Determination of trace element concentrations in natural freshwaters: How low is “low”, and how low do we need to go?
William
Shotyk
* and
Michael
Krachler
Institute of Earth Sciences, University of Heidelberg, INF 236, D-69120, Heidelberg, Germany. E-mail: William.Shotyk@geow.uni-heidelberg.de; Fax: +49 (6221) 54 5228; Tel: +49 (6221) 54 4803
First published on 10th September 2009
Abstract
There is an on-going need for reliable concentration data for trace elements in natural freshwaters, including soil solutions and groundwaters, surface waters (wetlands, streams, rivers, and lakes), precipitation (fog, rain, and snow), and drinking water (including natural spring water as well as bottled water and tap water). Some of the trace elements of interest may be present in these waters at elevated concentrations due either to natural processes such as mineral weathering (e.g. As and U in groundwater), or because of human activities (e.g. atmospheric contamination of snow with Pb and Sb).
Introduction
Regardless of the cause of the enrichment of any given trace element in a natural water, it is sometimes desirable and often useful to be able to determine the “baseline” or “background” value for that parameter, for comparison, to be able to quantify and better understand any changes which may have taken place as the water evolves. In the case of many trace elements, this means being able to reliably undertake determinations at the part per trillion (ng/L) concentration level: accurate and precise measurements in this range requires limits of detection (LOD) at least a factor of ten lower. Because the concentrations of many trace metals in natural freshwaters are extremely low, both sensitive analytical methods combined with clean lab methods and procedures are of paramount importance.A precedent was established for measuring Pb at extremely low concentrations in ancient layers of polar ice by Claire Patterson who documented in excruciating detail the extraordinary precautions that are needed to measure Pb reliably at the ng/L (part per trillion) concentration range.1 Subsequently, laboratories dedicated to studies of metals in polar ice have incorporated many of these pioneering discoveries and developments.2 Using metal-free “clean lab” methods combined with ICP-SMS allows the simultaneous determination of a broad range of trace elements, as well as Pb isotope ratios (206Pb, 207Pb, 208Pb) to be measured reliably in ancient layers of polar ice.3–5
The snow and ice which has accumulated since the Industrial Revolution, even in the most remote regions of the Arctic, is profoundly contaminated by such potentially toxic elements as Pb6 and Sb7 and therefore would provide a misleading starting point for any discussion of trace elements in “natural” freshwaters. In contrast, ancient layers of polar ice widely are considered to be the “cleanest water on earth”. To put trace element concentrations into perspective, it is helpful to use this material as a reference point, to provide a baseline against which other waters may be compared. In this commentary, we use ancient layers (ca. 3.3 to 7.9 K years old) of ice from Devon Island, Nunavut, Canada, as a starting point for the discussion. A detailed description of the purpose built Ti corer, and mechanical decontamination of the outer layers of the ice core samples, is given elsewhere.8
1. Ancient arctic ice
In general, for most trace elements, the lowest concentrations found during the past 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years are in the ice layers from the mid-Holocene.9–12 Here we present the average concentrations in six samples dating from 3278 to 7947 years BP i.e. Before Present (Table 1). For the present discussion, we ignore the fact that Ag (and Cd) are enriched during this period, compared to still older ice layers dating from the Late Glacial.10
000 years are in the ice layers from the mid-Holocene.9–12 Here we present the average concentrations in six samples dating from 3278 to 7947 years BP i.e. Before Present (Table 1). For the present discussion, we ignore the fact that Ag (and Cd) are enriched during this period, compared to still older ice layers dating from the Late Glacial.10
| Average ice, Devon Island, 3.3K to 7.9K BP (n = 6) | Std. dev. | LOD | Average Groundwater, Johnson (n = 1) | Std. dev | Average Groundwater, Parnell (n = 12) | Std. dev | Surface Water, Kawagama Lake (n = 13)d | Std. dev | Snow (Johnson n = 6. Parnell n = 3) | Std. dev | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a NOTES: Al, Ba, Fe, Li, Mn, Br, Ti and Zn not included because they are generally present in groundwaters in the part per billion concentration range and therefore less problematic. n.d. = not determined. b Re and W measured for the first time in these groundwaters in March of 2009 (Johnson, n = 3; Parnell, n = 6). c Detection limits for Ga, Mo, Ni and Th not yet determined. d Unfiltered Kawagama Lake surface water sampled in triplicate on 10.8.08. e All concentrations in ng/L (ppt). | |||||||||||
| Ag | 0.67 | 1.2 | 0.02 | 0.95 | 0.19 | 0.47 | 0.08 | n.d. | 2.8 | 1.0 | |
| As | 3.0 | 0.7 | 0.3 | 1591 | 73 | 244 | 38 | 137 | 3 | 71 | 22 |
| Be | n.d. | 0.03 | 0.55 | 0.06 | 0.56 | 0.11 | 6.4 | 0.5 | 1.4 | 0.5 | |
| Bi | 0.10 | 0.05 | 0.002 | 0.13 | 0.10 | 0.83 | 0.17 | 0.25 | 0.03 | 7.6 | 3.0 |
| Cd | 2.3 | 0.8 | 0.01 | 4.9 | 0.9 | 2.3 | 0.5 | 8 | 0 | 35 | 27 |
| Co | 2.5 | 1.0 | 0.01 | 8.6 | 0.9 | 17 | 3 | 12 | 0.1 | 18 | 9 |
| Cr | 4.1 | 1.3 | 0.015 | 7.7 | 3.9 | 4.6 | 2.2 | 75 | 4 | 116 | 39 |
| Cu | 19 | 8 | 0.1 | 64 | 77 | 17 | 9 | 470 | 112 | 852 | 584 |
| Ga | n.d. | 8.0 | 0.06 | 4.2 | 0.8 | n.d. | 8.4 | 2.2 | |||
| Ge | n.d. | 0.5 | 4.0 | 0.3 | 5.9 | 1.0 | 8.6 | 1.6 | 3.8 | 1.1 | |
| Mo | 0.82 | 0.09 | 0.01 | 673 | 108 | 453 | 122 | 8.1 | 1.2 | 75 | 26 |
| Ni | n.d. | 1.0 | 48 | 12 | 26 | 5 | 289 | 8 | 336 | 261 | |
| Pb | 5.1 | 1.4 | 0.06 | 5.9 | 3.6 | 3.4 | 1.9 | 57 | 672 | 264 | |
| Re b | n.d. | 0.001 | 1.4 | 0.0 | 5.2 | 0.1 | n.d. | n.d. | |||
| Sb | 0.10 | 0.04 | 0.006 | 1.4 | 0.2 | 2.2 | 0.4 | 30 | 1 | 31 | 18 |
| Sc | 0.65 | 0.17 | 0.006 | 1.3 | 0.5 | 0.75 | 0.13 | 6.5 | 1.4 | 2.3 | 0.9 |
| Te | n.d. | 0.2 | 12 | 2 | 2.6 | 0.8 | 1.4 | 0.1 | 1.8 | 0.7 | |
| Th | n.d. | 0.01 | 2.0 | 0.9 | 0.07 | 0.02 | 3.9 | 0.4 | 1.8 | 0.7 | |
| Ti | 0.24 | 0.05 | 0.008 | 0.67 | 0.29 | 0.43 | 0.06 | 4.9 | 0.1 | 2.5 | 1.4 |
| U | 0.50 | 0.18 | 0.002 | 843 | 28 | 1299 | 221 | 4.2 | 0.05 | 4.0 | 2.3 |
| V | 4.9 | 1.3 | 0.007 | 25 | 3 | 9.1 | 1.7 | 62 | 1 | 172 | 105 |
| W b | n.d. | 0.06 | 61 | 13 | 11 | 5 | 0.38 | 0.09 | n.d. |
First, notice that the average concentrations of trace elements during this time period may be very low e.g.Ag, Bi, Mo, Sb, Sc, Tl and U are all present at levels below 1 ng/L. Second, despite the obvious analytical challenge presented by these low concentrations, notice that the LODs obtained using appropriate clean lab methods and ICP sector-field mass spectrometry (ICP-SMS) are more than adequate; in all cases, the average concentration of any given trace element in the cleanest layers of ancient arctic ice is at least a factor of 10 greater than the LOD.
The average concentration of trace elements in ice layers from the mid-Holocene of the Arctic is the point of reference for the remainder of the discussion.
2. Groundwater
Also given in Table 1 is a summary of the average abundance of selected trace elements in groundwater from two artesian flows in Simcoe County, Ontario, Canada: the Johnson Farm in Springwater Township and the Parnell Farm in Tiny Township. Both of these artesian flows are described in more detail elsewhere.13 It is important to state clearly and to emphasize at the outset that none of the groundwater samples were filtered, and that waters were sampled only from flowing, artesian wells. The data for Al, Ba, Fe, Li, Mn, Sr, Ti and Zn are not presented here simply because they are present in the part per billion concentration range and therefore are less problematic to be measured reliably than most of the elements listed in Table 1; these data, however, are presented elsewhere.14Notice that the concentrations of Ag, Bi, Cd, Cr, Cu, Pb and Sc in groundwaters are effectively identical to those of ancient arctic ice (Table 1). Even Tl and V are within a factor of two of the values in ancient arctic ice. The point which we would like to stress here is that the extraordinary efforts which are needed to measure trace metals in ancient arctic ice,1 and the extreme care needed to avoid contamination of the samples,2 are also needed to reliably determine the abundance of many trace elements in natural groundwaters. Hodge et al.15 have compared the concentrations of trace elements in carbonate groundwaters to seawater, reminding us of the severity of analytical challenges facing chemical oceanographers.
As noted elsewhere,14 the differences between the groundwaters emanating from Parnell versus Johnson are reproducible and have been found consistently during the past five years of sampling and testing. Consider the case of Ag, for example: even though the average Ag concentrations are at or below 1 ng/L at both sites, frequent sampling and measurement has shown that the concentrations of Ag in the groundwaters from the Johnson flow are ca. twice those found at Parnell (Table 1); in other words, these are meaningful values which reflect real differences in the chemical composition of the waters; these can be clearly seen, even at extremely low concentrations, when great care is invested in sample collection, handling, preparation, and measurement.
The abundance of Re in these waters provides further illustration of the differences between these groundwaters. The concentrations of Re and U in replicate samples from these plus a third artesian flow (Archer) are shown in Fig. 1. Notice how reproducible these values are; precise data can be obtained even at extremely low concentrations, provided that all of the necessary precautions and prerequisites are employed. The detection limit for Re is 1 pg/L (i.e. part per quadrillion) and notice again the significant difference between the Johnson and Parnell flows. In the groundwaters at the Archer flow, the abundance of Re is only 0.40 ± 0.01 ng/L (n = 3).
 | ||
| Fig. 1 Comparison of Re and U concentrations in groundwaters from three artesian flows (Parnell, Johnson, Archer). Six samples were collected at the Parnell flow, while the other two were sampled in triplicate. | ||
Comparison with other groundwater data
It would be desirable to obtain other data for groundwaters in the area, but there is very little data available for comparison. For example, the Groundwater Monitoring Program of the Ontario Ministry of the Environment (MOE) has no data whatsoever for most of the trace elements listed in Table 1. For some of the trace elements given in Table 1 such as Co, Cr, and Cu, there is MOE data available for groundwaters in this region of Simcoe County, but the concentrations reported by the MOE are far higher. For example, the Cr concentrations in the groundwaters from the Johnson and Parnell flows are below 10 ng/L (Table 1), but the MOE data (unpublished) shows concentrations in the range of 0.3–3 µg/L ie values which are approximately 100 to 1000 times greater.16 A direct comparison of our data with that of the MOE is not possible because the samples are different, having been collected at different sites, and no consideration can be given to possible effects of the diverse Quaternary geological history of this region. Moreover, the MOE data is obtained from standing wells where the water has to be pumped and then filtered prior to testing. In contrast, our data is from flowing artesian wells, and represents unfiltered samples. Thus, we avoid altogether the temptation to compare the results, except to say that the concentrations are very different.Given that the concentrations of trace metals found in unfiltered groundwaters from flowing, artesian wells in this area are so low (Table 1), and in fact comparable to the concentrations found in ancient Arctic ice, one has to ask what procedures need to be in place in order to be able to pump and filter groundwaters of this quality, without contaminating them.
Effects of colloids on trace metal concentrations
Site 41 is an engineered landfill being constructed in Tiny Township, in an area of groundwater discharge, adjacent to the Parnell farm. In April of 2006 we were given permission to collect groundwater samples from three flowing wells on the Site 41 property, namely 3A-1, 5A-1 and GL-1-B; the latter is a natural, artesian flow. The two constructed wells (3A-1 and 5A-1) were purged and water allowed to run for one hour before collection into either pre-cleaned bottles provided by a consulting engineering firm (3A-1, 5A-1 and GL-1-B) or directly into our own bottles (3A-1-ME, 5A-1-ME and GL-1-B-ME).The results show that the two different bottles yield only small differences (Fig. 2). Of much greater importance is the fact that the water samples from the 3A well are distinctly different, yielding far greater concentrations of Al, Sc, Cr, V, Cu and Pb, as well as Cd and Tl (not shown). All of these waters originate in the same shallow aquifer, and we assume that the composition of the water underlying the landfill at Site 41 is fairly uniform; the similar Mg and Ca concentrations in the waters from all three wells supports this interpretation. The large differences in trace metal concentrations, therefore, appear to be entirely an artefact of the method of sample collection.
 | ||
| Fig. 2 Al, Sc, Cr, V, Cu, and Pb concentrations from three wells at Site 41, an engineered landfill now being constructed on the property. Aluminium concentrations given in µg/L, others in ng/L. Each bar represents the average of three samples. | ||
The groundwaters at Site 41, as well as those from the Johnson and Parnell artesian flows (Table 1) are in equilibrium with calcite and have a pH of 8. The expected concentration of Al at pH 8, assuming that the waters are in equilibrium with gibbsite, is approximately 10−7 M,17 or 3 µg/L. Groundwaters from the Johnson farm average 3.5 µg/L and from the Parnell flow 0.5 µg/L.14 Moreover, water samples collected from six additional artesian flows in the area (Belluz, Burgsma, Pigeon, Stone, Temolder, and Hwy 27) show similar Al values, and all yield Al concentrations below 3.5 µg/L (unpublished data). In contrast, the waters from the 5A and GL wells have significantly higher Al concentrations (ca. 10 to 20 µg/L). The 3A samples, in particular, containing up to 340 µg/L (ie 100 times more Al than the waters from any of the artesian flows), would be very difficult to explain based on either the geochemistry of the waters, or the geochemistry of Al. Instead, the elevated Al concentrations are an obvious indication of colloidal material having been introduced into the water, probably during purging of the wells. Notice that Sc, an element whose behaviour during hydrolysis is comparable to that of Al18 shows the same effect; at Johnson and Parnell, Sc concentrations are on the order of 1 ng/L (Table 1). In contrast, in the waters from the 3A well, the Sc concentrations exceed these values by 100× (Fig. 2). Again, we know of no logical explanation for such anomalous values based either on the geochemistry of the groundwaters or the geochemistry of Sc.
The introduction of colloidal material during purging and its effect on e.g.Al and Sc, would explain the anomalous concentrations of Cr and V (Fig. 2) as well as Cu and Pb (Fig. 2). Cd and Tl are similarly affected, but not shown. The data shown in Fig. 2, therefore, shows that the method of sample collection can have a profound effect on the apparent abundance of trace metals in groundwaters, with the trace metal concentrations a sensitive reflection of the abundance of colloidal materials.19 The data from the 3A well most certainly do not reflect the chemical composition of the groundwater, but rather the concentration of colloids added to the water by purging the well.
While the introduction of colloids has a profound effect on trace metals associated with them (such as Pb), notice that the anionic trace elements (As, Mo, U) are much less affected (Fig. 3). The introduction of colloidal matter during sampling, therefore, is especially problematic, because not all of the trace elements will be affected to the same extent.
 | ||
| Fig. 3 As, Mo, and U (ng/L) at the same site. Again, each bar represents the average of three samples. | ||
Regarding geochemical studies of the natural chemical composition of the groundwaters in the vicinity of Site 41, and the evolution of the fluids, we are confident that the data from the artesian flow on Parnell farm which is adjacent to Site 41, provides a reasonable indication of the quality of groundwater in this area today.
Effects of water sample filtration
The data in Table 1 shows that the natural abundance of many trace elements in several kinds of water samples (snow, surface water, groundwater) may be comparable to the cleanest layers of ancient arctic ice. While we certainly understand the importance of removing particulate material from water samples prior to testing, to allow the determination of “dissolved” concentrations of trace elements,20 this raises two important issues: first, the problem of introducing colloidal materials capable of passing through 0.45 µm membrane filters. Second, the challenges faced when attempting to clean the filters prior to use,21 to be able to achieve the blank values necessary to measure trace elements reliably at these extremely low concentrations. Although commercially-available filters for online groundwater monitoring are certified to yield a maximum blank value for any given element, in many cases these values are several orders of magnitude beyond the concentrations of many of the elements shown in Table 1. Thus, although commercially available, pre-cleaned, on-line membrane filters may be adequate for the kinds of compliance testing noted above, blank values must be carefully evaluated on an element by element basis for any rigorous scientific studies.Objectives of water sampling
In fairness to the MOE, it is their obligation to ensure that landfill sites are in compliance with water quality guidelines. The purpose of the monitoring wells, therefore, is simply to confirm that the water quality guidelines are being met, and the accuracy and precision of the chemical analyses must only be sufficient to complete such an assessment. Thus, from the perspective of a regulatory agency, it is not necessary to obtain the absolute value of any given parameter, but rather simply to ensure that the parameter has a concentration less than the guideline to complete the assessment.At the same time, however, it is important to realise that any trace element concentration data obtained from these kinds of monitoring programs, may provide little, if any information about the true chemical composition of the waters, and cannot be used in geochemical studies of the origin and evolution of the fluids.
3. Surface water
As noted elsewhere,14 contemporary snow in southern Ontario contains elevated Pb concentrations and Pb/Sc ratios, with values up to 1000× greater than crustal values, due to atmospheric pollution. Many other chalcophile trace elements show comparable enrichments, relative to crustal values, due to industrial emissions. Measuring the concentrations of chalcophile trace elements in contemporary snow in southern Canada is much less of a challenge compared to ancient Arctic ice.Despite the elevated atmospheric inputs, however, lake surface waters of southern Ontario today may exhibit Pb/Sc ratios approaching crustal values because of a variety of removal processes within the watershed. Taking the example of Kawagama Lake, the Pb concentrations in surface waters are sometimes as low as 10 ng/l, compared to 5 ng/l in ancient Arctic ice (Table 1). In massive water bodies such as the Great Lakes, such low Pb concentrations have been documented in the past,22–24 but these removal processes seem to also be operating effectively in much smaller watersheds.25
Further, in surface waters the concentrations of trace elements may also be extremely variable, because of particle removal and scavenging processes. At Kawagama Lake, for example, Pb concentrations and Pb/Sc in the streams entering the lake may easily be 100× greater than the values found in the lake waters themselves, simply because of the physical removal of Pb-bearing particles, probably reflecting the change in water velocity from stream to lake. Thus, the analytical methods which are adequate for measuring Pb in stream waters26–28 may be entirely inadequate for studies of lake waters within the same watershed. Cobalt is an excellent example of extreme variations in concentrations: at Kawagama Lake, for example, the variations in concentration extend over nearly three orders of magnitude, with nearly 10 µg/L (ppb) in seepage water and streams, and only a few ng/L (ppt) in the lake (Fig. 4). Geochemical studies of element flows in watersheds, therefore, also require the analytical sensitivity as well as the extreme care to avoid contamination as the great efforts which are needed to measure trace metals in ancient arctic ice.
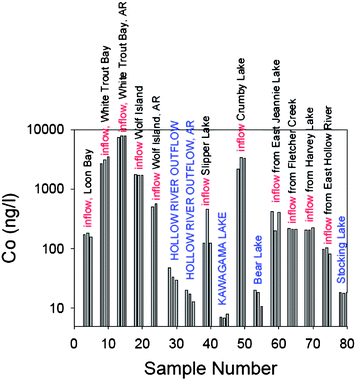 | ||
| Fig. 4 Co concentrations (ng/L) in surface waters from the Kawagama Lake watershed. Each site was sampled in triplicate. Although source waters contain up to 10 µg/L, the samples from the middle of Kawagama Lake itself averaged 7.4 ± 0.6 ng/L: this is comparable to the groundwater samples (Table 1) and within a factor of 3 of the values found in ancient Arctic ice (Table 1). AR = sampled after rain event. | ||
Comparison of surface water and groundwater
As noted elsewhere,14 As, Mo, and U are enriched in groundwater, relative to snow, thus the determination of these elements in groundwater is rendered somewhat less difficult. Recently we measured W in the groundwaters of Simcoe County for the first time, and the values (Table 1) are much greater than in snow from the area, suggesting that W, too, may be naturally enriched in the groundwaters. Despite this, W concentrations in the waters tested thus far are below 100 ng/L, so under no circumstances are these measurements trivial. In contrast, in the surface waters of Kawagama Lake, W concentrations are approximately two orders of magnitude lower than in the groundwaters (Fig. 5), with the average concentration only 0.38 ± 0.09 ng/L. Thus, the methods suitable for measuring some trace elements in groundwaters (such as As, Mo, U and W which become enriched in the example given here) may be unsuitable or inadequate for testing surface waters for the same elements. | ||
| Fig. 5 As, Mo, U, W in groundwaters (artesian flow, Johnson farm) versus surface waters (Kawagama Lake). These two areas are far removed from one another and hydrologically independent, and are compared only to illustrate the different concentration ranges characteristic of the waters. | ||
Bottled water
The concentrations of some trace elements in bottled waters (e.g.Ag, Te, Th) can be very low (a few ng/L or less), and many trace elements, in particular Li, Be, Ge and U, reveal very large variations in abundance.29 Although some constituents in bottled waters may reflect their abundance in the groundwater prior to packaging (assuming that the waters have not been filtered or otherwise treated), others simply reflect contamination from the packaging: PET plastic releases Sb30,31 whereas glass may release Pb32 as well as and Th and Zn.29 The median concentration of Pb in bottled waters packaged in PET plastic, however, is only a few ng/L32 and probably reflects the natural abundance of this element in the waters prior to packaging. Thus, depending on the packaging and duration and storage, some elements in bottled waters may reflect their natural abundance in groundwaters; this means that the range in concentrations may be comparable to the values shown in Table 1. Once again, therefore, the reliable determination of many trace elements in bottled water requires the sensitivity as well as the extreme care to avoid contamination as the great efforts which are needed to measure trace metals in ancient arctic ice and uncontaminated groundwaters.Conclusions
In natural waters, the concentrations of very many trace elements can be extremely low, in the range of a few ng/L or below. Although some chalcophile elements may be highly enriched in rain and snow because of atmospheric contamination, many of these may be efficiently removed by soils, leading to very low concentrations in groundwater (e.g.Ag, Bi, Cd, Cu, Pb, Sb, Tl). While some lithophile elements (Li, U) and chalcophile elements (As, Mo) can become naturally enriched in groundwaters due to chemical weathering, surface waters may exhibit very low concentrations of many trace elements because of the physical removal of metal-bearing particles within a watershed. Thus, a wide range of trace elements, including some commonly measured metals such as Cr, are present in many kinds of waters (surface waters and groundwaters) at concentrations comparable to those of ancient polar ice. The significance of this, of course, is that the extreme care required to measure trace elements in ancient arctic ice, is also needed for the reliable determination of trace elements in many other kinds of water samples.Using the clean lab methods and ICP-SMS which has been successfully applied to polar ice, a broad range of trace elements can be measured simultaneously and reliably, in all other natural waters, including rain and snow, surface waters, and groundwaters. Although the lower limits of detection provided by the ICP-SMS are more than adequate for accurate and precise measurements of virtually all trace elements found in natural freshwaters, sensitivity alone does not ensure representative data. In fact, the risk of sample contamination will always represent a far greater challenge than adequate limits of detection. Moreover, the need to remove particulate matter without contaminating the water sample or introducing colloidal materials, remains a daunting task.
References
- M. Murozumi, T. J. Chow and C. C. Patterson, Geochim. Cosmochim. Acta, 1969, 33, 1247–1294 CrossRef CAS.
- C. F. Boutron, J.-P. Candelone and U. Görlach, Analusis Mag., 1990, 20, M24.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, J. Anal. At. Spectrom., 2004, 19, 1017–1019 RSC.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, Anal. Chem., 2004, 76, 5510–5517 CrossRef CAS.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, Anal. Chim. Acta, 2005, 530, 291–298 CrossRef CAS.
- W. Shotyk, J. Zheng, M. Krachler, C. Zdanowicz, R. Koerner and D. Fisher, Geophys. Res. Lett., 2005, 32, L21814, DOI:10.1029/2005GL023860.
- M. Krachler, J. Zheng, C. Zdanowicz, R. Koerner, D. Fisher and W. Shotyk, J. Environ. Monit., 2005, 7, 1169–1176 RSC.
- J. Zheng, D. Fisher, E. Blake, G. Hall, J. Vaive, M. Krachler, C. Zdanowicz, J. Lam, G. Lawson and W. Shotyk, J. Environ. Monit., 2006, 8, 406–413 RSC.
- J. Zheng, W. Shotyk, M. Krachler and D. Fisher, Global Biogeochem. Cycles, 2007, 21, GB2027, DOI:10.1029/2005GL023860.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Global Biogeochem. Cycles, 2008, 22, GB1015, DOI:10.1029/2005GL023860.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Sci. Tot. Environ., 2008, 399, 78–89 Search PubMed.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Global Biogeochem. Cycles, 2009 DOI:10.1029/2009GB003471.
- W. Shotyk, M. Krachler, B. Chen and J. Zheng, J. Environ. Monit., 2005, 7, 1238–1244 RSC.
- W. Shotyk, M. Krachler, W. Aeschbach-Hertig, S. Hillier and J. Zheng, J. Environ. Monit., 2009 10.1039/b909723f.
- V. E. Hodge, K. J. Stetzenbach and K. H. Johannesson, Environ. Sci. Technol., 1998, 32, 2481–2486 CrossRef CAS.
- Ontario Ministry of the Environment, Provincial Groundwater Monitoring Program, unpublished data Search PubMed.
- G. Faure, Principles and applications of inorganic geochemistry: a comprehensive textbook for geology students. Upper Saddle River, N.J., London, Prentice Hall, 1998 Search PubMed.
- C. F. Baes, Jr. and R. E. Mesmer, The Hydrolysis of Cations. 512p., John Wiley and Sons, New York, 1976 Search PubMed.
- S. A. Sanudo-Wilhelmy, F. K. Rossi, H. Bokuniewicz and R. J. Paulsen, Environ. Sci. Technol., 2002, 36, 1435–1441 CrossRef CAS.
- A. J. Horowitz, K. R. Lum, J. R. Garbarino, G. E. M. Hall, C. Lemieux and C. R. Demas, Environ. Sci. Technol., 1996, 30, 954–963 CrossRef CAS.
- N. Rausch, L. Ukonmaanaho, T. Nieminen, M. Krachler, G. Le Roux and W. Shotyk, Anal. Chim. Acta, 2006, 558, 201–210 CrossRef CAS.
- J. O. Nriagu, G. Lawson, H. K. T. Wong and J. M. Azcue, J. Great Lakes Res., 1993, 19, 175–182 CAS.
- M. P. Field and R. M. Sherrell, J. Anal. At. Spectrom., 2003, 18, 254–259 RSC.
- G. Benoit, K. S. Hunter and T. F. Rozan, Anal. Chem., 1997, 69, 1006–1011 CrossRef CAS.
- W. Shotyk and M. Krachler, Geochim. Cosmochim. Acta, 2009 Search PubMed submitted.
- S. I. Vinogradoff, M. C. Graham, G. J. P. Thornton, S. M. Dunn, J. R. Bacon and J. G. Farmer, J. Environ. Monit., 2005, 7, 431–444 RSC.
- J. Klaminder, R. Bindler, H. Laudon, K. Bishop, O. Emteryd and I. Renberg, Environ. Sci. Technol., 2006, 40, 4639–4645 CrossRef CAS.
- M. C. Graham, S. I. Vinogradoff, A. J. Chipchase, S. M. Dunn, J. R. Bacon and J. G. Farmer, Environ. Sci. Technol., 2006, 40, 1250–1256 CrossRef CAS.
- M. Krachler and W. Shotyk, Sci. Tot. Environ., 2009, 407, 1089–1096 Search PubMed.
- W. Shotyk, M. Krachler and B. Chen, J. Environ. Monit., 2006, 8, 288–292 RSC.
- W. Shotyk and M. Krachler, Environ. Sci. Technol., 2007, 41, 1560–1563 CrossRef CAS.
- W. Shotyk and M. Krachler, Environ. Sci. Technol., 2007, 41, 3508–3513 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
