Surface modification of a solid-state cellulose matrix with lactose by a surfactant-enveloped enzyme in a nonaqueous medium
Shizuka
Egusa
a,
Shingo
Yokota
a,
Kyoko
Tanaka
a,
Kei
Esaki
a,
Yuri
Okutani
a,
Yukiko
Ogawa
a,
Takuya
Kitaoka
*a,
Masahiro
Goto
b and
Hiroyuki
Wariishi
a
aDepartment of Forest and Forest Products Sciences, Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka, 812-8581, Japan. E-mail: tkitaoka@agr.kyushu-u.ac.jp; Fax: + 81 92 642 2993; Tel: + 81 92 642 2993
bDepartment of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan
First published on 10th February 2009
Abstract
Glyco-modification of a solid cellulose surface with lactosevia an enzymatic reaction was successfully achieved using a surfactant-enveloped enzyme (SEE) in a lithium chloride/dimethylacetamide solvent system. The unique biocatalyst, SEE, which is active in organic media, was prepared by protecting the surface of cellulase with the specific nonionic surfactant dioleyl-N-D-glucona-L-glutamate. Lactose, a second cellulase substrate, was introduced onto cellulose surfaces viaSEE-mediated enzymatic reaction. Fluorescent labeling and imaging revealed the presence of galactose residues from lactose on the modified cellulose surface. X-Ray diffractometry showed that the crystal structure of cellulose remained unchanged even after glyco-modification. Cell adhesion assays were carried out using rat liver cells on which galactose-specific receptors are present, and the initial cell attachment (within 12 h) on the lactose-modified cellulose filter was greater than that on an original (unmodified) cellulose matrix. The SEE-mediated biocatalysis enabled efficient glyco-modification of the solid cellulose surface in a one-step reaction, without complicated pre-treatment of the cellulose matrix and donor sugar. Consequently, this simple and effective approach to surface modification of a solid cellulose matrix with bio-functional sugars would be expected to have wide potential applications in glycomaterials engineering.
Introduction
An area of research receiving increasing attention in recent times has been surface modification of bio-based materials, such as cellulose, to enhance their functional properties.1–3 Cellulose, which forms the main constituent of the cell wall in plants, is the most abundant biomacromolecule, and a variety of cellulosic materials have been widely applied in the housing, clothing, paper, polymer, food and medical industries.4–6 The surface morphology and functionality of cellulosic materials have also attracted much attention from physicochemical and biological engineering perspectives.7,8 Molecular derivatization of cellulosic polymers is possible, but more efficient approaches are required for the surface functionalization of cellulosic materials in their original solid-state forms. Direct modification of the solid cellulose surface is expected to lead to wider applications of cellulosic materials. However, it is difficult to effectively modify the surface of cellulose in the solid due to its strong intramolecular and intermolecular hydrogen bonding, resulting in the close molecular packing, high crystallinity, poor solubility and low reactivity towards chemicals that are characteristics of cellulose.5Many researchers have investigated chemical modification of solid cellulose surfaces by methods including direct acetylation of wood,9polymer grafting onto/from cellulosic substrates,10–15 and a variety of coating methods.16,17 Saito et al. reported topochemical oxidation of the cellulose crystal surface under moderate conditions, via an aqueous catalytic reaction.18,19 From different viewpoints, cellulose fiber materials, i.e. paper, have been surface-modified by many types of additives in various papermaking processes. Rosin and synthetic sizing agents to achieve water repellency,20 direct dyes for coloring,21 and various wet-end additives22–24 have been used. Surface activationvia irreversible adsorption of carboxymethylcellulose to cellulose fibers also has been reported.25 These surface treatments have imparted new functionality to solid cellulosic materials. Teeri et al. reported novel chemo-enzymatic modification of a cellulose fiber surface with xyloglucan using lipase and endotransglycosylase in combination for activation of solid cellulose surfaces.26
Of various functional substances for surface modification of solid materials, carbohydrates have attracted much interest from biological and medical standpoints because of their essential roles in all living things.27 Hence glyco-modification of solid materials has received increasing attention from academic and industrial circles, especially in the biomaterial engineering field. Akaike et al. reported the synthesis of a lactose (Lac)-pendant polymer and its application to surface modification of culture plates for tissue engineering, giving control of cell adhesion, cell proliferation and morphology.28 Cellular functions are closely related to the interaction between sugar residues on a culture matrix and diverse receptors on the cell surface.29 Consequently, glyco-modification of solid cellulose surfaces is attractive for the potential applications of cellulosic biomaterials.
In our previous study, nonaqueous enzymatic synthesis of cellulose from cellobiose was successfully achieved using a surfactant-enveloped enzyme (SEE) in a lithium chloride (LiCl)/dimethylacetamide (DMAc) system.30 The LiCl/DMAc system is a well-known nonaqueous solvent for dissolving crystalline solid cellulose, but its aprotic nature causes inevitable, strong inactivation of enzymes. The novel biocatalyst (SEE) was prepared by protecting the surface of cellulase with a specific nonionic surfactant, dioleyl-N-D-glucona-L-glutamate (2C18Δ9GE),31 thereby enabling it to be used in such an organic solvent as an enzymatic reaction medium. In the synthesis of cellulose, a long-chain cellulose with a degree of polymerization (DP) greater than 100 was first prepared via an efficient dehydration condensation reaction, whereas both conventional organic32 and enzymatic33 synthesis processes provided shorter-chain cellulose with DPca. 20. Furthermore, cellobiose without activation of the anomeric carbon atom at the reducing end was directly applicable as a starting sugar. Thus monomer design, which is indispensable for general glycosynthesis,33,34 was not necessary in our novel nonaqueous enzymatic system.
In the present study, we investigated direct surface modification of a solid cellulose matrix with Lac, using an SEE biocatalyst in the nonaqueous LiCl/DMAc solvent system. It was reported that in an aqueous transglycosylation reaction, the cellulase used in the present study recognized Lac as another substrate,35 thus Lac was used in its original form without any chemical modification. The cellulose powder and filter were used without pre-treatment. Fluorescent labeling and rough determination of galactose (Gal) residues on the Lac-modified cellulose surfaces, determination of the crystal structure of modified cellulose, and cell assay tests using rat hepatocytes were carried out. The purpose of those experiments was to pursue the potential applications of this method, and the possibility of using the modified surface as a novel bio-interface.
Experimental
Materials
Commercial cellulose samples, cotton powder (CF11, fibrous shape, Whatman International Co. Ltd.) and cotton fiber filter paper were used as matrices for glyco-modification. Crude cellulase consisting of 1,4-β-D-glucan 4-glucanohydrolase (EC 3.2.1.4) from Trichoderma viride was purchased from Wako Pure Chemical Industries Co. Ltd. Lac (Aldrich Co. Ltd.) as a donor sugar was dried at 105 °C overnight before use. Fluorescent isothiocyanate-labeled Ricinus communis agglutinin (RCA120-FITC, Seikagaku Co. Ltd.) was used for selectively labeling the Gal residues at the non-reducing ends of Lac molecules introduced onto the cellulose matrix surface. Complete Eagle's minimum essential medium (EMEM), penicillin-streptomycin and trypsin were purchased from Invitrogen Co. Ltd. The water used in this study was purified with a Milli-Q system (Millipore, Co. Ltd.). Other chemicals were of reagent grade and used without further purification.Synthesis of dioleyl-N-D-glucona-L-glutamate
Nonionic surfactant 2C18Δ9GE was synthesized according to Goto's method as summarized below.31 A mixture of L-glutamic acid (5.0 g), oleyl alcohol (20 g) and p-toluenesulfonic acid (8.0 g) was added to toluene solution (100 mL), then refluxed for 4 h, followed by removal of toluene under reduced pressure. The reaction mixture was dissolved in chloroform and washed with 5% sodium carbonate aqueous solution and Milli-Q water. After evaporation of chloroform, the intermediate, dioleylglutamate was obtained through a precipitation and purification process. Glucone-δ-lactone (2.0 g) was mixed with the purified dioleylglutamate (8.0 g) in ethanol (140 mL), and the mixture refluxed for 5 h. After solvent removal, the final product, 2C18Δ9GE, was purified by recrystallization at least three times from acetone.SEE preparation
Cellulase (20 mg) in 50 mM sodium phosphate solution (10 mL), and 2C18Δ9GE (25 mg) in toluene (30 mL) were mixed, and vigorously homogenized at 15000 rpm for 3 min. The cloudy water-in-oil (W/O) emulsion was immediately frozen with liquid nitrogen, then freeze-dried for at least 24 h. SEE was obtained as a white powder.30Lac modification
The cellulose powder and filter were thoroughly washed with Milli-Q water before use. The cellulose sample (0.2 g), Lac (1.0 g) and SEE (45 mg, containing 20 mg of cellulase) were poured into LiCl/DMAc (4.0 g per 50 mL), and the mixture was incubated with gentle stirring at 37 °C for 3, 6, 12, 24 or 48 h. The sample obtained was carefully washed with DMAc and water to remove SEE and physically adsorbed sugars. The Lac-modified cellulose matrix was dried at room temperature under reduced pressure. Control samples were prepared in a similar manner in the absence of either SEE or Lac.RCA120-FITC labeling
RCA120-FITC solution for labeling the Gal residues on the matrix surfaces was prepared by diluting the stock solution to 0.1 µg mL−1 with phosphate buffered saline (PBS, pH 7.2, Nissui Pharmaceutical Co. Ltd.) solution. FITC-conjugated lectin labeling was carried out according to the conventional method36 as summarized below. The samples were gently immersed in RCA120-FITC solution at 37 °C for 30 min, after non-specific binding sites were blocked with 1% bovine serum albumin at 37 °C for 30 min. Labeled samples were washed with PBS solution three times to remove the physical adsorbates, and imaged using a fluorescence microscope (DFC480, Leica Co. Ltd.). The sample preparation, fluorescent labeling and imaging procedures were repeated three times as independent experiments.Gal determination
The number of Gal residues on the Lac-modified cellulose filter was approximately estimated from the variation in the fluorescent intensity of the supernatant of RCA120-FITC solution before and after immersion of cellulose samples, using a fluorescence spectrophotometer (F-3010, Hitachi Co. Ltd) at 520 nm emission wavelength (excitation wavelength: 490 nm). The affinity of RCA120 (Gal-recognizing lectin) toward β-D-glucose, which is a component sugar of cellulose, is negligible (less than 0.3% as compared to β-D-Gal).37,38 The sample preparation and fluorescent analysis were repeated three times as independent experiments.X-Ray diffraction analysis
The crystal structure of the cellulose matrix was determined by XRD analysis using an XD-D1 X-ray diffractometer (Shimadzu Co. Ltd.). X-Ray diffraction (XRD) patterns were acquired using Ni-filtered CuKα radiation (λ = 1.5418 Å) at 30 kV and 40 mA. The scanning range was 5° ≤ 2θ ≤ 40°.Cell culture assay
Rat hepatocytes (IAR-20, JCRB Cellbank) were incubated in tissue culture flasks containing complete EMEM with 10% fetal bovine serum and 5% antigen (penicillin and streptomycin), under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Lac-modified cellulose filters were cut to size and set at the bottom of 24-well microtiter plates. Hepatocytes (1.25 × 105cells mL−1) in 1 mL medium were seeded to each well, and incubated at 37 °C for a designated time from 3 to 12 h in a 5% CO2 incubator. After the removal of suspended (unattached) cells by thoroughly rinsing with PBS solution, adherent cells were detached from the matrix surface by digestion with 0.05% trypsin in PBS solution at 37 °C for 15 min, then counted with a cell counter. The cell assay test was repeated three times.Results and discussion
Lac modification of cellulose surface
The surface modification of cellulose samples with Lac was first carried out in a nonaqueous medium via an SEE-mediated biocatalysis. Fig. 1 shows optical and fluorescent images of solid cellulose matrices before and after modification with Lac. Optical images show the fibrous shape of the cellulose powders and the cellulose fiber network of the filter paper, each bearing a close resemblance regardless of the Lac modification. However, RCA120-FITC labeling clearly showed stronger fluorescence of cellulose samples treated by 6 h incubation with Lac and SEE. Solid cellulose is chemically stable due to its strong intramolecular and intermolecular interactions, and the inherent stability has both advantages and disadvantages for material applications. Owing to the high chemical resistance of cellulose, surface modification of solid cellulose presents great difficulties, and there are, accordingly, few reports on direct sugar modification of the cellulose surface. The RCA120-FITC used in this study has high Gal-specific binding ability, thus these results suggested successful introduction of Lac onto the solid cellulose matrix. | ||
| Fig. 1 Optical (a–d) and fluorescence (e–h) images of fibrous cellulose powder and cellulose filter before and after SEE-mediated enzymatic Lac modification: (a, e) original powder, (b, f) Lac-modified powder, (c, g) original filter, and (d, h) Lac-modified filter. Scale bars = 100 µm. | ||
In this novel glyco-modification method, the LiCl/DMAc solvent for dissolving crystalline cellulose was used as a reaction medium. However, the cellulose samples remained unchanged without any apparent dissolution. Cellulose is known to be difficult to dissolve even in the LiCl/DMAc system, and pre-treatments such as a sequential solvent exchange, stepwise immersion in water (twice), acetone (twice), and DMAc (twice) in that order,39 are indispensable for complete dissolution. In this case, we deliberately omitted such solvent exchange procedures and other conditionings for complete dissolution to purposely maintain the solid form of the cellulose matrix. When subjected to partial solvent exchange, sequential immersion of the cellulose in water (twice) and acetone (twice) caused the cellulose to swell remarkably (after 6 h incubation), and it became a gel after 24 h (data not shown). LiCl addition was found to be an important factor for effective surface glyco-modification of solid cellulose, and this was further confirmed by the limited fluorescence observed without LiCl (data not shown). The LiCl/DMAc forms a complex with cellulose when it dissolves cellulose. Hence, such a complex would activate the surface of the solid-state cellulose matrix without molecule-level dissolution, and make a contribution to promotion of the SEE-mediated reaction, as illustrated in Scheme 1. Our previous achievement of enzymatic cellulose synthesis in a nonaqueous LiCl/DMAc system30 led to a speculation that the SEE-mediated biocatalysis could proceed via specific binding of SEE and substrate sugar that interacted with the LiCl/DMAc complex. At this stage, a linkage pattern of Lac and cellulose substrate is not clear; but it was presumed that some form of dehydration reaction occurred between Lac and cellulose molecules present on the surfaces of the cellulose matrix.
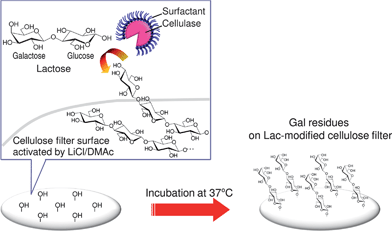 | ||
| Scheme 1 Conceptual schematic illustration of the Lac modification of the solid cellulose surface viaSEE-mediated biocatalysis in a LiCl/DMAc solvent system. | ||
Effect of water phase pH in SEE preparation on Lac modification
Enzymatic dehydration condensation using glycohydrolases has been reported by many researchers,33–35,40 but has an inevitable disadvantage for reaction media. General enzymes are active in water system, however the dehydration reaction is strictly inhibited in the presence of water. In our study, SEE has an enzymatic activity even in nonaqueous, aprotic LiCl/DMAc. Although the SEE-mediated reaction proceeds under nonaqueous conditions, the pH of the water phase of W/O emulsions in the SEE preparation is of great significance for effective dehydration condensation reactions,30,40 which seems paradoxical. Fig. 2 compares the fluorescence intensities of RCA120-FITC labeled cellulose powders that were incubated for 3 or 6 h using SEE prepared at different pHs. Stronger fluorescence intensity was observed for the Lac-modified cellulose samples compared with the original cellulose powder, thereby indicating successful Lac modification of the solid cellulose. Non-specific binding of RCA120-FITC on the matrix surface was also found to some extent, although the physical adsorption of Lac molecules was negligible. A pH dependence of the efficiency of Lac modification was apparent. A higher pH in the water phase of the W/O emulsion in the SEE preparation process brought about a stronger fluorescence intensity, probably resulting in larger amounts of Lac molecules introduced onto the cellulose matrix surface.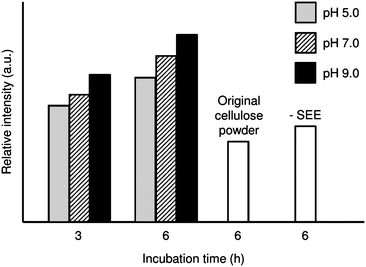 | ||
| Fig. 2 Fluorescence intensity of RCA120-FITC labeled cellulose powders with or without Lac modification. The pH of the water phase of the W/O emulsion in the SEE preparation was set at 5.0, 7.0 or 9.0. | ||
In relation to this glyco-modification method being carried out in a nonaqueous system, the pH of the water phase of the W/O emulsion during SEE preparation significantly influenced the catalytic efficiency. Zaks and Klibanov reported that enzymes in organic media possessed a memorized ionogenic state corresponding to the pH in the final aqueous solution.41 Such pH-dependent enzymatic activity was possibly involved in the memory of higher-order protein structures and the state of the active enzyme center in the water phase. In the present study, phosphate solution proved effective even in the pH region with no buffering effect, although the optimal pH of cellulase used in the hydrolysis reaction is 4.5–5.0 in an aqueous system. For the reverse dehydration reaction, similar phenomena have been reported during the synthesis of cellulose in a nonaqueous medium,30 and glycosylation with chitinase in an aqueous medium.34 The detailed mechanism is still unknown, but at this stage the water phase pH of the W/O emulsion in the SEE preparation was set at 9 for the work described in the following sections.
Lac modification behavior of cellulose filter
The Lac modification conditions examined in the previous section were applied to the surface modification of the cellulose filter, and the effect of incubation time on the introduction of Lac onto the filter matrix was investigated by RCA120-FITC labeling. As shown in Fig. 3, the fluorescence intensity, which indicated the presence of Gal residues, increased with increasing incubation time up to 24 h. The Lac-modified filter displayed fluorescence intensity several times stronger than that of the control samples incubated for 24 h in the absence of either SEE or Lac. The cellulose filter used in this study was composed of α-cellulose, which is highly resistant to organic chemicals, thus making it difficult, in general, to modify the solid surface. However, our novel approach enabled the significant introduction of Lac to the cellulose filter surface. | ||
| Fig. 3 Lac modification behavior of cellulose filters viaSEE-mediated biocatalysis. These samples were post-treated by RCA120-FITC labeling. The incubation times for Lac modification were 3, 6, 12, 24 or 48 h. The water phase pH of the W/O emulsion in the SEE preparation was set at 9.0. | ||
Accurate determination of the surface density of Gal residues on the matrix surface is required to elucidate the biofunction of Lac-modified cellulose, but surface density determination was difficult due to the relatively small quantity of Lac molecules that was introduced. The chromatographic quantification of unreacted Lac left in the medium, or Gal residue enzymatically separated from the matrix, was impossible owing to the extremely large or small amounts of analytes, respectively. Hence, we roughly estimated the abundance of Gal residues on the Lac-modified cellulose through the amounts of RCA120-FITC lectins adsorbed on the surface, by assuming that binding of RCA120-FITC to Gal residues was stoichiometric. As a result of the cellulose filter (after 24 h incubation) showing the strongest fluorescent intensity (Fig. 3), approximately 2.6–5.3 × 1013Gal residues (ca. 43–88 pmol) per g of Lac-modified cellulose was estimated from the spectrometric quantification of RCA120-FITC not attached to the modified cellulose surface. The RCA120 used has two binding sites in one tetra-complex protein, and thus can bind one or two Gal residues per RCA120-FITC molecule. In the case of two control samples, which one was prepared without SEE and another was without Lac, there was almost no change found in the supernatant of RCA120-FITC solution in which the cellulose samples were soaked. Physical adsorption of the adsorbent and other factors were negligible. The amounts of introduced Lac molecules were insufficient at this stage, and thus there is much room for further improvement.
Excess incubation of more than 24 h, however, brought about a decrease in the fluorescence intensity, indicating a decrease in the number of Gal residues on the cellulose surface. The recovery rate of each sample from the reaction medium was more than 97% by weight, and thus as a bulk material there was a negligibly small loss. Consequently, some variation of the matrix surface was assumed, and this phenomenon could have two possible causes. 1) Partial hydrolysis of cellulose by the inherent enzymatic reaction via cellulase, owing to the water that was inevitably generated as the dehydration reaction proceeded, and 2) during long-time incubation, the solid cellulose surface, especially the Lac-modified portion of the cellulose surface, was gradually dissolved in the reaction medium. The introduction of Lac might weaken the molecular association between the cellulose chains, resulting in an increase in solubility. Water removal from the reaction medium must be investigated for more effective glyco-modification of the solid cellulose matrix.
In this study, Lac as a donor sugar was directly used for the glyco-modification reaction without the need for time-consuming monomer derivatization. In general, activation of the anomeric carbon of sugar substrates is required for enzymatic glyco-synthesis,31,32 but in our novel method, no tedious steps were necessary. This strongly indicates that such a nonaqueous enzymatic glyco-modification has potential as a new approach to use enzymes and functional carbohydrates. Moreover, the Lac-modified cellulose was easy to separate from the reaction medium with almost 100% recovery because the solid cellulose matrix is insoluble in the reaction medium. Thus we anticipate that this simple, one-step method will have great advantages for effective surface glyco-modification of a solid cellulose matrix.
Crystal structure of Lac-modified cellulose filter
Fig. 4 compares the XRD patterns of the original cellulose and the Lac-modified filter. Both displayed identical XRD patterns of cellulose I with diffraction angles at 2θ = 14.8, 16.3, 22.6 and 34.3°, which are assigned to the (1−10), (110), (200) and (004) diffraction planes, respectively. The degree of crystallinity and the size of the crystallites calculated from the full width at half maximum at 2θ = 22.6° remained constant at ca. 73% and 6.3 nm, respectively, before and after modification for 24 h. Even after 48 h, the degree of crystallinity and crystallite size were virtually unchanged (72% and 6.2 nm), showing that the crystal structure was not changed by Lac modification. As shown in Fig. 3, 48 h incubation brought about smaller introduced amounts of Gal residues on the matrix surface, and thus the superficial region of the modified matrix might be partially dissolved in the LiCl/DMAc solvent. However, the remaining Lac-modified cellulose preserved the inherent crystal structure during immersion for a long time in a solvent for cellulose. In this system, the cellulose samples (filter paper and cellulose powder with crystallinities 73% and >90%, respectively) were both insoluble in the LiCl/DMAc solvent system. The non-crystalline domain might be swollen to some extent as a result of DMAc solvation, but addition of LiCl was indispensable for achieving efficient Lac modification, although such treatment did not affect the crystal structure of the cellulose filter. These results suggest that the Lac modification occurred not only in the non-crystalline domain, but also on the crystal surface of the cellulose matrix. LiCl/DMAc can dissolve a variety of polysaccharides, and it would perform, therefore, as a versatile surface activator for solid polysaccharide materials in SEE-mediated biocatalytic systems. | ||
| Fig. 4 XRD profiles of cellulose filters before and after Lac modification. | ||
Direct enzymatic modification of crystalline cellulose is particularly difficult due to the lower reactivity at the solid/liquid interface, and the insolubility of cellulose in aqueous media. It has been reported that the 2,2,6,6-tetramethylpiperidine-1-oxy radical enabled topochemical oxidation of primary alcohol groups on the crystal surface of native cellulose samples, resulting in formation of a cellulose single nanofiber with an inherent crystal structure.19 Teeri et al. have reported a unique chemo-enzymatic modification technique using xyloglucan endotransglycosylase, whereby the xyloglucan pre-adsorbed on the solid cellulose surface was modified.26 This two-step approach is effective for surface modification of solid cellulose, but an oligosaccharide mediator with high affinity for cellulose is indispensable. By contrast, in the present work the LiCl/DMAc system could directly enhance the accessibility of enzymes to the surface of the solid cellulose matrix (Scheme 1). The organic media-active biocatalyst SEE allowed us to use a wide range of reaction media for enzymatic synthesis and modification. This novel approach is expected to provide potential applications for surface glyco-modification of solid-state polysaccharides matrices.
Cell adhesion assay on Lac-modified cellulose filter
The Lac-modified cellulose filter prepared in this study comprises a number of Gal residues (a particular bioactive sugar) on the surface. Thus, a cell adhesion assay was carried out to investigate novel functions of this modified surface as a bio-interface. Fig. 5 shows the cell adhesion behavior of rat hepatocytes IAR-20 on cellulose filters. The Lac-modified cellulose filter, which was incubated for 24 h for surface glyco-modification, demonstrated significantly higher cell adhesion compared with the original, and SEE-free treated cellulose filters. After 12 h culture, cell attachment on the Lac-modified cellulose filter was more than twice that on the control cellulose filters. There was little difference in cell attachment between the original cellulose filter and the cellulose filter treated without SEE, showing that physical adsorption of Lac molecules had negligible influence on the cellulose filter for cell attachment. It is well known that initial hepatocyte attachment is dominated by the presence of Gal residues that directly interact with the asialoglycoprotein receptors on the rat liver cell surface.28 Consequently, such an increasing trend in cell attachment on the Lac-modified cellulose filter must be attributed to direct recognition of Gal residues on the cellulose filter by rat liver cells. Significant dissolution of the Lac-modified cellulose matrix was not observed during the modification reaction since the cellulose matrix preserved its inherent crystal structure (cellulose I) and crystallinity before and after Lac modification (Fig. 4), although slight dissolution/rearrangement of cellulose chains might occur for long-time incubation (Fig. 3). Hence the Gal residues were presumably present on the outer surface of the fiber-network matrix, and could perform effectively as a sugar ligand for rat hepatocyte receptors although slight amounts of Gal residues were introduced. The Lac-modified cellulose filter is a flexible, easy-to-handle material in paper form, and is stable during cell culture studies in an aqueous medium. The SEE-mediated surface glyco-modification process is expected to produce paper and other cellulosic materials with novel bio-functionality that is attractive for further practical applications. | ||
| Fig. 5 Cell adhesion behavior on (●) the Lac-modified filter, (○) cellulose filter treated without SEE, and (▼) original cellulose filter. | ||
Conclusions
Surface modification of a solid-state cellulose matrix with Lac was successfully achieved via a nonaqueous enzymatic reaction using SEE as a biocatalyst that is active in the LiCl/DMAc solvent system. Lac components were directly introduced into the non-crystalline domain and on the crystal surface of the cellulose matrix, retaining its original external appearance and paper-like properties. Favorable cell adhesion was observed on the Lac-modified cellulose filter when rat liver cells were used. The major advantages of this novel method are i) effective surface glyco-modification is possible in a one-step reaction, ii) chemical design of both the sugar donors and the matrix is unnecessary, and iii) the cellulose matrix can be modified without changing its appearance or handling. For those reasons, this nonaqueous SEE-mediated biocatalysis method is expected to be applicable to the development of new types of polysaccharide-based biomaterials.Acknowledgements
This research was supported by Research Fellowships for Young Scientists from the Japan Society for the Promotion of Science (S.E. and S.Y.), and by Grants-in-Aid for Young Scientists (A: 17688008 to T.K.; Start-up: 20880021 to Y. Ogawa) and Scientific Research (B: 20380104 to T.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
- H. H. Yuan, Y. Nishiyama, M. Wada and S. Kuga, Biomacromolecules, 2006, 7, 696–700 CrossRef CAS.
- T. T. Teeri, H. Brumer, G. Daniel and P. Gatenholm, Trends Biotechnol., 2007, 25, 299–306 CrossRef CAS.
- S. Park, R. A. Venditti, D. G. Abrecht, H. Jameel, J. J. Pawlak and J. M. Lee, J. Appl. Polym. Sci., 2007, 103, 3833–3839 CrossRef CAS.
- J. Schurz, Prog. Polym. Sci., 1999, 24, 481–483 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem. Int. Ed., 2005, 44, 3358–3393 CrossRef CAS.
- M. J. John and S. Thomas, Carbohydr. Polym., 2008, 71, 343–364 CrossRef CAS.
- W. Czaja, A. Krystynowicz, S. Bielecki and R. M. Brown, Biomaterials, 2006, 27, 145–151 CrossRef CAS.
- S. Yokota, T. Kitaoka and H. Wariishi, Appl. Surf. Sci., 2007, 253, 4208–4214 CrossRef CAS; S. Yokota, K. Matsuyama, T. Kitaoka and H. Wariishi, Appl. Surf. Sci., 2007, 253, 5149–5154 CrossRef CAS; S. Yokota, T. Kitaoka, J. Sugiyama and H. Wariishi, Adv. Mater., 2007, 19, 3368–3370 CrossRef CAS; S. Yokota, T. Kitaoka and H. Wariishi, Carbohydr. Polym., 2008, 74, 666–672 CrossRef CAS; S. Yokota, T. Kitaoka, M. Opietnik, T. Rosenau and H. Wariishi, Angew. Chem. Int. Ed., 2008, 47, 9866–9869 CrossRef CAS.
- M. Jebrane and G. Sebe, Carbohydr. Polym., 2008, 72, 657–663 CrossRef CAS.
- T. Kondo, H. Kubota and R. Katakai, J. Appl. Polym. Sci., 1999, 71, 251–258 CrossRef CAS.
- A. Carlmark and E. Malmstrom, J. Am. Chem. Soc., 2002, 124, 900–901 CrossRef CAS; A. Carlmark and E. E. Malmstrom, Biomacromolecules, 2003, 4, 1740–1745 CrossRef CAS.
- E. Sipahi-Saglam, M. Gelbrich and E. Gruber, Cellulose, 2003, 10, 237–250 CrossRef CAS.
- M. K. Zahran, M. Morsy and R. I. Mahmoud, J. Appl. Polym. Sci., 2004, 91, 1261–1274 CrossRef CAS.
- S. Perrier, P. Takolpuckdee, J. Westwood and D. M. Lewis, Macromolecules, 2004, 37, 2709–2717 CrossRef CAS.
- H. Lonnberg, Q. Zhou, H. Brumer, T. T. Teeri, E. Malmstrom and A. Hult, Biomacromolecules, 2006, 7, 2178–2185 CrossRef.
- I. H. Tan, M. L. P. Silvab and N. R. Demarquette, J. Mater. Chem., 2001, 11, 1019–1025 RSC.
- C. Y. Yin, J. B. Li, Q. Xu, Q. Peng, Y. B. Liu and X. Y. Shen, Carbohydr. Polym., 2007, 67, 147–154 CrossRef CAS.
- T. Saito and A. Isogai, Colloids Surf. A, 2006, 289, 219–225 CrossRef CAS.
- T. Saito, Y. Nishiyama, J. L. Putaux, M. Vignon and A. Isogai, Biomacromolecules, 2006, 7, 1687–1691 CrossRef CAS.
- F. Wang, T. Kitaoka and H. Tanaka, Colloids Surf. A, 2003, 221, 19–28 CrossRef CAS; H. Yamamoto, T. Kitaoka and H. Tanaka, J. Pulp Pap. Sci., 2004, 30, 136–140 Search PubMed.
- J. Shore, Ind. J. Fib. Text. Res., 1996, 21, 1–29 Search PubMed.
- J. Yoshizawa, A. Isogai and F. Onabe, J. Pulp Pap. Sci., 1998, 24, 213–218 Search PubMed.
- F. Wang, T. Kitaoka and H. Tanaka, Tappi J., 2003, 2, 21–26 Search PubMed.
- L. Gardlund, M. Norgren, L. Wagberg and A. Marklund, Nord. Pulp Pap. Res. J., 2007, 22, 210–216 CrossRef.
- M. Blomstedt, E. Kontturi and T. Vuorinen, Nord. Pulp Pap. Res. J., 2007, 22, 336–342 CrossRef CAS.
- H. Brumer, Q. Zhou, M. J. Baumann, K. Carlsson and T. T. Teeri, J. Am. Chem. Soc., 2004, 126, 5715–5721 CrossRef CAS; M. T. Gustavsson, P. V. Persson, T. Iversen, M. Martinelle, K. Hult, T. T. Teeri and H. Brumer, Biomacromolecules, 2005, 6, 196–203 CrossRef CAS.
- C. J. Thibodeaux, C. E. Melancon and H. W. Liu, Nature, 2007, 446, 1008–1016 CrossRef CAS; A. Varki, Nature, 2007, 446, 1023–1029 CrossRef CAS.
- A. Kobayashi, M. Goto, T. Sekine, A. Masumoto, N. Yamamoto, K. Kobayashi and T. Akaike, Artif. Organs, 1992, 16, 564–567 CAS; S. H. Kim and T. Akaike, Tissue Eng., 2007, 13, 601–609 CrossRef CAS.
- I. Geffen and M. Spiess, Int. Rev. Cytol., 1992, 137B, 181–219 Search PubMed.
- S. Egusa, T. Kitaoka, M. Goto and H. Wariishi, Angew. Chem. Int. Ed., 2007, 46, 2063–2065 CrossRef CAS.
- M. Goto, M. Matsumoto, K. Kondo and F. Nakashio, J. Chem. Eng. Jpn., 1987, 20, 157–164 CrossRef CAS.
- F. Nakatsubo, H. Kamitakahara and M. Hori, J. Am. Chem. Soc., 1996, 118, 1677–1681 CrossRef.
- S. Kobayashi, K. Kashiwa, T. Kawasaki and S. Shoda, J. Am. Chem. Soc., 1991, 113, 3079–3084 CrossRef CAS.
- S. Kobayashi, T. Kiyosada and S. Shoda, J. Am. Chem. Soc., 1996, 118, 13113–13114 CrossRef CAS; S. Shoda, T. Kiyosada, H. Mori and S. Kobayashi, Heterocycles, 2000, 52, 599–602 CAS.
- K. Totani, N. Yasutake, H. Ohi, T. Murata and T. Usui, Arch. Biochem. Biophys., 2001, 385, 70–77 CrossRef CAS; N. Yasutake, K. Totani, Y. Harada, S. Haraguchi, T. Murata and T. Usui, Biochim. Biophys. Acta, 2003, 1620, 252–258 CrossRef CAS.
- M. Inbar, H. Benbassa and L. Sachs, Int. J. Cancer, 1973, 12, 93–99 CrossRef CAS.
- G. L. Nicolson and J. Blaustein, Biochim. Biophys. Acta, 1972, 266, 543–547 CAS.
- S. Olsnes, E. Saltvedt and A. Pihl, J. Biol. Chem., 1974, 249, 803–810 CAS.
- T. R. Dawsey and C. L. McCormick, J. Macromol. Sci. Rev. Macromol. Chem. Phys., 1990, C30, 405–440 Search PubMed; A. Mayumi, T. Kitaoka and H. Wariishi, J. Appl. Polym. Sci., 2006, 102, 4358–4364 CrossRef CAS.
- S. Okazaki, N. Kamiya and M. Goto, Biotechnol. Prog., 1997, 13, 551–556 CrossRef CAS.
- A. Zaks and A. M. Klibanov, Science, 1984, 224, 1249–1251 CAS.
| This journal is © The Royal Society of Chemistry 2009 |
