Thylakoids entrapped within porous silica gel: towards living matter able to convert energy
Christophe F.
Meunier†
a,
Pierre
Van Cutsem
b,
Young-Uk
Kwon
ac and
Bao-Lian
Su
*ad
aLaboratory of Inorganic Materials Chemistry (CMI), The University of Namur (FUNDP), 61 Rue de Bruxelles, B-5000 Namur, Belgium. E-mail: bao-lian.su@fundp.ac.be
bUnit of Research in Plant Cellular and Molecular Biology (URBV), The University of Namur (FUNDP), 61 Rue de Bruxelles, B-5000 Namur, Belgium
cDepartment of Chemistry, BK-21 School of Chemical Materials Science and SKKU Advanced Institute of Nanotechnology, Sungkyunkwan University, Suwon 440-746, Korea
dState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 122 Hongshan Luoshi Road, 430070, Wuhan, China
First published on 3rd February 2009
Abstract
Thylakoids, photosynthetic sub-cellular plant structures, have been entrapped within silica network using the sol-gel process. The photocatalytic splitting of H2O into O2 by these structures has been studied using a Clark cell reactor. The influence of the silica precursor concentration on the chemical, morphological and diffusion properties of the matrix have been investigated and correlated with the enzymatic activity of entrapped thylakoids. Compared to the free thylakoid suspension, the bioactivity of entrapped thylakoids can be extended during several weeks. The addition of stabilizing agents (i.e.glycerol and bovine serum albumin) does not essentially improve the oxygen production. This work clearly demonstrates that through the immobilization of photosynthetic membranes, photocatalytic reactors capable of biomimicking photosynthetic processes, such as harvesting solar energy and splitting water molecules, can easily be targeted.
Introduction
The intricate organisation and efficient functionalities of natural species continue to fascinate scientists in many fields, including chemists. Over the past few years, great efforts have been made to understand and reproduce artificially these structures, leading to a wealth of bio-inspired materials.1–3 One important challenge (or even dream) for scientists is to create materials able to mimic these biological processes. Living cells and, more generally, biological species are able to perform a series of complex and attractive catalytic reactions. However, biological entities are very sensitive to environmental conditions. Therefore, one way to exploit nature's potential is to develop hybrid systems that incorporate biological species and confer protection while maintaining the biological functions. In this sense, encapsulation has to be more than just a simple protection, but it must allow the control of the interfacial properties of the biological entities. This control requires a three-dimensional scaffold topography.4 As a consequence, 3D encapsulation has emerged as the key point in the development of novel “living materials” with the ability to perform biological functions. This direction of research is expected to have a major impact in many fields, from bio-catalysis5–8 to bio-sensing9–11 and even to medical applications,12–19 since the regulation of these bio-reactions is essentially directed by the cell environments.One important biological function is the conversion of solar energy into chemical energy. Eukaryotic algae, bryophytes, ferns, gymnosperms and angiosperms, all of which perform oxygenic photosynthesis, possess photosynthetic membranes (thylakoids) that are enclosed within an envelope consisting of a double membrane. This forms a structure known as the chloroplast, the centre for photosynthetic reactions. Chloroplasts perform carbon dioxide conversions with great efficiency. This organelle achieves all the primary processes (viz. light capture and electron transport are located within thylakoids, leading to NADPH and ATP syntheses) and most of the secondary processes (e.g. the synthesis of 3-carbon phosphorylated compounds from CO2) of photosynthesis. They also synthesize many proteins and other components.20 Thus, this bio-structure displays some self-sufficiency.
With the uprising problems of depleting fossil fuels and global warming, we sought solutions through exploiting nature, namely, the generation of a photosynthetic bioreactor that uses CO2. This may be achieved by realizing a fully controlled and stable photo-bioreactor through combining these organelles with an appropriate and protecting three-dimensional scaffold. The challenge is to control the environment of chloroplasts and thylakoids whilst maintaining their photochemical activity, since the instability of isolated photosystems is a crucial limitation.
In the literature, the encapsulation of bacteria has been widely reported.11,21–30 However, unlike bacteria, the photosynthetic organelles have a low resistance to abiotic environments and, hence, only a few examples can be found on the entrapment of chloroplasts and thylakoids within different organic materials.31 For example, chloroplasts and an oxidoreductase were immobilized together within calcium alginate to form a bioreactor capable of decomposing various hydrophobic chemicals including pollutants and carcinogens.32Thylakoid membranes, encapsulated within albumin-glutaraldehyde crosslinked matrices were also used to design a cheap and straightforward amperometric biosensor to detect toxic chemicals.33 However, organic matrices have several problems in terms of being a stable and controlled environment for cells and organelles. They possess a poor thermal (even at low temperature) and mechanical stability, an uncontrolled porosity and, moreover, their synthesis is not always fully biocompatible, as in the case of albumin-glutaraldehyde crosslinked matrices as it will be detailed in the following manuscript. In this regard, inorganic materials are more appealing.
Although there are examples of using other inorganic materials such as alumina,24 porous silica has been the prime material for encapsulating cells and biological species. Silica has many desirable properties for such a goal. It is biocompatible as evident from its existence in nature in the construction of frustules, the exterior cell wall of diatoms.34,35 It is also inexpensive to synthesize, chemically inert, optically transparent,36 resistant to microbial attack, mechanically strong and thermally stable. Many studies have shown that proteins,37,38antibodies39 and enzymes40–42 can be entrapped within a wide range of silica matrices. Particularly, an in situ immobilization technique allows for the creation of a cage surrounding the biological moieties and thus protecting them from aggregation and unfolding. It has also been shown that bacteria and yeasts in porous silica can retain or even intensify their enzymatic activity.22,43 However, the entrapment of whole cells within inert frameworks, yielding hybrid materials that can potentially be used as biocatalysts, is still in its infancy. Encapsulation of cells, especially plant and animal cells, is a more delicate task compared to proteins.44,45 Maintaining the integrity of biological membrane systems (e.g. entire cells) requires the preservation of the hydrophobic interactions occurring between phospholipids during the entrapment process. As a consequence, the use of organic solvents should be strictly avoided for these systems. This is contrary to the case of proteins, which, in some cases, are reported to maintain their activity in a methanolic solution.46 Thus, one has to develop synthesis method to form silica networks around biological membranes without causing cellular damage. The reactions have to be conducted under mild and biocompatible conditions (physiological pH, isotonic medium, adequate salts composition) in order to preserve functional biological membranes. Moreover, the resulting matrices have to present physicochemical and biochemical environments favorable for the interactions with the entrapped biological species.
Compared to other biological systems such as bacteria or fungi that can resist or adapt to their environments (e.g. presence of alcohols, salts, pH) up to some extent, thylakoids are very sensitive to their surroundings. Consequently, they are not stable in an isolated state. However, these photosynthetic entities are extremely efficient since the quantum yield of the primary process of photochemical reaction is close to 100%.47 Preserving such sub-structures after their isolation is thus a challenge and constitutes the purpose of this study. In the present work, we report, for the first time, the encapsulation of functional thylakoids within a porous silica network, resulting in a photosynthetic biomaterial. This study can be taken as the first step towards the design of “living materials”.
Experimental
Isolation of thylakoids
For the sake of clarity, Fig. 1 schematically demonstrates the location and shape of thylakoids in a chloroplast. All steps dedicated to the isolation of thylakoids were performed at 2 °C in order to preserve the photosynthetic activity. Spinach plants were first kept in the dark for 24 hours prior to the isolation process. A thylakoid suspension was obtained by the two-step process detailed in the following.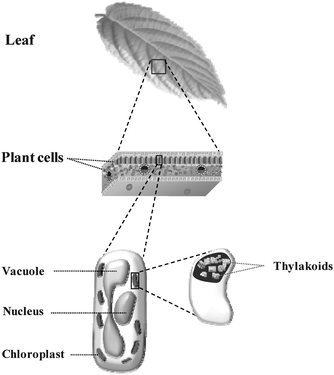 | ||
| Fig. 1 Schematic representation of thylakoid location within a plant. | ||
Synthesis of photosynthetic materials: entrapment of thylakoids in porous silica gel
Porous silica gels were synthesized using tetraethoxysilane (TEOS) in a two-steps process.49 TEOS (≥99.0%, Fluka) was mixed with acidified distilled water (pH 2.3) under vigorous stirring. The by-product ethanol was removed by fractional distillation over a period of 15 h. The hydrolyzed sol (10 mL) was neutralized by adding a small amount of KOH solution (50 µL 1.0 M) and cooled to 10 °C before gently adding the buffer that contained the thylakoids. In some cases a protecting agent (glycerol and BSA) was added to the sol prior to the addition of the biological materials. The gelation time takes between a few minutes and one hour depending mainly on the ratio between the inorganic source and water. A longer polymerization time allows the formation of a complete framework silica gel around the thylakoids. To study the effect of the silica network (density, porosity, and chemical environment of silica) on the enzymatic activity of thylakoids entrapped in silica gel, photosynthetic materials with different TEOS concentrations were prepared while keeping the amount of thylakoids constant. Hybrid gels HG1, HG2 and HG3 correspond to TEOS concentrations of 1.0, 1.2 and 1.6 M respectively.Using UV-visible spectroscopy, the efficiency of entrapment was checked by determining the concentration of chlorophyll leached into the supernatant solutions. In all cases, no significant amount was detected.
Characterization techniques
In order to get information about the morphological properties of the matrix, the aerogels were studied by SEM and TEM. The aerogels were sputter-coated with gold for the SEM samples. The TEM samples were prepared by dispersing the aerogel in an epoxy resin LX112, followed by microsectioning.
The hybrid gels were also studied by solid state 29Si MAS NMR spectroscopy on a Bruker MSL 400 spectrometer. The spectra were recorded on samples aged for 2 days after synthesis.
The shrinkage rates of the gels were obtained by following the dimensional changes of bulk samples (aged at 10 °C) with a micrometer. The v/v data, compared to the initial volume of gels, provides the magnitude of shrinkage.
Information about the diffusion properties of silica hosts was obtained using different sizes of monolithic gels previously prepared. For each sample, the oxygen concentration released in the supernatant, after its biosynthesis from water and diffusion through the silica network, was evaluated using a Clark cell vessel.
Results and discussion
The conversion of solar energy into chemical energy in plant cells is realized in the thylakoids. Thylakoids are located inside of chloroplast envelope, which itself is found within the cytosol of plant cells where the physiological conditions are always well maintained (Fig. 1). Thus isolated thylakoids are not stable. This work demonstrates the first attempts to stabilize and control the environment of fragile thylakoids by encapsulating them within an artificial three-dimensional inert solid matrix.Isolation of thylakoids
The thylakoids were extracted from plant tissue. Particularly, spinach was selected due to its softness (allowing easily disruption of cells) and its low content in phenolics and other inhibitory compounds which are prevalent in most of the other cultivated species. A key feature of the isolation procedure is the purification step, since the inevitable release of the central vacuolar sap during cell disruption is probably the most damaging event during thylakoid extraction. The release of free fatty acids and hydrolytic enzymes can lead to a structural breakdown and functional impairment of photochemical activity. One way to overcome this problem is to first isolate chloroplasts, purify them and then extract thylakoids from these chloroplasts. The exposure of thylakoids to the vacuolar sap is thus minimized. As a preliminary step, the purity of the chloroplast fraction was verified by SEM. Fig. 2A shows a sample of high purity, obtained from spinach plants with no cellular debris. The average size of a chloroplast is about 5 μm and their envelope (the inner and outer membrane) is well preserved (Fig. 2B). After extraction, the thylakoid stacking is also intact as evidenced in the TEM image (Fig. 2C). These observations suggest that the photochemical activity of the isolated thylakoids in the present study is well maintained. | ||
| Fig. 2 Electron microscopy images of isolated organelles: scanning electron microscopy image of chloroplasts (A), transmission electron microscopy images of whole chloroplast (B) and thylakoid stacks (C). | ||
Effect of sol-gel by-products
Oxygen production under a light source is a direct indicator of the enzymatic activity and the photochemical conversion efficiency of thylakoids. Thylakoids are very sensitive to fluctuations of ionic strength, osmotic pressure, pH and also to the composition of the aqueous medium. Conventional silica synthesis pathways use alkoxysilane precursors (e.g. TMOS, TEOS, TPrOS) for silica sources, liberating large amounts of alcohols (e.g.methanol, ethanol, and propanol). Unfortunately, all these by-products are unsuitable for thylakoid encapsulation because aliphatic alcohols dissolve the fatty acids and phospholipids of biological membranes. Livage and co-workers studied the encapsulation of bacteria in silica gel and demonstrated that alcohol molecules damaged the cell membrane.21Fig. 3 shows the effects of deliberately added methanol and ethanol to a thylakoid suspension. The oxygen production rapidly decreases with increasing concentration of ethanol or methanol. This chart evidences the damaging effect of methanol and ethanol to thylakoids. | ||
| Fig. 3 Effect of sol-gel by-products on the oxygen production of thylakoids after 24 h of incubation at 10 °C. The activity of the thylakoid suspension conserved in an appropriate biological medium for 24 h after isolation was taken as the reference (100%). | ||
Livage and co-workers also reported that silica gels obtained by an aqueous route using a sodium silicate solution as a silica source are less damaging to bacteria than those obtained by an alkoxide route using TMOS as the silica precursor.21 However, the high ionic strength caused by the presence of Na+ ions is also detrimental to biological species. Therefore, the desirable biocompatible silica gels have to be free of both Na+ and alcoholic by-products. For the simplicity and easy control of the hydrolysis and polycondensation, the alkoxide route could be quite attractive if a way can be found to eliminate the alcohol molecules.
Novel hybrid silica gels
The pathway developed here to immobilize thylakoids uses conventional alkoxy precursors (TEOS). One of the key points of this immobilization process is that the cytotoxic ethanol liberated is removed49 by fractional distillation before the entrapment of thylakoids. An IR study showed that more than 95% of ethanol in the gel could be really eliminated by fractional distillation.Fig. 4 shows the variation in the enzymatic activity of hybrid gels HG1, HG2 and HG3 obtained using different concentrations of silica precursor (1.0, 1.2 and 1.6 M, respectively) and that of free thylakoids in a suspension. The activity of thylakoids in the free suspensions declined very quickly and became undetectable after 3 days. To the sharp contrast, encapsulation in silica gel can significantly extend the enzymatic activity. Even though approximately 30% of the initial enzymatic activity is lost due to the osmotic and shear stresses encountered during the silica polymerization step, oxygen production could be detected over the course of one month at 10 °C (hybrid gel HG1 in Fig. 4). This result constitutes a major advance in this field, considering that the longest bioactivity of thylakoids so far reported is 8 days at 4 °C for thylakoids immobilized in a crosslinked albumin polymer.53 Organic matrices perform much worse and require very low temperature, ca. −18 °C, just for the thylakoid preservation.33 Thus, the present silica matrices unequivocally confer real protection on the thylakoid membranes; a leap forward in the construction of an artificial bioreactor that operates in the same way as a leaf to split H2O to O2 and to convert CO2 to useful carbohydrates.
![Comparison of enzymatic stability (10 °C) of entrapped thylakoids within different ratios of silica gels to free thylakoids. The gels are synthesized with different silica precursor concentrations: HG1: [Si]sol = 1.0 M; HG2: [Si]sol = 1.2 M; HG3: [Si]sol = 1.6 M. Time zero corresponds to the moment which thylakoids have been isolated.](/image/article/2009/JM/b817172f/b817172f-f4.gif) | ||
| Fig. 4 Comparison of enzymatic stability (10 °C) of entrapped thylakoids within different ratios of silica gels to free thylakoids. The gels are synthesized with different silica precursor concentrations: HG1: [Si]sol = 1.0 M; HG2: [Si]sol = 1.2 M; HG3: [Si]sol = 1.6 M. Time zero corresponds to the moment which thylakoids have been isolated. | ||
Fig. 4 also shows that the biological activity of thylakoids is in the order of HG3 (about 10 days) < HG2 (20 days) < HG1 (30 days), increasing with the decrease of the concentration of TEOS used. A possible explanation is due to the residual ethanol in the hybrid gels. Although the fractional distillation eliminates around 95% of the ethanol by-product, the amount of residual ethanol in the hybrid gels should vary in the order HG3 > HG2 > HG1. Hence, it is possible that higher ethanol concentrations result in a more rapid deterioration rate of thylakoids. In order to verify this, an experiment was carried out by adding ethanol to hybrid gels HG1 and HG2 to reach the same level of ethanol found in the hybrid gel HG3. The enzymatic activity was decreased because of the added ethanol; however, this decrease in activity was only by a few days, suggesting that the improved stability of hybrid gels HG1 and HG2 had a different origin, which we believe is associated with the silica frameworks of the hybrid gels.
One important feature concerning the preservation of physiological function of thylakoids relies on the textural properties of the synthesized matrices for two reasons. Firstly, porosity is essential in order to allow light and matter to permeate through the silica network and then be delivered to the biological systems. Secondly, the silica framework itself could act as a scaffold in some conditions, maintaining the structural organisation of thylakoids. However with aging time, this scaffold could also restrict the environment of thylakoids, placing them under increased stress. Fine control of silica network properties is thus critical to the successful encapsulation of thylakoids.
The concentration of silica precursor can control the textural properties as well as the interactions between silica walls and biological materials. Verification of this would require studies on the texture of hybrid gels, which was not easy because of the liquid phase. Therefore, we chose to study the aerogels obtained by treating the hybrid gels under critical conditions of CO2. In these conditions the silica gel structure can be preserved during the drying procedure. The obtained silica materials were characterized by N2adsorption-desorption measurements, SEM, and TEM. The N2adsorption-desorption data of all the materials showed the type II isotherms with large surface areas and macro-porosity with pores larger than 50 nm (Table 1). The concentration of silica precursor is reflected in the silica density and in the macroscopic texture of the silica framework as revealed by TEM and SEM (Fig. 5). From the SEM images (Fig. 5A), one can clearly see that a lower silica precursor concentration leads to a more spongelike framework. Upon increasing the TEOS concentration, the silica network becomes denser. The TEM images (Fig. 5B) show that the silica framework is more scaffold-like, formed by the aggregation of silica colloids. These data all agree in that HG1 has a larger porosity leading to a lower surface area while HG3 is denser with a smaller pore size and yields a higher surface area. It appears that the varying bioactivity data of the hybrid gels are correlated with their textures. One possible mechanism would be that the different porosities of the hybrid gels influence the diffusion of matter through the hybrid gels. However, the diffusion experiments proved that the pore size generated in the hydrogel is large enough to ensure the diffusion of the nutrients and the bio-production of oxygen. The oxygen production rate is not dependent on the pore size of the gel (Table 2). Therefore, we can conclude that the diffusion is not the limiting parameter of thylakoid activity. These results corroborate the conclusions concerning the influence of gel porosity on the enzymatic activity of entrapped bacteria.21
 | ||
| Fig. 5 Micrographs obtained by scanning electron microscopy (A) and transmission electron microscopy (B) of aerogels prepared from hybrid gels HG1, HG2 and HG3 by critical point drying of CO2. | ||
| Silica gel | [Si] in sol (M) | SBET (m2/g) | Pore size (nm) | Pore volume (cm3/g) |
|---|---|---|---|---|
| HG1 | 1.0 | 880 | >50 nm | 3.48 |
| HG2 | 1.2 | 907 | >50 nm | 4.46 |
| HG3 | 1.6 | 1000 | >50 nm | 4.63 |
| Monolithic gel size (mm3) | Hybrid gels | ||
|---|---|---|---|
| HG1 | HG2 | HG3 | |
| Oxygen production (µmoles g of gel−1 min−1) × 103 | |||
| 5 | 17.1 | 13.1 | 11.8 |
| 3 | 16.9 | 13.0 | 11.5 |
| 1 | 17.2 | 12.8 | 11.6 |
The other possibility to correlate the porosity of the hybrid gel with the photoactivity would be the stresses that the matrices impose on the thylakoids because the density and compactness of the macro-framework is related to the porosity of the matrices. The porosity affects also the capillary effect that can occur during the slow evaporation of water. Therefore, with increasing silica concentration, the capillary effect is stronger and the surface silanol groups are nearer to one another which leads to a higher degree of condensation. The 29Si MAS NMR data on the hybrid gels support this possibility (Fig. 6). The spectra of all the wet gels aged for 2 days show a peak at −112 ppm, which is assigned to the presence of quaternary SiO4 units (Q4) within the gels. The two other peaks at −102 ppm and −90 ppm are characteristic of SiO3(OH) units (Q3) and SiO2(OH)2 units (Q2) respectively. By comparison of the three spectra, it appears that the amount of Q4 units, i.e. the degree of condensation, is directly proportional to the TEOS concentration. A higher degree of condensation means a larger amount of shrinkage of the matrix which exerts a pressure on the entrapped thylakoids, sometimes causing their destruction. The kinetics of gel contraction support this explanation. The rate of gel contraction depends on the silica content (Fig. 7). During the first few hours of silica polymerization, the hybrid gels undergo a rapid shrinkage. The rate is increased with the increase of TEOS concentration, which corroborates with the trend in the bioactivity. After five days of aging, the rate of contraction slows down and remains more or less constant from that point on.
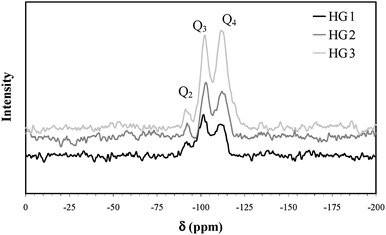 | ||
| Fig. 6 Solid state 29Si MAS NMR spectroscopy of wet gels. | ||
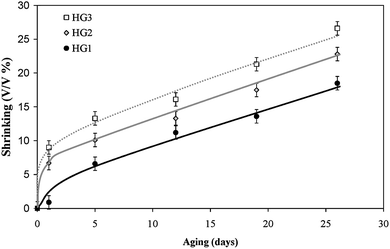 | ||
| Fig. 7 Kinetics of gel shrinking of hybrid gels at 10 °C. | ||
Based on these observations, one can conclude that the bioactivity of thylakoids within silica materials depends mainly on the silica density and chemical environment which are controlled by the precursor concentration. By minimizing the shrinking phenomenon, thylakoid activity can be increased.
Effect of additives
In order to further extend the photo-enzymatic activity of thylakoids and thus the production of oxygen by the hybrid gels, we studied the effect of some additives that could modify the density, the chemical environment of the silica framework and thus the interactions between thylakoids and silica.Glycerol, a well-known cryoprotectant, is often used to maintain biological materials in the frozen state.54 Moreover, glycerol has been used to protect bacteria during their encapsulation within silica gels. This osmolyte seems to protect cell membranes against the stress arising from encapsulation.22 The addition of glycerol to a pre-hydrolyzed TEOS sol was thus investigated in order to decrease the mechanical stress on the photosynthetic membranes during aging, as too low concentrations of silica (<0.9M) did not yield stable macroporous frameworks. For the sake of clarity, two graphs are presented: the first one (Fig. 8A) corresponds to the effect of additives on thylakoids in suspension; the second one (Fig. 8B) represents their effects on the hybrid gel HG1. Whereas glycerol stabilizes the thylakoids in suspension, the opposite effect is surprisingly observed for the encapsulated membranes. In fact, the enzymatic activity of hybrid gels is about two fold lower in the presence of glycerol. This observation leads us to suppose that glycerol, present in the porous media instead of water affects the diffusion properties and modifies the interactions between the matrix and thylakoids. 29Si MAS NMR (Fig. 9) shows that the amount of quaternary units (Q4) is lower in the presence of glycerol, suggesting less shrinking. However, the very low degree of condensation within the silica framework for the glycerol-doped matrix can affect the scaffold stabilization effect, leading to an irreversible biosystem deformation. Thus in the present case, glycerol does not give a favorable effect in extending the photoactivity of thylakoids.
 | ||
| Fig. 8 Influence of glycerol (150 mM) and BSA (15 mg/mL) on the oxygen production by thylakoid suspension (A) and by hybrid gel HG1 (B) at 10 °C. | ||
 | ||
| Fig. 9 Solid state 29Si MAS NMR spectroscopy of hybrid gels with an additive: glycerol (150 mM) or BSA (15 mg/mL). | ||
The addition of BSA in suspension or in the hybrid gel was also studied and stabilized the oxygen production. BSA does not seem to significantly modify the degree of condensation of silica. Indeed, the intensity distribution of silica species is not changed in the 29Si MAS NMR spectra from that of HG1 (Fig. 9). This protein is known to adsorb free fatty acids which can suppress the activity of photosystem II embedded within thylakoid membranes, decreasing the oxygen production.55,56 Moreover, BSA does not affect other enzymes. It is thus an excellent protecting agent. However, the stabilizing effect cannot be prolonged in the case of the present hybrid gel. After 6 days, BSA no longer has an effect as the oxygen production is the same as in gel HG1 (Fig. 8B, right). This is probably due to the confinement of the BSA within silica altering its adsorptive properties.
Conclusions
The encapsulation of fragile membrane systems like thylakoids is a real challenge. This work demonstrates, using an alcohol-free sol-gel route based on the gentle fractional distillation of alcohol, that silica gels can provide an adapted environment to stabilize thylakoids. These first results are very promising and provide a proof of concept that biocompatible porous matrices, if optically transparent, are ideal supports to confer protection to photosynthetic membranes and extend their enzymatic activity. The silica gel in the present work allows photo-induced oxygen production from thylakoids even after 30 days whereas no oxygen production can be detected after 3 days in a free suspension of thylakoids. It was shown that the bioactivity of thylakoids depends on some key factors such as the kinetics of silica polymerization, the chemical environment and the silica density.Further work is in progress to improve the immobilization of thylakoids and also of whole chloroplasts and plant cells, a step forward in the development of an artificial photosynthetic bioreactor. New sensors, catalysts and air-cleaning systems could be fabricated by coupling inert materials with living matter. The present hybrid gels are good examples that may be used in the design of photosynthetic fuel cells and be extended to biosensing applications to detect herbicides and various environmental pollutants.
Acknowledgements
C. Meunier thanks the Belgian FNRS (Fonds National de la Recherche Scientifique) for his Research Fellow position. This work was realized in the frame of the Belgian Federal Government IUAP-PAI programme (INANOMAT P6/17) project.References
- C. Sanchez, H. Arribart, M. Madeleine and G. Guille, Nat. Mater., 2005, 4, 277 CrossRef CAS.
- A. Dittmar and G. Delhomme, ARAGO, OFTA, Paris, 1999, pp. 123–160 Search PubMed.
- R. R. Naik and M. O. Stone, Mater. Today, 2005, 8, 18 CrossRef CAS.
- M. M. Stevens and J. H. George, Science, 2005, 310, 1135 CrossRef CAS.
- M. Petre, G. Zarnea, P. Adrian and E. Gheorghiu, Resour., Conserv. Recycling, 1999, 64, 185 Search PubMed.
- C. J. Cunningham, I. B. Ivshina, V. I. Lozinsky, M. S. Kuyukina and J. C. Philp, Int. Biodeter. Biodegr., 2004, 54, 167 Search PubMed.
- K. Ishihara, H. Hamada, T. Hirata and N. Nakajima, J. Mol. Catal. B: Enzym., 2003, 23, 145 CrossRef CAS.
- A. Giri, V. Dhingra, A. G. Giri, A. Singh, O. P. Ward and M. L. Narasu, Biotechnol. Adv., 2001, 19, 175 CrossRef CAS.
- Y. Lei, W. Chen and A. Mulchandoni, Anal. Chim. Acta, 2006, 568, 200 CrossRef CAS.
- S. Daunert, G. Barret, J. S. Feliciano, R. S. Shetty, S. Shrestha and W. Smith-Spencer, Chem. Rev., 2000, 100, 2705 CrossRef CAS.
- H. K. Baca, C. Ashley, E. Carnes, D. Lopez, J. Flemming, D. Dunphy, S. Singh, Z. Chen, N. Liu, H. Fan, G. P. Lopez, S. M. Brozik, M. Werner-Washburne and C. J. Brinker, Science, 2006, 313, 337 CrossRef CAS.
- T.-A. Read, D. R. Sorensen, R. Mahesparan, P. Enger, R. Timpl, B. R. Olsen, M. H. B. Hjelstuen, O. Haraldseth and R. Bjerkvig, Nat. Biotechnol., 2001, 19, 29 CrossRef CAS.
- T. Joki, M. Machluf, A. Atala, J. Zhu, N. T. Seyfried, L. F. Dunn, T. Abe, R. S. Carroll and P. McL. Black, Nat. Biotechnol., 2001, 19, 35 CrossRef CAS.
- S. Prakash and M. L. Jones, J. Biomed. Biotechnol., 2005, 1, 44 Search PubMed.
- D. W. Green, I. Leveque, D. Walsh, D. Howard, X. Yang, K. Partridge, S. Mann and R. O. C. Oreffo, Adv. Funct. Mater., 2005, 15, 917 CrossRef CAS.
- S. Sakai, T. Ono, H. Ijima and K. Kawakami, Biomaterials, 2002, 23, 4177 CrossRef CAS.
- G. Orive, R. M. Hernández, A. R. Gascón, R. Calafiore, T. M. S. Chang, P. De Vos, G. Hortelano, D. Hunkeler, I. Lacík, A. M. J. Shapiro and J. L. Pedraz, Nat. Med., 2003, 9, 104 CrossRef CAS.
- J. Lee, S. Shanbhag and N. A. Kotov, J. Mater. Chem., 2006, 16, 3558 RSC.
- M. P. Lutolf, J. L. Lauer-Fields, H. G. Schmoekel, A. T. Metters, F. E. Weber, G. B. Fields and J. A. Hubbell, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5413 CrossRef CAS.
- D. W. Lawlor, Photosynthesis, Bios, Oxford, 3rd edn, 2003, 386 pp Search PubMed.
- A. Coiffier, T. Coradin, C. Roux, O. M. M. Bouvet and J. Livage, J. Mater. Chem., 2001, 11, 2039 RSC.
- N. Nassif, C. Roux, T. Coradin, M.-N. Rager, O. M. M. Bouvet and J. Livage, J. Mater. Chem., 2003, 13, 203 RSC.
- N. Nassif, O. Bouvet, M. N. Rager, C. Roux, T. Coradin and J. Livage, Nat. Mater., 2002, 1, 42 CrossRef CAS.
- M. Amoura, N. Nassif, C. Roux, J. Livage and T. Coradin, Chem. Commun., 2007, 4015 RSC.
- M. L. Ferrer, L. Yuste, F. Rojo and F. Del Monte, Chem. Mater., 2003, 15, 3614 CrossRef CAS.
- M. L. Ferrer, Z. Y. Garcia-Carvajal, L. Yuste, F. Rojo and F. Del Monte, Chem. Mater., 2006, 18, 1458 CrossRef CAS.
- D. Fiedler, U. hager, H. Franke, U. Soltmann and H. Böttcher, J. Mater. Chem., 2007, 17, 261 RSC.
- J. C. Rooke, A. Léonard and B. L. Su, J. Mater. Chem., 2008, 18, 1333 RSC.
- J. C. Rooke, A. Léonard, H. Sarmento, J.-P. Descy and B. L. Su, J. Mater. Chem., 2008, 18, 2833 RSC.
- J. Chen, Y. Xu, J. Xin, S. Li, C. Xia and J. Cui, J. Mol. Catal. B: Enzym., 2004, 30, 167 CrossRef CAS.
- J. C. Rooke, C. F. Meunier, A. Léonard and B. L. Su, Pure Appl. Chem., 2008, 80, 2345 CrossRef CAS.
- M. Hare, S. Iazvoskaia, H. Ohkawa, Y. Asada and J. Miyake, J. Biosci. Bioeng., 1999, 6, 793 CrossRef.
- P. Euzet, M. T. Giardi and R. Rouillon, Anal. Chim. Acta, 2005, 539, 263 CrossRef CAS.
- I. V. Ikhoshvai, E. G. Sorokovikova, N. L. Bel'kova, O. I. Belykh, A. T. Titov, M. V. Sakirko and V. V. Parfenova, Dokl. Biol. Sci., 2006, 407, 201 Search PubMed.
- E. G. Vrieling, T. P. M. Beelen, R. A. van Santen and W. W. C. Gieskes, J. Biotechnol., 1999, 76, 39 CrossRef.
- C. Bouvy, W. Marine, R. Sporken and B. L. Su, Chem. Phys. Lett., 2006, 420, 225 CrossRef CAS.
- W. Jin and J. D. Brennan, Anal. Chim. Acta, 2002, 461, 1 CrossRef.
- T. R. Besanger and J. D. Brennan, J. Sol-Gel Sci. Technol., 2006, 40, 209 CrossRef CAS.
- H. Yang and Y. Zhu, Anal. Chim. Acta, 2005, 554, 92 CrossRef CAS.
- J. D. Brennan, Nat. Mater., 2005, 4, 189 CrossRef CAS.
- T. J. M. Luo, R. Soong, E. Lan, B. Dunn and C. Montemagno, Nat. Mater., 2005, 4, 220 CrossRef CAS.
- H. R. Luckarift, J. C. Spain, R. R. Naik and M. O. Stone, Nat. Biotechnol., 2004, 22, 211 CrossRef CAS.
- E. Bressler, O. Pines, L. Goldberg and S. Braun, Biotechnol. Prog., 2002, 18, 445 CrossRef CAS.
- G. Carturan, R. Dal Toso, S. Boninsegna and R. Dal Monte, J. Mater. Chem., 2004, 14, 2087 RSC.
- G. Pressi, R. Dal Toso, R. Dal Monte and G. Carturan, J. Sol-Gel Sci. Technol., 2003, 26, 1189 CrossRef CAS.
- S. Wu, L. Ellerby, J. Cohan, B. Dunn, M. El-Sayed, J. Valentine and J. Zink, Chem. Mater., 1993, 5, 115 CrossRef CAS.
- T. Hiyama, Physiol. Veg., 1985, 23, 605 Search PubMed.
- D. I. Arnon, Plant Physiol., 1949, 24, 1 CrossRef CAS.
- M. L. Ferrer, F. Del Monte and D. Levy, Chem. Mater., 2002, 14, 3619 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 5102 CrossRef.
- S. Brunauer, P. H. Emmer and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- T. Delieu and D. A. Walker, New Phytol., 1972, 71, 201 CrossRef CAS.
- M. F. Cocquempot, B. Thomasset, J. N. Barbotin, G. Gellf and D. Thomas, Appl. Microbiol. Biotechnol., 1981, 11, 193 CrossRef CAS.
- N. Shuji, S. Akira, A. Yoshihiko and M. Tsunetomo, Plant Sci., 1993, 91, 67 CrossRef.
- P. A. Siegenthaler, Biochim. Biophys. Acta, 1972, 275, 182 CAS.
- T. Galliard, Methods Enzymol., 1980, 31, 520 Search PubMed.
Footnote |
| † Research fellow position: Fonds National de la Recherche Scientifique, 5 rue d'Egmont, B-1000, Bruxelles, Belgium. |
| This journal is © The Royal Society of Chemistry 2009 |
