of CNT_1000, (d) TEM image of a metal nano-particle trapped inside a DWCNT grown at 1000 °C, (e) the corresponding SEM image of high density nanotubes grown at 1000 °C. | ||
When the synthesis temperature was increased to 1000 °C, the STEM analysis indicated that the Fe/Co nano-particles have a wide diameter distribution. These metal nano-particles with various sizes nucleate the growth of nanotubes with a very broad diameter distribution. Fig. 3(c) shows the TEM image of SWCNTs with various diameters synthesized at 1000 °C. In addition to SWCNTs, about 10–20% of DWCNTs were also present in the sample synthesized at 1000 °C. In this case, the amount of DWCNTs synthesized is higher compared to the nanotubes grown at lower temperatures. This may be due to the large catalytically active nano-particles present on the MgO support at 1000 °C. It has also been shown by others that larger metal nano-clusters yield DWCNTs as well as nanotubes with large diameters.26 The Fe/Co nano-particles with diameters between 4.5–6.5 nm present in the MgO support possibly nucleate DWCNT growth. Fig. 3(d) shows a TEM image of a metal nano-cluster with a diameter of 4.5 nm trapped inside a DWCNT grown at 1000 °C. On the other hand, small nano-particles in the range of 1–2.5 nm yield SWCNT growth with small diameters.27 Although nano-particles with diameter of approximately 4 nm may seem slightly large for typical SWCNT synthesis, it was reported that they were utilized to produce SWCNTs with large diameters.28Fig. 3(e) shows the SEM images of high density CNTs synthesized at 1000 °C. These images reveal a large number of entangled nanotubes grown on the Fe-Co/MgO catalyst system.
Thermogravimetric analysis is a useful technique for characterizing the purity and the thermal stability of CNTs. Fig. 4(a) shows the weight loss profile curves of the CNTs grown at 800 and 1000 °C. The quantitative analysis revealed that only after one purification step, both CNT_800 and CNT_1000 had a purity of higher than 95%. The remaining quantity left after the burning of carbon nanotubes (less than 5%) represents oxides of the metallic nano-particles that are still present within the bundles of SWCNTs or inside the walls of DWCNTs as shown in Fig. 3(d). The inset in Fig. 4(a) presents the first derivatives of the normalized TGA curves indicating the nanotube combustion temperatures of each sample. The TGA curves and the corresponding differential thermal analysis (DTA) show significant weight losses at 568 °C and 585 °C for the purified CNT_800 and CNT_1000. The CNTs grown at 1000 °C decompose at a higher temperature than the ones grown at 800 °C due to the presence of more DWCNTs in CNT_1000. It has been shown that the thermal decomposition temperature of the CNTs depends on their morphological properties and as the number of their graphitic walls increases so does their combustion temperature.29,30 As previously reported, purified DWCNTs decompose at around 700 °C, which is much higher than the SWCNT combustion temperature.26 Therefore, our TGA analysis correlates well with the TEM findings which indicate a higher percentage of DWCNTs grown at 1000 °C compared to 800 °C. Fig. 4(b) shows the TGA curves of the as-produced CNTs grown at different temperatures (700–1000 °C, in 50 degree increments). The inset shows the combustion temperatures for the CNTs generated from the first derivative curves of the corresponding TGA curves. The decomposition temperature of the CNTs grown at 1000 °C is higher than the combustion temperature of the CNTs grown at lower temperatures. In addition, the purified CNT_800 and CNT_1000 burn at higher temperatures than the as-produced samples. This is due to the presence of metal nano-particles trapped next to the nanotube walls (in between the bundles) of the un-purified samples, which act as thermal catalysts in the burning process.31 The un-purified samples grown at 800 °C and 1000 °C were found to burn at 503 and 527 °C, respectively.
 | ||
| Fig. 4 (a) TGA curves of the CNTs grown at 800 and 1000 °C, (inset) the first derivative curves indicating the corresponding combustion temperatures for CNT_800 and CNT_1000, (b) TGA curves of the as-produced CNTs, (inset) the corresponding combustion temperatures of the CNTs grown between 700–1000 °C, in 50 degree increments, (c) yield of CNTs produced through pyrolysis of methane utilizing the catalytic system Fe-Co/MgO (2.5:2.5:95 wt.%) at different temperatures. | ||
The carbon nanotube synthesis efficiency was calculated in order to estimate the activity of the catalyst systems thermally treated at different temperatures. To determine the intrinsic weight loss of the catalyst, a “blank” experiment without the hydrocarbon source and only with argon was performed. The efficiency or the yield (η (%)) of the carbonaceous products that resulted after each synthesis was calculated according to the following formula:32
 | (1) |
Raman spectroscopy was used to analyze the crystallinity and the diameter distribution of CNTs grown at various reaction temperatures. The vibrational modes observed in the nanotube Raman spectra are the radial breathing mode (RBM), the D band, G band and the 2D band.34 The RBM peaks are usually observed between 100 to 500 cm−1, and their positions strongly depend on the diameter of the nanotubes.35 Often, the TEM images reveal that most of the nanotubes are bundled together which affects their RBM peak positions when compared to isolated nanotubes. Theoretical calculations of SWCNTs have shown that tube diameter d and the radial mode frequency ωRBM exhibit the following straightforward relationship:
 | (2) |
Raman analyses of the CNTs were performed before and after purification to make sure that the nanotubes still remained structurally undamaged after being exposed to hydrochloric acid. Fig. 5(a) shows the Raman spectrum of the CNTs grown at 800 °C collected with 633 nm laser excitation. The inset in Fig. 5(a) shows the TEM image of SWCNTs with very uniform and small diameters. These SWCNTs have a very intense peak in the RBM region at 190 cm−1 which correspond to the nanotube diameter of 1.3 nm. Fig. 5(b) shows the Raman spectrum of the CNTs grown at 1000 °C collected with 633 nm laser excitation. There is a large number of peaks in the RBM region corresponding to a broad nanotube diameter distribution. Fig. 5(b) inset shows a TEM image of SWCNTs with many different diameters varying from 1 to 5 nm grown at 1000 °C. Fig. 6 shows the Raman analysis spectra collected with 514 nm laser excitation of the nanotubes grown at 800 and 1000 °C. The RBM regions of the CNT_1000 Raman spectra (collected with 514 and 633 nm laser excitations) have additional peaks compared to the spectrum of CNT_800, which might correspond to the diameters of the DWCNTs present in the sample. In addition, Fig. 6(b) shows that the ratio of the G over D band intensity values calculated from the Raman spectrum of CNT_1000 collected with the 514 nm laser is higher compared to the nanotubes grown at 800 °C.
 | ||
| Fig. 5 (a) Raman spectrum of the CNTs grown at 800 °C collected with 633 nm laser excitation. The inset shows the TEM image of SWCNTs with very small diameters. (b) Raman spectrum of the CNTs grown at 1000 °C collected with 633 nm laser excitation. The inset shows the TEM image of SWCNTs with many different diameters. | ||
 | ||
| Fig. 6 Raman analysis spectra collected with 514 nm laser excitation of the low (a) and high (b) frequency domains, which indicate the presence of all the corresponding bands for the CNTs grown at two different temperatures. | ||
Table 2 presents the RBM peaks from the Raman spectra collected with two laser excitations (514 and 633 nm wavelengths) and the corresponding diameters of CNTs calculated using equation (2). The dominant diameter distribution of CNT_800 ranges from 0.98 to 1.91, whereas the nanotubes grown at 1000 °C have a wider diameter distribution between 1.15 to 2.49 nm. Fig. 7(a) shows the RBM region of the Raman spectra collected with 633 nm laser excitation for carbon nanotubes grown at different temperatures. Most of the CNTs grown at 800 °C have diameters around 1.3 nm which correspond to the very strong RBM peak at 190 cm−1. The Raman spectrum of CNT_1000 has a larger number of RBM peaks corresponding to different diameters of SW or DWCNTs. For example, the RBM peak at 118 cm−1 (of the Raman spectrum collected with 633 nm laser excitation) might correspond to the outer wall of the DWCNT with diameter 2.16 nm shown in Fig. 3(d) (the arrow indicates a small DWCNT grown at 1000 °C). The Raman scattering of its corresponding inner wall might correlate with the 190 cm−1RBM peak (collected with 633 nm laser), or with the Raman shift at 184 cm−1 present in the spectrum of CNT_1000 collected with 514 nm laser excitation. The RBM peaks of DWCNTs and SWCNTs with large diameters cannot be seen in these Raman spectra. Furthermore, it is worth mentioning that the presence of the RBM peaks does not represent the actual quantity of the various nanotube diameters.34 This is due to the fact that the Raman scattering of SWCNTs occurs though a resonance phenomenon associated with the wavelength of the laser excitations.36Fig. 7(b) shows the ratios of the G over D band intensities (IG/ID) calculated from the Raman spectra of the corresponding CNTs grown at different temperatures. The nanotubes grown at 800 °C have the highest IG/ID ratio which decreases as the synthesis temperature raises. At 800 °C the D band present in the Raman spectrum is very weak and its linewidth is relatively small (35 cm−1) indicating the presence of a very small amount of amorphous carbon.38 Therefore, the CNTs grown at 800 °C have the highest crystallinity which is reflected by the absence of high carbonaceous products with structural defects.37 This demonstrates that the reaction temperature has an effect not only on the diameter distribution, but also on their crystallinity and morphological properties.
| Sample | ω RBM(cm−1) | Diameter (nm) |
|---|---|---|
| λexc. = 633 nm | ||
| CNT_800 | 132; 190; 211; 247 | 1.91; 1.3; 1.16; 0.98 |
| CNT_1000 | 104; 118; 133; 148; 164; 190; 213 | 2.49; 2.16; 1.9; 1.69; 1.51; 1.3; 1.15 |
| λexc. = 514 nm | ||
| CNT_800 | 182; 200 | 1.36; 1.23 |
| CNT_1000 | 184; 203; 243; 261; 301; | 1.34; 1.21; 1.004; 0.93; 0.8; |
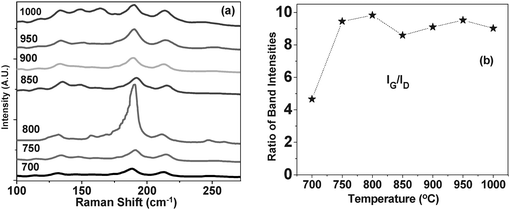 | ||
| Fig. 7 (a) The RBM region of the Raman spectra collected with 633 nm laser excitation for CNTs grown at different temperatures. (b) The IG/ID ratios of the corresponding nanotubes calculated from their high frequency domain Raman spectra. | ||
Since the results from the previous techniques indicate major differences in the diameter distribution between the CNTs synthesized at 800 and 1000 °C, the UV-Vis-NIR spectroscopy was utilized to study the optical properties of these samples. The Kataura plot (calculated for sodium cholate solution) was used to assign the diameters of CNT_800 and CNT_1000.39 As shown in Fig. 8 the homogenous solutions prepared from both samples indicate several absorption bands between 600 to 1300 nm wavelengths. These bands correspond to the absorption of the isolated SWCNTs and possibly to a few DWCNT absorptions.40 Usually, the isolated DWCNTs demonstrate very weak absorbance peaks because during the ultracentrifuge process their high weight density causes them to sediment at the bottom of the aqueous solution.
 | ||
| Fig. 8 The UV-Vis-NIR spectra of CNTs dispersed in sodium cholate solution and synthesized at 800 and 1000 °C. | ||
The absorption spectrum of the nanotubes grown at 800 °C indicates a small number of peaks assigned to the following (n,m) values: (8,7); (12,1); (9;2) and (7,5) which correspond to the SWCNT diameters ranging between 0.8 and 1.03 nm. The first van Hove optical transitions (S11) for these semiconducting SWCNTs were 1265, 1180, 1128 and 1022 nm, whereas their corresponding second van Hove transition peaks were found between 809 and 643 nm. For the CNTs grown at 1000 °C, the UV-Vis-NIR spectrum indicates several absorption peaks assigned to specific semiconducting nanotubes with diameters 0.829, 0.884, 0.895, 0.916, 1.014, and 1.103 nm, which belong to the following chiral families: (7,5); (10,2); (7,6); (11,1); (11,3); and (9,7). The first van Hove optical transition (S11) wavelengths for the semiconducting CNTs grown at 1000 °C were 1320, 1267, 1187, 1122, 1063, and 1029 nm. Their corresponding second van Hove transition (S22) peaks varying between 612 to 791 nm were also assigned. The absorption spectrum of CNT_1000 shows a larger amount of peaks dominating in the 1000 nm wavelength region compared to that of CNT_800. This could be due to the presence of small nanotubes with different diameters grown at 1000 °C, whereas the nanotubes with larger diameters exhibit weak absorbance peaks making them very difficult to detect. The UV-Vis-NIR spectrum of CNT_800 shows only a few absorbance peaks corresponding to SWCNTs with very small and uniform diameters. Therefore, the UV-Vis-NIR results are in good agreement with the analysis from the other techniques which indicate that the CNTs grown at 1000 °C have a wider diameter distribution than the ones grown at 800 °C.
Furthermore, it is worth mentioning that the results presented for this particular catalyst can be expanded to other similar catalytic systems. Thermal treatments can be applied to various mono- or bimetallic catalysts which could be later utilized for CNT growth. Similar reaction conditions presented in this work may be followed for high quality CNT synthesis.
Experimental details
The Fe-Co/MgO catalyst system with a stoichiometric composition of 2.5:2.5:95 wt.% was prepared by the impregnation method as previously described.41 First, weighed amount of metal salts, Fe(NO3)3·9H2O and Co(NO3)2·6H2O, were dissolved separately in ethanol with agitation. Next, MgO with surface area 130 m2/g was completely dispersed into 30 ml of ethanol and the metal salt mixtures were added to this MgO solution. The final mixture was sonicated for approximately 1 hour. Next, the ethanol was evaporated under continuous agitation, and the catalyst system was further dried overnight at 60 °C. Finally, the catalyst was calcined in air at 500 °C for 2 hours.CNTs were grown by radio frequency (RF) catalytic chemical vapor deposition (cCVD) on the MgO supported Fe-Co bimetallic catalyst system utilizing methane as a hydrocarbon source.42 Approximately 100 mg of the catalyst was uniformly spread into a thin layer on a graphite susceptor and placed in the center of a quartz tube with an inner diameter of 1 inch. First, the tube was purged with the carrier gas (argon) for 10 minutes at 150 ml/min. Next, the RF generator (which provides a very fast heating rate of 300–350 °C/min) was turned on and when the temperature of the graphite boat reached the desired synthesis temperature, methane (CH4) was introduced at 40 ml/min for 30 minutes. The temperature at which the nanotubes were grown was varied between 700 to 1000 °C in 50 degree increments. At the end of each reaction, the system cooled in the presence of argon for 10 minutes. The catalyst systems were thermally treated for the same duration of time under only argon atmosphere without introducing the hydrocarbon source at 800 °C and 1000 °C. The as-produced CNTs were purified in one easy step using diluted hydrochloric acid solution and sonication. To burn the amorphous carbon, the purified samples were heated in air at approximately 400 °C for 15 minutes.
Characterization techniques
To analyze the structure of the Fe-Co/MgO (2.5:2.5:95 wt.%) catalyst system and carbon nanotube morphology, several techniques such the microscopy, thermogravimetric analysis, Raman scattering spectroscopy, X-ray diffraction, BET surface area analysis and UV-Vis NIR spectroscopy were utilized.Transmission electron microscopy (TEM) images were collected on a field emission JEM-2100F TEM (JEOL Inc.) equipped with a CCD camera. The acceleration voltages were set to 200 kV for the catalyst systems and 100 kV for the nanotube analysis. CNTs were homogeneously dispersed in 2-propanol and ultrasonicated for 30 minutes. Next, a few drops of the suspension were deposited on the TEM grid, dried, and evacuated before analysis. Scanning electron microscopy (SEM) images were obtained using a JEOL 7000F high-resolution scanning electron microscope. The samples were mounted on aluminium pins with double-sided carbon tape, and their corresponding SEM images were obtained.
Raman scattering spectra were recorded at room temperature using a Horiba Jobin Yvon LabRam HR800 equipped with a CCD, a spectrometer with a grating of 600 lines/mm and a He-Ne laser (633 nm) as excitation source. The laser beam intensity measured at the sample was kept at 5 mW and Raman shifts were calibrated with a silicon wafer at a peak of 521 cm−1.
Thermogravimetric analyses (TGA) were performed under airflow of 150 ml/min using a Mettler Toledo TGA/SDTA 851e. In every TGA experiment, approximately 3 mg of each sample was heated from 25 to 850 °C at a heating rate of 5 °C/min.
The X-ray powder diffraction profiles of the catalyst systems were recorded in the θ-2θ mode on the Bruker D8 Discovery diffraction system (Rigaku Corporation). The monochromatic Cu Kα radiation line and scintillation counter were used as excitation source and detector, respectively. The experiments were carried out in Bragg-Brentano geometry. Quantitative analysis was performed with whole pattern fitting and Rietveld refinement.
Brunauer-Emmett-Teller (BET) and Langmuir surface area analyses were determined by recording nitrogen adsorption/desorption isotherms at 77 K using a static volumetric technique (Micromeritics ASAP 2020).
The optical absorption spectra at UV-Vis-NIR range were recorded using the Shimadzu double beam spectrophotometer UV-3600 with three detectors. In order to analyze their diameter distributions, the CNTs were individually dispersed in a sodium cholate (Sigma-Aldrich) aqueous solution. The samples were first ultrasonicated in a bath sonicator (FS20, Fisher Scientific) for one hour to attain a homogeneous solution, which was later centrifuged for 2 hours at 15000 × g speed using a high revolution centrifuge (Galaxy 16 Micro-centrifuge, VWR International) after which only the supernatant was utilized for optical measurements.
Conclusions
By using the highly selective Fe-Co/MgO catalytic method, high quality SWCNTs were synthesized at various temperatures utilizing methane as a hydrocarbon source. The synthesis temperature was varied between 700–1000 °C and its effects on the morphology of the bimetallic Fe/Co catalyst supported on porous MgO and on the CNT growth were systematically studied. Increasing the reaction temperature from 800 to 1000 °C broadens the diameter distribution of Fe/Co nano-particles and hence affects the nanotube diameters. The STEM analysis of cat_800 show that the diameters of the Fe/Co nano-particles present on the MgO support vary between 1–2.5 nm hence yielding SWCNT growth with small and uniform diameters. When the synthesis temperature was increased to 1000 °C, the STEM analysis indicated that the Fe/Co nano-particles have a wider diameter distribution (between 1.5–6.5 nm), which nucleate the growth of nanotubes with many different diameters. Therefore, an increase in synthesis temperature leads to SWCNT growth with a wider diameter distribution as well as a small amount of DWCNTs. These results are also supported by thermogravimetric and spectroscopic (Raman scattering, optical absorption) analysis. Compared to monometallic catalysts, this bimetallic catalyst system (Fe-Co/MgO) provides a more efficient synthesis of high quality SWCNTs and can be proposed as an alternative way for commercially available catalysts. Furthermore, these finding provide a simple method to optimize the diameter distribution of SWCNTs by choosing a proper reaction temperature.References
- R. Kumashiro, N. Hiroshiba, N. Komatsu, T. Akasaka, Y. Maeda, Sh. Suzuki, Y. Achiba, R. Hatakeyama and K. Tanigaki, J. Phys. and Chem. of Solids, 2007, 69(5–6), 1206–1208 Search PubMed.
- Z. Li, H. R. Kandel, E. Dervishi, V. Saini, Y. Xu, A. R. Biris, D. Lupu, G. J. Salamo and A. S. Biris, Langmuir, 2008, 24(6), 2655–2662 CrossRef CAS.
- A. R. Biris, D. Lupu, E. Dervishi, Z. Li, V. Saini, D. Saini, S. Trigwell, M. K. Mazumder, R. Sharma and A. S. Biris, Particulate Science and Technology, 2008, 26(4), 297–305 CrossRef CAS.
- P. Stokes and S. I. Khondaker, Nanotechnology, 2008, 19(17), 175202 CrossRef.
- T. Kim, S. Kim, E. Olson and J. M. Zuo, Ultramicroscopy, 2008, 108(7), 613–618 CrossRef CAS.
- T. W. Ebbesen and P. M. Ajayan, Nature, 1992, 358, 220–222 CrossRef CAS.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit, J. Robert, C. Xu, Y. H. Lee, S. G. Kim, A. G. Rinzler, D. T. Colbert, G. E. Scuseria, D. Tománek, J. E. Fischer and R. E. Smalley, Science, 1996, 273, 483–487 CrossRef CAS.
- M. Terrones, N. Grobert, J. Olivares, J. P. Zhang, H. Terrones, K. Kordatos, W. K. Hsu, J. P. Hare, P. D. Townsend, K. Prassides, A. K. Cheetham, H. W. Kroto and D. R. M. Walton, Nature, 1997, 388, 52–55 CrossRef CAS.
- Z. F. Ren, Z. P. Huang, J. W. Xu, J. H. Wang, P. Bush, M. P. Siegal and P. N. Provencio, Science, 1998, 282, 1105–1107 CrossRef CAS.
- J. Kong, H. T. Soh, A. M. Cassell, C. F. Quate and H. Dai, Nature, 1998, 395, 878–881 CrossRef CAS.
- S. Iijima, Nature (London, United Kingdom), 1991, 354(6348), 56–58 Search PubMed.
- Ch. Laurent, E. Flahout, A. Peigney and A. Rousset, New J. Chem., 1998, 22, 1229–1237 RSC.
- T. Guo, P. Nikolaev, A. Thess, D. T. Colbert and R. E. Smalley, Chem. Phys. Lett., 1995, 243, 49–54 CrossRef CAS.
- E. Flahaut, A. Govindaraj, A. Peigney, Ch. Laurent, A. Rousset and C. N. R. Rao, Chem. Phys. Lett., 1999, 300, 236–242 CrossRef CAS.
- Z. Li, E. Dervishi, Y. Xu, X. Ma, V. Saini, A. S. Biris, R. Little, A. R. Biris and D. Lupu, J. Chem.l Phys., 2008, 129(7), 074712 Search PubMed.
- E. Dervishi, Z. Li, A. R. Biris, D. Lupu, S. Trigwell and A. S. Biris, Chem. Mater., 2007, 19(2), 179–184 CrossRef CAS.
- C. Guerret-Piécourt, Y. Le Bouar, A. Lolseau and H. Pascard, Nature, 1994, 372, 761–765 CrossRef CAS.
- R. B. Little, J. Cluster Science, 2003, 14, 135–185 Search PubMed.
- Y. Y. Wei, G. Eres, V. I. Merkulov and D. H. Lowndes, Appl. Phys. Lett., 2001, 78, 1394–1396 CrossRef CAS.
- A. Ansaldo, M. Haluskac, J. Cechc, J. C. Meyerc, D. Riccia, F. Gattib, E. Di Zittia, S. Cincottia and S. Rothc, Physica E: Low-dimensional Systems and Nanostructures, 2007, 37, 6–10 Search PubMed.
- H. Ago, S. Imamura, T. Okazaki, T. Saito, M. Yumura and M. Tsuji, J. Phys. Chem. B, 2005, 109, 10035–10041 CrossRef CAS.
- J.-C. Charlier and S. Iijima, Topics in Applied Physics, 2001, 80, 55–80 CAS.
- H. Dai, Topics in Applied Physics, 2001, 80, 29–53 CAS.
- H. Ago, K. Nakamura, N. Uebara and M. Tsuji, J. Phys. Chem. B, 2004, 108, 18908–18915 CrossRef CAS.
- D. Feng, R. Arne and B. Kim, Carbon, 2005, 43(10), 2215–2217 CrossRef CAS.
- H. Ago, K. Nakamura, S. Imamura and M. Tsuji, Chem. Phys. Lett., 2004, 391, 308–313 CrossRef CAS.
- T. Yamada, T. Namai, K. Hata, D. N. Futaba, K. Mizuno, J. Fan, M. Yudasaka, M. Yumura and S. Iijima, Nature Nanotechnology, 2006, 1, 131–136 CrossRef CAS.
- Y. Li, W. Kim, Y. Zhang, M. Rolandi, D. Wang and H. Dai, J. Phys. Chem. B, 2001, 105, 11424–11431 CrossRef CAS.
- G. S. B. McKee and K. S. Vecchio, J. Phys. Chem. B, 2006, 110(3), 1179–1186 CrossRef CAS.
- S. Arepalli, P. Nikolaev, O. Gorelik, V. G. Hadjiev, W. Holmes, B. Files and L. Yowell, Carbon, 2004, 42, 1783–1791 CrossRef CAS.
- A. C. Dillon, T. Gennett, K. M. Jones, J. L. Alleman, P. A. Parilla and M. J. Heben, Adv. Mater., 1999, 11, 1354–1358 CrossRef CAS.
- D. Méhn, A. Fonseca, G. Bister and J. B. Nagy, Chem. Phys. Lett., 2004, 393, 378–384 CrossRef CAS.
- T. Saito, S. Ohshima, W. Xu, H. Ago, M. Yumura and S. Iijima, J. Phys. Chem. B, 2005, 109, 10647–10652 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus, A. Jorio, A. G. Souza Filho and R. Saito, Carbon, 2002, 40, 2043–2061 CrossRef CAS.
- H. Kuzmany, W. Plank, M. Hulman, Ch. Kramberger, A. Gruneis, Th. Pichler, H. Peterlik, H. Kataura and Y. Achiba, Euro Phys J.B., 2001, 22, 307–320 CrossRef.
- A. M. Rao, J. Chen, E. Richter, U. Schlecht, P. C. Eklund, R. C. Haddon, U. D. Venkateswaran, Y.-K. Kwon and D. Tománek, Phys. Rev. Lett., 2001, 86, 3895–3898 CrossRef CAS.
- K. L. Strong, D. P. Anderson, K. Lafdi and J. N. Kuhn, Carbon, 2003, 41, 1477–1488 CrossRef CAS.
- T. Saito, S. Ohshima, W. Xu, H. Ago, M. Yumura and S. Iijima, J. Phys. Chem. B, 2005, 109, 10647–10652 CrossRef CAS.
- R. B. Weisman and S. M. Bachilo, Nano Lett., 2003, 3, 1235–1238 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strane, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. H. Smalley, Science, 2002, 297, 593–596 CrossRef CAS.
- S. C. Lyu, B. C. Liu, S. H. Lee, C. Y. Park, H. K. Kang, C. W. Yang and C. J. Lee, J. Phys. Chem. B, 2004, 108, 1613–1616 CrossRef CAS.
- A. R. Biris, A. S. Biris, E. Dervishi, D. Lupu, S. Trigwell, Z. Rahman and P. Marginean, Chem. Phys. Lett., 2006, 429, 204–208 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
