DOI:
10.1039/B820310E
(Paper)
J. Mater. Chem., 2009,
19, 2135-2140
In situ synthesis of silver nanoparticles on zinc oxide whiskers incorporated in a paper matrix for antibacterial applications
Received
13th November 2008
, Accepted 21st January 2009
First published on 23rd February 2009
Abstract
Silver nanoparticles (AgNPs) were successfully synthesized in situ on a paper matrix composed of ceramic fibers as the main framework and zinc oxide (ZnO) whiskers as a selective support for AgNPs. Paper-like ceramic fiber/ZnO whisker composites were prepared in advance using a high-speed, low-cost papermaking technique, then immersed in an aqueous solution of silver nitrate for 6 h. AgNPs with particle size 5–20 nm were spontaneously formed on the ZnO whiskers through selective ion-exchange between Ag and Zn species, and simultaneous ZnO-mediated photo-reduction under natural light irradiation. As-prepared material (AgNPs@ZnO paper) was subjected to antibacterial assay by the disk diffusion method using Escherichia coli. The AgNPs@ZnO paper exhibited excellent antibacterial activity and durability for repeated use, as compared with paper composites containing either ionic Ag components or commercial crystalline Ag microparticles. The facile and direct synthesis of AgNPs on a paper-like matrix is a unique approach for the immobilization of highly active metal NPs onto easy-to-handle support materials, and the AgNPs@ZnO paper is expected to be a promising bioactive material having both antibacterial function and paper-like utility.
Introduction
Nanosized metal particles have attracted increasing attention due to a wide range of potential applications in optoelectronic,1catalytic2 and biomedical3 fields. However, practical implementation of each original nanostructured form involves considerable difficulties because metal nanoparticles (NPs) very easily aggregate to minimize their surface area. Such inevitable aggregation of metal NPs lessens their inherent functionality, and eventually yields ordinary bulk metals. Consequently, an area of ongoing research has focused on effective immobilization of metal NPs on easy-to-handle supports such as porous membranes4 and nanostructured inorganic sheets.5
Of various metallic elements, silver (Ag) is well known as exhibiting antibacterial properties with low toxicity for humans and other animals.6,7Ag and Ag-compounded materials are effective for both Gram-negative and Gram-positive bacteria, whereas the efficacy of general antibiotics depends on the type of bacteria.6 Many researchers have reported that Ag nanoparticles (AgNPs) have significant antibacterial activity;8–10 but practical methods for AgNP immobilization have been insufficiently advanced. Incorporation of AgNPs into various matrices has recently been investigated to extend their utility in practical, biomedical applications.5,6,11–13 For example, it was reported that AgNPs were synthesized on polyethyleneglycol-polyurethane-titanium dioxide (TiO2) films via TiO2-mediated photocatalysis under UV light irradiation, which facilitated photo-reduction of silver nitrate (AgNO3) to form AgNPs on the polymer-inorganic hybrid matrix.6 Ag-nanocoated cotton fabric was also successfully developed by an ion-exchange method.11 Such types of Ag-organic polymer complexes are generally sensitive to external stimuli (e.g. heat, light and pH), leading to insufficient material stability. Thus Ag-doped antibacterial inorganics such as Ag-hydrogen titanate nanobelt sheets5 and thin silica films of ionic Ag-incorporated tetraethyl orthosilicate12 have been developed using various approaches, e.g.sol-gel processing, ion-exchange and surface modifications. Despite these efforts, however, there is still a need to find more facile ways of using AgNPs, without sacrificing their excellent bioactive functionality, in antibacterial applications. Thus one of the present challenges is to develop a novel method for immobilization of highly-active AgNPs on multipurpose, familiar support materials.
In a previous study we carried out direct in situ synthesis of copper nanoparticles (CuNPs) on an inorganic paper matrix composed of ceramic fibers and zinc oxide (ZnO) whiskers, through selective ion-exchange between Cu and Zn species.14 As-prepared CuNPs@ZnO paper was lightweight, flexible and easy to handle (similar to cardboard), and demonstrated excellent catalytic performance in the methanol reforming process for producing hydrogen for fuel cell applications. This facile technique has great possibilities for potential applications in the ‘on-paper’ synthesis of metal NPs such as Ag, Pt and Au, which have lower ionization tendency than Cu. Paper-like materials on which functional metal NPs are supported would be expected to have wide applications.
In this study, in situ synthesis of AgNPs on a ZnO whisker-containing matrix was investigated both for facile immobilization of AgNPs on a paper-like material, and for development of a bioactive material with antibacterial activity. Paper-like ceramic fiber/ZnO whisker composites (ZnO paper) as a supporting matrix for the AgNPs synthesis were prepared by our established papermaking technique.14 Direct synthesis of AgNPs on ZnO paper was performed in a simple and cost-effective procedure as follows. ZnO paper composites were immersed in an aqueous solution of AgNO3, followed by pick-up, washing and drying. Antibacterial properties, including activity and durability in repeated use, were investigated with respect to (1) Ag-free ZnO paper as control, (2) AgNP-supported ZnO paper (AgNPs@ZnO paper), and two ZnO whisker-free paper composites containing either (3) ionic Ag components (AgNO3-impregnated ZnO-free paper) or (4) commercial crystalline Ag microparticles (Ag powder-containing paper), by disk diffusion assay using the typical Gram-negative bacterium Escherichia coli (E. coli).
Experimental
Materials
Ceramic fibers and ZnO whiskers were purchased from IBIDEN, Ltd. and Matsushita Amtec, Ltd., respectively. Pulp fibers as a matrix component in the paper fabrication process were obtained by refining commercial bleached hardwood kraft pulp (>90% Eucalyptus grandis natural hybrids, Brazil) to a Canadian Standard Freeness of 300 mL with a Technical Association of the Pulp and Paper Industry standard beater. Two types of flocculants were used as retention aids: cationic polydiallyldimethylammonium chloride (PDADMAC; molecular weight: ca. 3 × 105 g mol−1; charge density: 5.5 meq g−1; Aldrich, Ltd.) and anionic polyacrylamide (A-PAM, HH-351; molecular weight: ca. 4 × 106 g mol−1; charge density: 0.64 meq g−1; Kurita, Ltd.). An alumina sol (Snowtex 520, Nissan Chemicals, Ltd.) was used as a binder to improve the physical strength of the paper composites after calcination. AgNO3 and fine Ag powders (particle size: ca. 1 µm) were obtained from Wako Pure Chemical Industries, Ltd. and Soekawa Chemical, Ltd., respectively. Other chemicals were reagent grade and were used without further purification.
Papermaking procedure
The preparation details of paper composites using organic and inorganic fibers, through a dual polyelectrolyte retention system, have been described in previous reports.14–18 In summary, a water suspension of ceramic fibers and ZnO whiskers was mixed with PDADMAC (0.5 wt% of total solids), an alumina sol binder and A-PAM (0.5 wt% of total solids), in that order. The mixture was added to a pulp fiber suspension, and solidified by dewatering using a 200-mesh wire. The wet-state handsheets were pressed at 350 kPa for 3 min, then dried in an oven at 105 °C for 1 h. The resulting paper composite (2 × 104 mm2) consisted of ceramic fibers (5.0 g), ZnO whiskers (0.0 or 3.1 g), alumina sol (0.50 g) and pulp fibers (0.25 g). Ag powder-containing paper composites were prepared by substituting Ag powder (0.6 g) for ZnO whiskers. The paper composites obtained were calcined at 350 °C for 12 h to remove pulp fibers and to improve the physical strength by binder sintering.14–18
Preparation of AgNPs@ZnO paper and AgNO3-impregnated ZnO-free paper
The preparation procedure for AgNPs@ZnO paper was as follows. As-prepared ZnO whisker-containing paper composite was cut into disk-shaped pieces (8 × 102 mm2) and immersed in an aqueous solution of AgNO3 (1.3 × 102 mM, 100 mL) for 6 h. The treated disks were removed from the AgNO3 solution using tweezers, thoroughly washed with deionized water, then dried at 105 °C for 2 h. AgNPs@ZnO whiskers (not a paper shape) were prepared in a similar manner. In the case of AgNO3-impregnated paper, disk-shaped ZnO whisker-free paper composite (8 × 102 mm2) was immersed in aqueous AgNO3 (73 mM, 30 mL), followed by evaporation of water to dryness at 105 °C for 30 min to force precipitation of Ag components on the paper composite.
Characterization
Ag and Zn contents were determined by atomic absorption spectrophotometric analysis using a Shimadzu AA-6600F instrument. The concentrations of Ag+ or Zn2+ extracted from the samples with 35% nitric acid were quantified by flame atomic absorption. Transmission electron microscopy (TEM) was carried out using a JEM1010 instrument (JEOL, Ltd.) at an accelerating voltage of 80 kV. The chemical states of the component elements were analyzed by X-ray photoelectron spectroscopy (XPS, AXIS-HSi spectrometer, Shimadzu/Kratos, Ltd.). X-Ray diffractometry (XRD) was performed using an XD-D1 X-ray diffractometer (Shimadzu, Ltd.) with Ni-filtered CuKα radiation (λ = 1.5418 Å) with scanning angle (2θ) range 30–60° at 30 kV voltage and 40 mA current. The Scherrer formula was used to calculate the Ag crystallite size on the basis of the full width at half maximum of the Ag(111) reflection (2θ ≈ 38°).12 Surface observation of paper samples was conducted using a scanning electron microscope (SEM, JSM-5600, JEOL, Ltd.).
Antibacterial assay
The antibacterial activity of paper composites was determined by disk diffusion assay using E. coli (JM109, a Gram-negative bacterium) as a model pathogenic bacterium.5,6,11 Luria-Bertani (LB) agar (20 mL) was poured into a sterilized Petri dish, and solidified within 10 min. E. coli bacterial suspension (LB medium, 100 µL, 3.5 × 105 colony forming units per mL) was uniformly inoculated on the solidified agar gel. Each disc-shaped piece (10 mm in diameter and 1 mm in thickness) of Ag-free ZnO paper, AgNPs@ZnO paper, AgNO3-impregnated paper or Ag powder-containing paper was placed on the LB agar plate, then incubated at 37 °C for 24 h. The Ag content of the latter three samples was 2.0 mg per disc. The antibacterial activities were compared by the diameter of the zone of inhibition around each paper disk. In addition, the antibacterial durability for repeated use was evaluated by the variations in the diameter of the zone of inhibition during five-cycle antibacterial tests.
Results and discussion
AgNPs synthesis and characterization
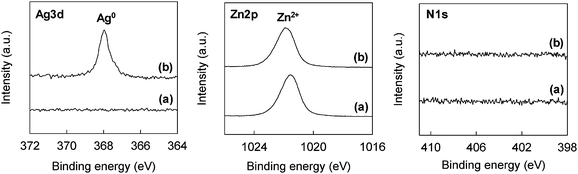
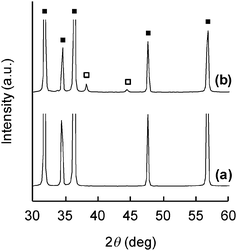
AgNPs synthesis on ZnO whiskers was successfully achieved by a simple technique whereby the ZnO whiskers were suspended in an aqueous solution of AgNO3, followed by continuous stirring for 6 h, filtration, washing with deionized water, and drying. Fig. 1 displays TEM images of the original and the AgNO3-treated ZnO whiskers. The ZnO whiskers possessed a tetrapod-like nanostructure composed of four ZnO needles (Fig. 1a and b). After the soaking treatment with AgNO3 solution, a number of NPs of 5–20 nm diameter were clearly observable on the surface of the ZnO needles (Fig. 1c). The chemical states of the component elements were analyzed by XPS (Fig. 2). The ZnO whiskers treated with AgNO3 solution displayed a well defined Ag3d5/2 peak at ca. 368 eV which was assigned to Ag0.6,19 The Zn2p peak at ca. 1022 eV was derived from the divalent Zn2+ component of ZnO whiskers. No nitrogen-related species were found. XRD analysis was conducted to elucidate the crystal structure of the component elements (Fig. 3). The XRD pattern of ZnO whiskers treated with AgNO3 showed typical peaks of crystalline ZnO and two weak peaks (2θ = 38 and 44°) which correspond to the characteristic (111) and (200) reflections, respectively, of crystalline Ag.11,19 This result indicated successful formation of Ag nanocrystals (with crystallite size ca. 16 nm, calculated by the Scherrer formula). The TEM, XPS and XRD data strongly suggested that the NPs spontaneously formed on the ZnO whiskers were AgNPs. This phenomenon presumably arises from the difference in ionization tendency between Ag and Zn, similar to the finding reported in a previous study.14 In essence, monovalent Ag+ ions in acidic nitrate solution were substituted by a portion of the Zn species in the ZnO whiskers. However, in this case, the atomic absorption analysis determined that the amount of Zn2+ ions eluted from ZnO whiskers was ca. 4.6 mmol mol-ZnO−1, whereas that of Ag adsorbed on the ZnO whiskers was ca. 15.1 mmol mol-ZnO−1. Hence, a simple ion-exchange mechanism does not adequately explain the observations. Furthermore, Ag components present on the ZnO whiskers were neither ionic nor oxidized species, and had metallic Ag0 crystal structure, as shown in Fig. 2b and 3b. ZnO-mediated photocatalysis has recently become the center of attention in relation to optical20 and photoelectronic21 applications. The band gap of ZnO crystals is 3.37 eV,20 similar to that of the anatase-type TiO2 crystals which can reduce Ag ions to AgNPs. Thus it is presumed that rapid ion-exchange between Ag and Zn occurred on ZnO whiskers, and simultaneously photo-reduction of adsorbed Ag species proceeded on the ZnO crystal surface to form AgNPs under natural light irradiation. The molar ratio of Ag to Zn estimated from XPS and atomic absorption analyses was ca. 0.049 and ca. 0.015, respectively, indicating that a lot of AgNPs preferentially existed on the surface of ZnO whiskers.
 |
| | Fig. 1
TEM images of original ZnO whiskers (a, b) and AgNO3-treated ZnO whiskers (c). | |
 |
| | Fig. 2
XPS spectra of original ZnO whiskers (a) and AgNO3-treated ZnO whiskers (b). | |
 |
| | Fig. 3
XRD patterns of original ZnO whiskers (a) and AgNO3-treated ZnO whiskers (b). □: Ag, ■: ZnO. | |
In situ synthesis of AgNPs on ZnO paper
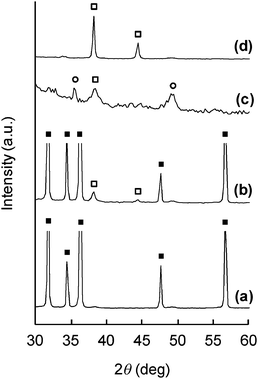
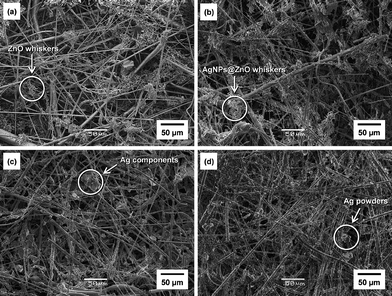
The direct synthesis of AgNPs on a paper composite was performed by using ZnO whiskers as a scaffold for AgNPs. ZnO whiskers and ceramic fibers were first fabricated into a paper composite by a papermaking technique;14 the retention of inorganic materials was approximately 100%. Subsequently, as-prepared ZnO paper was immersed in an aqueous solution of AgNO3. Fig. 4 shows optical images of original Ag-free ZnO paper, ZnO paper treated with AgNO3 solution, AgNO3-impregnated ZnO-free paper and Ag powder-containing ZnO-free paper. These paper composites were cardboard-like materials, and were lightweight, flexible and easy to handle. Fig. 5 and 6 show the XPS spectra and XRD patterns, respectively, of each paper composite. The profiles of AgNO3-treated ZnO paper shown in Fig. 5b and 6b were similar to those of AgNPs@ZnO whiskers in Fig. 2b and 3b, respectively. AgNO3-treated paper without ZnO whiskers was prepared in a similar manner, and then the amount of Ag adsorbed on the paper composite was less than 2.8%, as compared with AgNO3-treated ZnO paper, suggesting that AgNPs were synthesized selectively on the ZnO whiskers incorporated in the paper composite. In the case of AgNO3-impregnated paper, the formation of crystalline AgNO3 precipitates was confirmed by the XPS data (Ag3d5/2: ca. 369 eV, N1s: ca. 407 eV)19 and the XRD assignment (reflections with 2θ values approximately 36 and 49°),22 as shown in Fig. 5c and 6c. Ag powder-containing paper proved to contain Ag0 crystals (Fig. 5d and 6d). Fig. 7 displays SEM images of the surface of each paper composite, revealing a characteristic porous fiber network microstructure composed of ceramic fibers.14–17 The ZnO whiskers are fine fillers, and they were entangled with and scattered on the ceramic fiber networks, although they were not easily identified in the SEM images (Fig. 7b). From the result of XRD analysis (Fig. 6b), Ag crystallite size of AgNPs@ZnO paper was ca. 17 nm, which was similar to that of AgNPs@ZnO whiskers (ca. 16 nm). Consequently, it was presumed that as-synthesized AgNPs with their original nanometer sizes existed in the macro-scale paper composite. AgNO3-impregnated paper and Ag powder-containing paper exhibited relatively large aggregates, possibly originating from AgNO3 precipitates and Ag powders, respectively. Thus direct in situ synthesis of AgNPs on easy-to-handle paper composites was successfully achieved by using ZnO whiskers as a selective support in a facile and cost-effective manner.
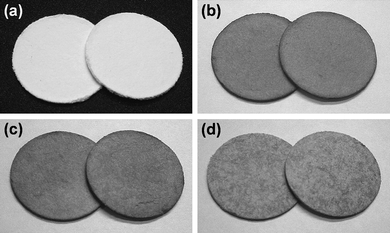 |
| | Fig. 4 Optical images of (a) Ag-free ZnO paper; (b) AgNO3-treated ZnO paper; (c) AgNO3-impregnated ZnO-free paper; (d) Ag powder-containing paper. The size of each paper composite is 8 × 102 mm2. | |
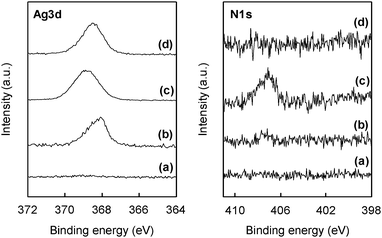 |
| | Fig. 5
XPS spectra of (a) Ag-free ZnO paper; (b) AgNO3-treated ZnO paper; (c) AgNO3-impregnated ZnO-free paper; (d) Ag powder-containing paper. | |
 |
| | Fig. 6
XRD patterns of (a) Ag-free ZnO paper; (b) AgNO3-treated ZnO paper; (c) AgNO3-impregnated ZnO-free paper; (d) Ag powder-containing paper. □: Ag, ○: AgNO3, ■: ZnO. | |
 |
| | Fig. 7
SEM images of the surfaces of (a) Ag-free ZnO paper; (b) AgNO3-treated ZnO paper (AgNPs@ZnO paper); (c) AgNO3-impregnated ZnO-free paper; (d) Ag powder-containing paper. | |
Antibacterial properties of AgNPs@ZnO paper
The antibacterial activity of AgNPs@ZnO paper was compared with that of Ag-free ZnO paper, AgNO3-impregnated paper and Ag powder-containing paper. The Ag content was adjusted to be identical (ca. 2.0 mg) for each sample. Fig. 8 displays the optical images of the zone of inhibition against E. coli for each paper composite. Ag-free ZnO paper had no antibacterial activity (Fig. 8a). By contrast, the three paper composites containing Ag species showed a clear zone of inhibition around each paper disc (Fig. 8b–d), indicating significant antibacterial activity. Of the test samples, AgNPs@ZnO paper demonstrated the largest zone of inhibition, i.e. the highest antibacterial activity, although the same amounts of Ag components were retained in each paper sample. The antibacterial mechanism of Ag species has been a matter of debate for decades. Recently, researchers have reported credible rationales for the antibacterial activity of Ag, as follows. (1) Ag+ ions interact with phosphorus moieties in DNA, resulting in inactivation of DNA replication, and/or (2) they react with sulfur-containing proteins, leading to inhibition of enzyme functions.23,24 Though the mechanism of antibacterial action of AgNPs is still insufficiently understood, many researchers have reported that AgNPs could be toxic because they release Ag+ ions which play an essential role in antibacterial effects.8,25–27 On the other hand, Pal et al. reported that spherical AgNPs had greater antibacterial activity against E. coli than Ag+ ions in the form of AgNO3, and proposed that the nanometer size and the presence of Ag(111) crystal faces synergistically promoted the antibacterial effect of AgNPs.28 Lok et al. also suggested that spherical AgNPs were significantly more efficient against E. coli than Ag+ ions (AgNO3) in mediating their antimicrobial activities.29 Besides, Navarro et al. have recently reported that the toxicity of AgNPs appeared to be much higher than that of AgNO3 as a function of the Ag+ concentration.30 Hence, the elution of Ag+ ions from and the nanomorphology of AgNPs would synergistically contribute to the excellent antibacterial activity of AgNPs@ZnO paper.
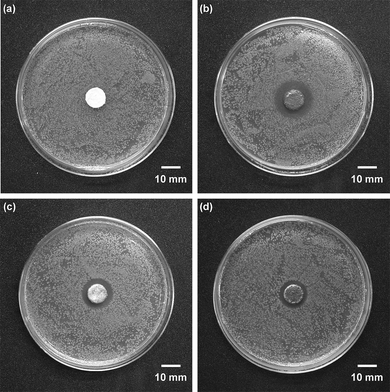 |
| | Fig. 8 Optical images of the zone of inhibition for (a) Ag-free ZnO paper; (b) AgNPs@ZnO paper; (c) AgNO3-impregnated ZnO-free paper; (d) Ag powder-containing paper. Each paper composite is 10 mm in diameter. Ag content: 0.0 mg (a) or ca. 2.0 mg (b, c, d). Incubation condition: 37 °C, 24 h. | |
Fig. 9 compares antibacterial behavior for repeated use of AgNPs@ZnO paper, AgNO3-impregnated paper and Ag powder-containing paper. Initially AgNPs@ZnO paper was clearly superior to the other paper composites. In all of the test cases, the diameter of the zone of inhibition gradually decreased in successive test cycles. The Ag contents also decreased due to gradual release from each paper composite during the antibacterial tests, in accordance with the decrease in antibacterial effects. However, although the final Ag contents after the five-cycle test were very similar, namely 0.2, 0.2 and 0.3 mg for AgNPs@ZnO paper, AgNO3-impregnated paper and Ag powder-containing paper, respectively, AgNPs@ZnO paper maintained considerably higher antibacterial activity than the other samples. For AgNPs@ZnO paper the diameter of the zone of inhibition was ca. 17 mm after the fifth cycle, thus almost the same as those for fresh AgNO3-impregnated paper and Ag powder-containing paper. Such good durability of performance of AgNPs@ZnO paper can possibly be attributed both to the nanometer size and to the exposure of an active crystal face of AgNPs, leading to efficient antibacterial activity. Thus, the AgNPs@ZnO paper with paper-like flexibility and convenience in handling is expected to be a promising bioactive material.
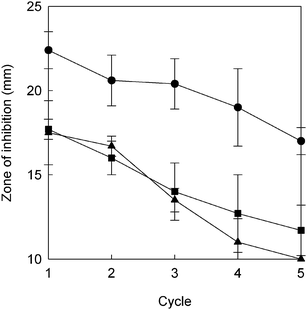 |
| | Fig. 9 Antibacterial activity for repeated use: AgNPs@ZnO paper (circles), AgNO3-impregnated ZnO-free paper (squares), and Ag powder-containing paper (triangles). | |
Conclusions
Bioactive AgNPs were successfully synthesized in situ on ZnO whiskers as a selective support, which were pre-incorporated into a ceramic paper matrix, via ion-exchange between Ag and Zn species and simultaneous ZnO-mediated photo-reduction. The easily fabricated AgNPs@ZnO paper demonstrates excellent antibacterial activity and durability against the bacterium E. coli. The inorganic AgNPs@ZnO paper composites with a paper-like porous structure and practical utility are expected to be promising antibacterial materials, and this novel technique has great potential for wide applications in the ‘on-paper’ synthesis of other metal NPs.
Acknowledgements
This research was supported by a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science (H. K.). The authors sincerely thank Dr H. Ichinose for his technical support with E. coli assay.
References
- G. Zotti, B. Vercelli and A. Berlin, Chem. Mater., 2008, 20, 397–412 CrossRef CAS.
- T. Ishida and M. Haruta, Angew. Chem. Int. Ed., 2007, 46, 7154–7156 CrossRef CAS.
- D. Ma, J. Guan, F. Normandin, S. Dénommée, G. Enright, T. Veres and B. Simard, Chem. Mater., 2006, 18, 1920–1927 CrossRef CAS.
- D. M. Dotzauer, J. Dai, L. Sun and M. L. Bruening, Nano Lett., 2006, 6, 2268–2272 CrossRef CAS.
- Y. Wang, G. Du, H. Liu, D. Liu, S. Qin, N. Wang, C. Hu, X. Tao, J. Jiao, J. Wang and Z. L. Wang, Adv. Funct. Mater., 2008, 18, 1131–1137 CrossRef CAS.
- M. S. A. S. Shah, M. Nag, T. Kalagara, S. Singh and S. V. Manorama, Chem. Mater., 2008, 20, 2455–2460 CrossRef CAS.
- V. Alt, T. Bechert, P. Steinrücke, M. Wagener, P. Seidel, E. Dingeldein, E. Domann and R. Schnettler, Biomaterials, 2004, 25, 4383–4391 CrossRef CAS.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS.
- S. K. Gogoi, P. Gopinath, A. Paul, A. Ramesh, S. S. Ghosh and A. Chattopadhyay, Langmuir, 2006, 22, 9322–9328 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS.
- H. Y. Lee, H. K. Park, Y. M. Lee, K. Kim and S. B. Park, Chem. Commun., 2007, 2959–2961 RSC.
- H.-J. Jeon, S.-C. Yi and S.-G. Oh, Biomaterials, 2003, 24, 4921–4928 CrossRef CAS.
- H. Dong, D. Wang, G. Sun and J. P. Hinestroza, Chem. Mater., 2008, 20, 6627–6632 CrossRef CAS.
- H. Koga, T. Kitaoka and H. Wariishi, Chem. Commun., 2008, 5616–5618 RSC.
- H. Koga, S. Fukahori, T. Kitaoka, A. Tomoda, R. Suzuki and H. Wariishi, Appl. Catal. A: Gen., 2006, 309, 263–269 CrossRef CAS.
- H. Koga, S. Fukahori, T. Kitaoka, M. Nakamura and H. Wariishi, Chem. Eng. J., 2008, 139, 408–415 CrossRef CAS.
- S. Fukahori, H. Koga, T. Kitaoka, A. Tomoda, R. Suzuki and H. Wariishi, Appl. Catal. A: Gen., 2006, 310, 138–144 CrossRef CAS.
- S. Fukahori, H. Koga, T. Kitaoka, M. Nakamura and H. Wariishi, Int. J. Hydrogen Energy, 2008, 33, 1661–1670 CrossRef CAS.
- P. D. Godbole, A. Mitra, R. Pasricha, A. B. Mandale and K. R. Patil, Mater. Lett., 2005, 59, 1958–1961 CrossRef CAS.
- P. Yang, H. Yan, S. Mao, R. Russo, J. Johnson, R. Saykally, N. Morris, J. Pham, R. He and H.-J. Choi, Adv. Funct. Mater., 2002, 12, 323–331 CrossRef CAS.
- X. An, C. Cao and H. Zhu, J. Cryst. Growth, 2007, 308, 340–347 CrossRef CAS.
- T.-X. Liu, X.-Z. Li and F.-B. Li, Environ. Sci. Technol., 2008, 42, 4540–4545 CrossRef CAS.
- A. Gupta, M. Maynes and S. Silver, Appl. Environ. Microbiol., 1998, 64, 5042–5045 CAS.
- Y. Matsumura, K. Yoshikata, S. Kunisaki and T. Tsuchido, Appl. Environ. Microbiol., 2003, 69, 4278–4281 CrossRef CAS.
- D. Lee, R. E. Cohen and M. F. Rubner, Langmuir, 2005, 21, 9651–9659 CrossRef CAS.
- Y. H. Lv, H. Liu, Z. Wang, L. J. Hao, J. Liu, Y. M. Wang, G. J. Du, D. Liu, J. Zhan and J. Y. Wang, Polym. Adv. Technol., 2008, 19, 1455–1460 CAS.
- S. H. Jeong, Y. H. Hwnag and S. C. Yi, J. Mater. Sci., 2005, 40, 5413–5418 CrossRef CAS.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef.
- C.-N. Lok, C.-M. Ho, R. Chen, Q.-Y. He, W.-Y. Yu, H. Sun, P. K.-H. Tam, J.-F. Chiu and C.-M. Che, J. Proteome Res., 2006, 5, 916–924 CrossRef CAS.
- E. Navarro, F. Piccapietra, B. Wagner, F. Marconi, R. Kaegi, K. Odzak, L. Sigg and R. Behra, Environ. Sci. Technol., 2008, 42, 8959–8964 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here. 








