Polynuclear aromatic hydrocarbons (PAHs) in global background soils
Received
8th August 2008
, Accepted 3rd October 2008
First published on 22nd October 2008
Abstract
The levels and distribution of polynuclear aromatic hydrocarbons (PAHs) were investigated in global background soil samples. Total PAH concentrations (sum of 15 compounds) ranged over 5 orders of magnitude, from <1 to 7,840 (mean 328) ng g−1 dry weight. The order was generally Europe > North America > Asia > Oceania > Africa > South America. Proximity to long-term emissions sources and locations susceptible to high atmospheric depositional inputs tended to have higher concentrations. A broad positive correlation was obtained between population density and soil PAH concentrations (i.e. source-related factors). However, concentrations were also influenced by the holding capacity of the soils (i.e. sink-related factors) with statistically significant correlations observed between PAHs and soil organic matter (SOM), and black carbon (BC).
Introduction
The distribution of polynuclear aromatic hydrocarbons (PAHs) in the environment is of interest with respect to their source identification, environmental partitioning, and possible ecotoxicological significance. Soils can be key sources, reservoirs and sinks of PAHs and other persistent organic pollutants (POPs). Several papers have described regional soil contaminant distribution e.g.,1–4 but there is little information on global scale variations.5 Anthropogenic combustion activities are a major source of atmospheric PAHs in industrialized countries, which may be deposited onto soils. PAHs accumulate in C-rich top soils and are often associated with soil organic matter (SOM) and soot-like C, commonly called black carbon (BC).6–8 The role of SOM and BC in PAH partitioning is well established. Other factors may also affect the PAH concentrations in soil at the global scale, such as the climatic zone, which can influence the amounts and turnover of SOM and propensity for degradation. Samples of global background soils which had previously been analysed for PCBs and HCB9 were used in this study.
Materials and methods
Soil sampling
Surface soil samples (0–5 cm) were collected from a range of background locations worldwide in 1998 (Fig. 1). Sites were chosen to be remote from potential sources to ensure that they were representative of background levels in the area from which they were collected. Details of the method, sample information, and properties of the soils have been published previously.9 Soil sampling kits and instructions were sent to volunteers around the world, who were asked to choose sites that were far away from towns and cities, >2 km from busy roads, and >500 m from small dwellings and tracks. All samples were collected from the surface 0–5 cm in triplicate using a hand-held coring device. Overlying vegetation was removed prior to collection of the sample. Samples were double-wrapped in solvent-rinsed Al foil, sealed in plastic bags, and frozen prior to sending them to Lancaster University, where they were stored frozen until required for extraction. Separate samples were also collected at most sites to determine the bulk density. A total of 108 samples was selected for analysis.
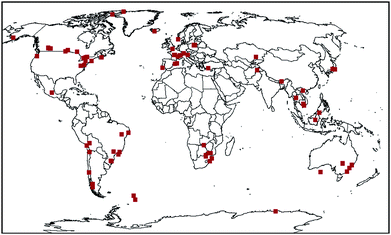 |
| | Fig. 1 Sample location map. | |
Chemicals and instrumentation
A mixture containing 15 PAHs (naphthalene (Nap), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzanthracene (BzA), chrysene (Chr), benzo(b)fluoranthene (BaA), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenzo(a,h)anthracene (DaA), benzo(ghi)perylene (BgP), and coronene (Cro)) at 100 µg/ml hexane was purchased from LGC Promochem (Middlesex, UK) and diluted to make calibration standards for HPLC-fluorescence analysis. Silica gel 60 (0.063–0.200 mm) and alumina (neutral, 0.063–0.200 mm) for column chromatography was obtained from Merck (Poole, UK). All solvents were of HPLC grade (Merck, Poole, UK). An HPLC ISS 200 (Perkin Elmer, USA) equipped with an FP-2020 fluorescence detector (Jasco, Japan) was used for PAH analysis.
PAH analysis
About 10 g of soil was mixed with anhydrous sodium sulfate and then extracted with dichloromethane (DCM) for 16 hours by Soxhlet extraction. Cleanup was performed on a silica and alumina gravity fed chromatography column. After clean-up the solvent was evaporated and exchanged to acetonitrile. PAH standards at seven concentrations (from 0.01 to 0.4 µg/ml) were prepared. Separation was achieved on a ChromSpher5 150 × 4.6 mm HPLC column (Varian, UK). Quantification was by an external calibration method.
BC and TOC
Total organic carbon (TOC) and BC were determined at Stockholm University. An elemental analyzer (Carlo Erba, Italy) was used for the TOC analysis after the samples were treated with HCl. The detection limit of the analyzer was 4.9 µg for C, measured from the long-term running average (n = 41) of the blank, plus three times the standard deviation of the blank. The BC content was determined with the chemothermal oxidation method.10 The soil was first finely ground for 20 min in an automatic ball grinder. Then, inorganic carbon (IC) was removed by a mild acidificationin situ in Ag capsules. Amorphous non-pyrogenic organic carbon (OC) was subsequently removed in a thermal oxidation procedure at 375 °C in a tube furnace continuously supplied with an atmosphere of air.11 Total SOM was measured by loss-on-ignition at 450 °C at Lancaster University.9,12
Quality control
For every twelve samples, a laboratory blank and a duplicate sample were incorporated in the analytical procedure. Method detection limits were derived from the blanks and quantified as three times the standard deviation of the mean concentration of the blanks. The method detection limit ranged from 0.1–17 ng ml−1 for the 15 PAHs. Overall recovery was determined by analyses of spiked samples into blank samples. The average recovery was 102% (range 50–132%) and the average coefficient of variation (CV) of the duplicate samples was 17% (range 12–24%).
Results and discussion
Levels and spatial distribution of PAHs
Differences of over 5 orders of magnitude were observed between sampling sites. The lowest and highest PAH concentrations ranged from <1 to 7,840 ng g−1 dry weight soil (Table 1); they were found in the samples from Antarctic/Australia and mainland Europe, respectively. The mean and median Σ15PAH concentrations were 328 and 44 ng g−1, respectively.
Table 1 Summary of PAH concentrations in the global background soils (ng g−1 soil dw)a
Fig. 2 depicts the broad spatial distribution of soil concentrations while Table 2 makes a comparison between continents. Highest concentrations were generally in the temperate industrialized latitudes of the Northern Hemisphere (ca. 30–70° N), particularly mainland Europe and eastern North America (Table 2), consistent with the trend noted previously for PCBs.9 These areas are relatively densely populated, but also have a long history of industrial activity. Values in Asia, where economic development and expansion is generally more recent, had average values typically a factor of 5–10 below those of Europe and North America. South America and Africa were typically a factor of 2 lower again.
Table 2 Sum of 15 PAH concentrations (ng g−1 dw) by continents
| |
Mean |
Median |
No. of samples |
| Africa |
30 |
33 |
7 |
| Asia |
65 |
21 |
11 |
| Europe |
714 |
136 |
23 |
| North America |
347 |
91 |
21 |
| South America |
21 |
5.8 |
7 |
| Oceania |
60 |
13 |
14 |
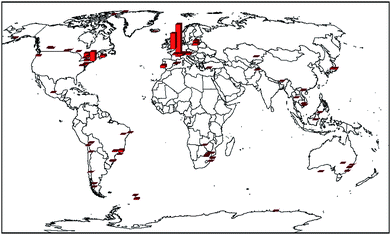 |
| | Fig. 2 PAH distribution map of the global background soils (key: largest bar = 7,840 ng g−1). | |
Relationship between PAHs and population density
Atmospheric concentrations of PAHs have been previously correlated to human activity and hence population density is often used to provide spatial information on sources. For example, Hafner et al. (2005) found positive correlations between population density and atmospheric PAH concentrations for several regions of the world.13 In order to investigate this relationship further, the soil data presented in this paper were correlated with population density within a 50 km radius of the sampling location. Prior to this, the soil concentration data were normalized to 1% SOM (see later), to take this variable out of the correlation. These data were correlated against mean population data14 taken from a 50 km circular buffer around the sampling location. Some of the resulting correlations are shown in Fig. 3, for Σ15PAH, pyrene and B(a)P. These plots show that there is a weak positive relationship, with the strongest evident for BaP. This broad relationship may be useful for global scale modeling purposes.
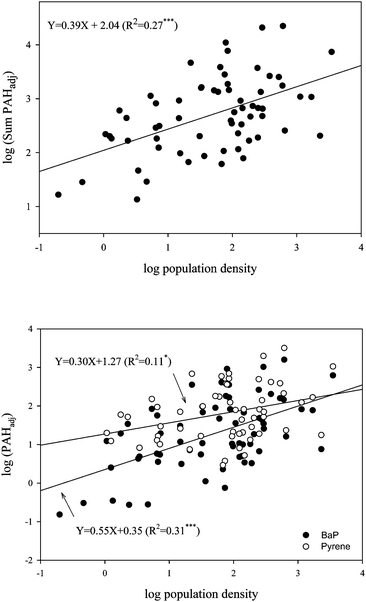 |
| | Fig. 3 Regression between (above) ΣPAH, and (below) pyrene and benzo(a)pyrene (normalised for OM) and mean population density. | |
There are numerous categories of combustion sources of PAHs, such as burning of coal and wood for space heating and electricity production, waste incineration, vehicle and factory emissions, and natural sources such as forest fires.15,16 The intensity and relative importance of these sources varies between regions and countries, and on the degree of industrial development. Further work could consider the refinement of this relationship, with more detailed knowledge of the dominant source types in different regions (e.g. vehicle emissions versus fuel burning). Natural sources such as forest fires and biogenic production will also be important in some parts of the world.
Correlation with OM and BC
Clearly part of the explanation of varying concentrations relates to proximity to sources, source types, and the time for cumulative deposition. However, it is also important to consider the retentive capacity of soil.17
The SOM, TOC and BC contents of the soils varied widely - reflecting the wide range of environments, land cover and climatic conditions sampled. The ranges (and means) were in line with other published data1–4,18,19 as follows: SOM, 0.2–98.6 (18.2)%; TOC, 11.9–406 (92.3) mg g−1; BC, 0.2–5.1 (1.43) mg g−1 (see Table 3). Relationships between PAHs and SOM and BC were explored. PAH concentrations were natural log transformed to reduce scatter and allow linear fits before analysis. Data are plotted in Figs. 4 (with SOM) and 5 (with BC). Statistically significant correlations (p<0.001) between PAHs and SOM varied with the numbers of aromatic rings, with the most significant relationship occurring for the lightest (3-ringed) species (see Fig. 4). Statistically significant correlations also occurred between PAHs and BC, but here there was no relationship for the 3-ringed species (see Fig. 5) and strong ones for the 4-, 5-and 6-ringed compounds. A strong interaction was observed between SOM and BC (p<0.01), so it is not straightforward to interpret the data. However, it is well established that PAHs sorb strongly to BC in air and soils6–8 and that SOM represents an important sink for compounds which can undergo repeated air-soil exchange.17
Table 3 Organic matter, TOC, and black carbon content in the global background soils
| |
Mean |
Median |
Range |
No. of samples analysed |
| Organic matter (%) |
18.2 |
11.5 |
0.2–98.6 |
84 |
| TOC (mg g−1) |
92.3 |
62.8 |
11.9–406 |
27 |
| Black carbon (mg g−1) |
1.43 |
1.13 |
0.2–5.1 |
27 |
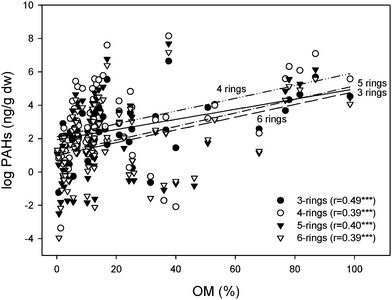 |
| | Fig. 4 Regression of PAHs grouped by the number of rings versus soil organic matter (SOM). Note: PAH data were natural log transformed. N = 80. | |
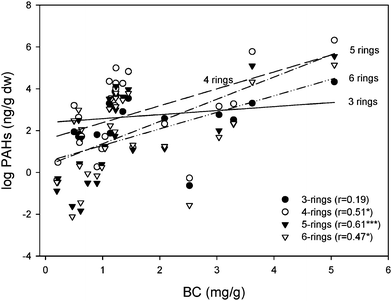 |
| | Fig. 5 Regression of PAHs grouped by the number of ring versus black carbon (BC). Note: PAH data were natural log transformed. N = 27. | |
Acknowledgements
We are grateful to the UK Department of the Environment, Food and Rural Affairs (Defra) and the European Union AQUATERRA project for funding research on Persistent Organic Pollutants at Lancaster University. J. J. Nam acknowledges the Civil Service Commission (Korea) for financial support to perform research as a visiting scientist in Lancaster University.
References
- J. J. Nam, B. H. Song, K. C. Eom, S. H. Lee and A. Smith, Chemosphere, 2003, 50, 1281–1289 CrossRef CAS.
- T. D. Bucheli, F. Blum, A. Desaules and O. Gustafsson, Chemosphere, 2004, 56, 1061–1076 CrossRef CAS.
- W. Wilcke and W. Amelung, Soil Sci. Soc. Am. J., 2000, 64, 2140–2148 CAS.
- E. Aamot, E. Steinnes and R. Schmid, Environ. Pollut., 1996, 92, 275–280 CrossRef CAS.
- W. Wilcke, Geoderma, 2007, 141, 157–166 CrossRef CAS.
- J. Dachs and S. J. Eisenreich, Environ. Sci. Technol., 2000, 34, 3690–3697 CrossRef CAS.
- G. Cornelissen, G. D. Breedveld, S. Kalaitzidis, K. Christanis, A. Kibsgaard and A. M. P. Oen, Environ. Sci. Technol., 2006, 40, 1197–1203 CrossRef CAS.
-
O. Gustafsson and P. M. Gschwend, in Molecular Markers in Environmental Geochemistry, ed. R. P. Eganhouse, ACS Symposium Series, 1997, vol. 671, pp. 365–381 Search PubMed.
- S. N. Meijer, W. A. Ockenden, A. J. Sweetman, K. Breivik, J. O. Grimalt and K. C. Jones, Environ. Sci. Technol., 2003, 37, 667–672 CrossRef CAS.
- O. Gustafsson, F. Haghseta, C. Chan, J. Macfarlaneand and P. M. Gschwend, Environ. Sci. Technol., 1997, 31, 203–209 CrossRef CAS.
- O. Gustafsson, T. D. Bucheli, Z. Kukulska, M. Andersson, C. Largeau, J. N. Rouzaud, C. M. Reddy and T. I. Eglinton, Global Biogeochem. Cycles., 2001, 15, 881–890 CrossRef CAS.
- S. N. Meijer, E. Steinnes, W. A. Ockenden and K. C. Jones, Environ. Sci. Technol., 2002, 36, 2146–2153 CrossRef CAS.
- W. D. Hafner, D. L. Carlson and R. A. Hites, Environ. Sci. Technol., 2005, 39, 7374–7379 CrossRef CAS.
- Center for International Earth Science Information Network (CIESIN), Columbia University, United Nations Food and Agriculture Programme (FAO) and Centro Internacional de Agricultura Tropical (CIAT), 2005, Gridded Population of the World: Future Estimates (GPWFE). Palisades, NY: Socioeconomic Data and Applications Center (SEDAC), Columbia University. http://sedac.ciesin.columbia.edu/gpw.
- Y. Zhang, S. Tao, J. Cao and R. M. Coveney, Environ. Sci. Technol., 2007, 41, 683–687 CrossRef CAS.
- S. R. Wild and K. C. Jones, Environ. Pollut., 1995, 88, 91–108 CrossRef CAS.
- A. J. Sweetman, M. Dalle Valle, K. Prevedouros and K. C. Jones, Chemosphere, 2005, 60, 959–972 CrossRef CAS.
- J. J. Nam, G. O. Thomas, F. M. Jaward, E. Steinnes, O. Gustafsson and K. C. Jones, Chemosphere, 2008, 70, 1596–1602 CrossRef CAS.
- J. A. González-Pérez, F. J. González-Vila, G. Almendros and H. Kinicker, Environ. Int., 2004, 30, 855–870 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here. 




