Rapid new methods for paint collection and lead extraction
William F.
Gutknecht
*a,
Sharon L.
Harper
b,
Wayne
Winstead
a,
Kristen
Sorrell
a,
David A.
Binstock
a,
Cynthia A.
Salmons
a,
Curtis
Haas
a,
Michelle
McCombs
a,
William
Studabaker
a,
Constance V.
Wall
a and
Curtis
Moore
a
aRTI International, P.O. Box 12194, Research Triangle Park, NC 27709, USA. E-mail: wfg@rti.org; Fax: +91-541- 5929; Tel: +919-541-6883
bU.S. Environmental Protection Agency, National Exposure Research Laboratory, U.S. EPA Mail Room, Mail Code D205-05, Research Triangle Park, NC 27711, USA. E-mail: harper.sharon@epa.gov; Fax: +919-541-3527; Tel: +919-541-2443
First published on 14th November 2008
Abstract
Chronic exposure of children to lead can result in permanent physiological impairment. In adults, it can cause irritability, poor muscle coordination, and nerve damage to the sense organs and nerves controlling the body. Surfaces coated with lead-containing paints are potential sources of exposure to lead. In April 2008, the U.S. Environmental Protection Agency (EPA) finalized new requirements that would reduce exposure to lead hazards created by renovation, repair, and painting activities, which disturb lead-based paint. On-site, inexpensive identification of lead-based paint is required. Two steps have been taken to meet this challenge. First, this paper presents a new, highly efficient method for paint collection that is based on the use of a modified wood drill bit. Second, this paper presents a novel, one-step approach for quantitatively grinding and extracting lead from paint samples for subsequent lead determination. This latter method is based on the use of a high-revolutions per minute rotor with stator to break up the paint into approximately 50 micron-size particles. Nitric acid (25%, v/v) is used to extract the lead in <3 minutes. Recoveries are consistently >95% for real-world paints, National Institute of Standards and Technology's standard reference materials, and audit samples from the American Industrial Hygiene Association's Environmental Lead Proficiency Analytical Testing Program. This quantitative extraction procedure, when paired with quantitative paint sample collection and lead determination, may enable the development of a lead paint test kit that will meet the specifications of the final EPA rule.
Introduction
Lead-based paint is a major source of lead poisoning in children, and it can also affect adults. In children, lead poisoning can cause irreversible brain damage and can impair mental functioning. It can retard mental and physical development and reduce attention span. In adults, it can cause irritability, poor muscle coordination, and nerve damage to the sense organs and nerves controlling the body. In 2002, approximately 38 million houses in the United States still contained leaded paint.1 The lead-containing paint must be removed or controlled by some approved means if lead is present at or above the Federal Action Levels (Title X of the Housing & Community Development Act, 1992), which are 0.5% lead by weight or 1.0 mg lead/cm2.2Environmental Protection Agency (EPA) has finalized a new rule, the Lead: Renovation, Repair, and Painting Program3 (RRP), requiring that those involved in renovation and repair take special safety measures during their work if building structures being worked on contain lead above the Federal Action Level of 1.0 mg lead/cm2 or 0.5% lead by weight. The EPA rule also requires that the lead concentration be determined with no more than 5% false negative results at levels greater than or equal to the Federal Action Level and no more than 10% false positive results at levels less than the Federal Action Level.3 New analytical procedures that would meet this need must also be inexpensive, take less than an hour per sample, and be easy to perform. One field measurement method that does have the potential to meet the requirements is anodic stripping voltammetry (ASV),4–7 but it is moderately complicated and expensive. Additionally, ASV results show a negative bias due to paint matrix effects for some paint samples.8
The variation in paint properties can influence testing accuracy and precision. Lead paint found in older dwellings might be multilayered with the lead-containing layers usually at the bottom of the stack, which will be closest to the substrate. The multilayered paints might be 1/8-inch thick or more and a mix of oil-based and water-based paints. The amount of lead in paint that is available for an in-place chemical reaction will depend upon the following:
• The amount of lead exposed, which depends on the type of cut or lack thereof into the paint
• The extractability of the lead, which depends on the age of the leaded paint and its composition
• The chemical form of the lead pigment and the time allowed for the lead extraction.
The only known instrumental methods for measuring lead in paint in place are field-portable X-ray fluorescence (FPXRF)9–12 and portable laser-induced breakdown spectroscopy (LIBS) spectrometers.13 The accuracy of FPXRF is dependent upon the thickness of the old paints, and one cannot be assured of meeting the requirements described previously.10 Furthermore, FPXRF instrumentation is complicated, relatively expensive, and will represent a significant financial investment for a small repair and renovation company. The same can be said for LIBS, which will most likely be difficult to use for routine lead testing. In light of these limitations, there is still a need for a new, simple, inexpensive method for analysis that meets the analytical requirements of the RRP. Most likely, a test kit that meets this need will be based on a quantitative process, for example, electrochemical reduction/oxidation (ASV), complexation (colorimetry) or precipitation (gravimetry or turbidimetry); this means solubilization of the lead in the paint. The application of such a kit first requires removing the paint from the substrate, then grinding/homogenizing it, and finally solubilizing the lead. This paper describes our attempts at developing procedures for these three tasks that are consistent in peformance with the goals of the RRP.
This paper first describes a new method for collecting paint from surfaces that yields fine paint particles well suited for extraction. It should be noted that a standard method, ASTM E 1729-05, is already available for the collection of paint samples.14 This method is well suited for collection of paint samples of known area and is especially suited for collection of paint from hard surfaces, such as metal and masonry (see further details in following section). It does not, however, result in paint ground directly into a powder, as does the new method described below. Second, this paper describes a new, rapid, and relatively inexpensive procedure for grinding the paint samples removed from the substrate and simultaneously extracting the lead from the paint in only one step in preparation for quantitative analysis.
Experimental
Development of a method for paint collection
Collecting paint from surfaces can pose a variety of challenges.8,15 For instance, the surface can be vertical or horizontal; the paint can be thick (sometimes more than 3 mm) or thin; the paint can be brittle or rubbery, or a combination of the two in different layers; and the paint can be on a variety of substrates that affect the ease of removing the paint. Substrates can be wood (which can vary from hard to soft and partially decayed), wall board or plaster, masonry, or metal. The goal of this work was to quantitatively collect a “chip” of paint of known area. Several different tools for removing paint from substrate, including a wood-carving knife, a steel chisel, a wood-burning tool, and a hot air gun, were tested for ease of use and efficiency using a variety of old painted boards.Of the removal methods evaluated, the easiest to use is ASTM E 1729-05, which describes the use of the combination of a hot air gun and a steel chisel.14 The hot air gun is used to soften the paint, and the chisel is pushed under the paint and the sample removed without removing any significant amount of substrate; the paint is circumscribed using the chisel before or after heating with the hot air gun. The resultant samples are whole or broken pieces of paint. Concurrent research (see the next section, Development of a method for grinding paint samples) indicated the need for crushed/ground paints to improve the lead extraction efficiency, so a method for crushing/grinding the whole pieces of paint was needed. Procedures are available for manually grinding paint chips in the field. One of these procedures is to cool the paint sample, its container, and a glass rod with dry ice for several minutes and then crush the paint with the rod; this cooling makes the paint brittle and relatively easy to crush into small particles.8 The method does require 5–15 minutes of grinding for each sample. As an improvement to these procedures, a drill-based paint collection procedure was developed that includes simultaneously grinding the paint as it is being collected. The new drill-based collection procedure consists of using a 1/2-inch (1.27 cm) diameter wood drill bit that has had its centering pin ground down to a size only large enough to hold the bit in place (see Fig. 1). The bit was used in a variable speed drill at approximately 200 revolutions per minute (rpm), which provided control so that paint could be collected with little to no substrate while minimizing any paint chip scatter. When sampling was performed on a vertical surface, a throw-away rectangular paper funnel normally used for putting oil in an engine (see Fig. 2) was taped below the sampling point. Notches were made at opposite sides of the top of the funnel for placement of the drill bit. The funnel can be held in place manually, but handling is easier if the funnel is taped to the vertical surface along the edges of the cut-out. As drilling is performed, the paint particles fall through the funnel into the collection/extraction tube held or taped to the bottom of the funnel. When the surface is horizontal, the paint particles are brushed onto a slick analytical weighing paper or sticky note, and then transferred into the test tube. When hard surfaces, such as metal or masonry are sampled, a small pilot hole may be needed for placement of the wood bit centering pin, though the hot air gun and chisel method (ASTM E 1729-05) is more likely to be used in this situation to avoid architectural damage. If whole pieces of paint are collected, they will need to be crushed into small pieces before being finely ground and extracted following the procedure described in the next section of this paper.
 | ||
| Fig. 1 Drill bits with reduced and standard centering pins. | ||
 | ||
| Fig. 2 Paper funnel attached to a wall for collecting drilled-out paint samples. | ||
Recovery efficiency experiments were conducted involving spiking the funnel and horizontal surfaces with known amounts of powdered paint. Replicate samples of approximately 0.1 g, 0.2 g, and 0.3 g were used, and all tests for both vertical and horizontal collection methods showed recoveries >99%. As noted later in this paper, this latter method proved successful for collecting paint from National Institute of Standards and Technology (NIST) Paint on Fiberboard, Reference Material (RM) 8680.16
Development of a method for grinding paint samples
Grinding paint to achieve high extraction efficiency has long been a challenge. In the past, methods used have included mechanical grinders, mortar and pestle, and cryogenic pulverizers.17,18 For the sake of simplicity, it would seem that the mortar and pestle would be easiest to use with small paint samples, typically 1 cm2. The problem is that variable amounts of any small paint sample will be left behind on the surfaces of the mortar and pestle. This can amount to 20% of the sample.17,18 Also, cleaning the mortar and pestle is a tedious job and cannot be easily performed in the field. It should be noted that it was these problems with mortar and pestle grinding that led to the development of the cryogenic grinding method using dry ice.19,20The authors of this paper developed a new idea for grinding paint, which is to use the type of tissue homogenizer employed in the biological/medical research field. The primary component of this device consists of two concentric plastic tubes; the inner tube (rotor) has slats at the end and is rotated at high speed (15,000 rpm–30,000 rpm) while the outer tube (stator), which also has slats at the end, remains stationary (see Fig. 3). The second component is a high-speed motor to spin the rotor. The rotor/stator configuration is common in high-shear homogenizers and mixers. This high-shear process is at the heart of the grinding efficiency of this approach. The sample, which should already be in small pieces, is placed in a liquid medium, such as water. When the system is operated using a high-speed motor (10,000 rpm–30,000 rpm), the sample pieces are drawn up into the center of the rotor and then spun out with great force. The small pieces are caught between the rotating and static “blades” and are cut into smaller pieces. With continued grinding, the pieces become smaller and smaller.
 | ||
| Fig. 3 Photograph of rotor and stator separated and combined as normally used (rotor/stator probe shown manufactured by Omni International, Marietta, GA). | ||
One brand of tissue homogenizer found in several different supply catalogues is the Omni model TH homogenizer/grinder (Omni International, Marietta, GA), and one of these was purchased. First to be tested was the capability of this tissue homogenzier to grind paint chips. A quick test with a paint chip in a 50-mL plastic test tube indicated that grinding dry chips with the Omni tissue homogenizer would generate powdered paint, so subsequent tests were performed with chips placed in several milliliters of water in the test tube. The grinder transformed paint chips from diameters of approximately 0.3 cm–0.5 cm down to fine particles, although some larger particles (approximately 2 mm) remained after 1 minute of constant grinding. This paint chip material and those paint materials used in subsequent tests were taken from RTI's Environmental Lead Proficiency Analytical Testing (ELPAT) repository.21 This program sponsored by the American Industrial Hygiene Asociation (AIHA) is a component of EPA's Environmental Lead Laboratory Accreditation Program (ELLAP) that involves sending real-world paint, soil, and dust samples to more than 200 laboratories for performance testing.
In the next set of experiments, researchers used the Omni grinder with various amounts of water in the 50-mL plastic centrifuge tube. Approximately 2 mL of water was found (visually) to yield the maximum number of small particles. Though many particles were less than approximately 50 µm in diameter, as determined with a light microscope, a number were still greater than approximately 200 µm in diameter. Noting that a coffee grinder yields finer particles when it is stopped and started several times, the mode of grinding the paint was also varied. It was determined that stopping and starting the grinder yielded the greatest grinding efficiency. Though not totally optimized, it was determined that 30 seconds on, followed by 15 seconds off, and then repeating this cycle four times yielded the largest number of small particles. It appeared that during the off time, the smaller particles settled to the bottom of the tube first, and then larger particles settled on top of these. Then, when the grinder was restarted, the large particles were preferentially pulled into the rotor/stator probe.
The size of the tube was also varied. It was determined that a 15-mL conical plastic centrifuge tube yielded superior samples when compared to the 50-mL plastic centrifuge tube. With the 15-mL tube, the vast majority of the particles were found microscopically to be <50 µm (see Fig. 4) when the stop and start method was used.
 | ||
| Fig. 4 Micrograph of paint particles resulting from grinding using a rotor/stator system. | ||
Lead extraction/solubilization
The next step to analysis was lead extraction/solubilization. Standard ASTM methods are available for this task, including ultrasonic extraction22 and hot plate or microwave digestors,23 as well as EPA standard operating procedures.24,25 Although these procedures yield quantitative recovery (>90%) of the lead from paint, they require additional apparatus and handling of the samples. Therefore, with the rotor/stator system producing fine paint particles, the water was replaced with 10% (v/v) nitric acid (HNO3) to determine if grinding and extraction could be performed in one step. To properly challenge this potential new method, it was considered essential that real-world paint materials be used in the testing. Only real-world paints, with their variability in lead pigments, base matrix (oil or water), number and thickness of layers, and presence of other metals, could reveal the universality of the method. It would be highly preferable to use RMs, but their availability is very limited. A first choice is the powdered paints available from NIST; included are NIST Standard Reference Materials (SRMs) 2580 at nominally 4% lead,26 2581 at nominally 0.5%,27 and 2582 at nominally 200 mg/kg.28 A second choice for a test material is the powdered ELPAT paint samples, which have lead concentration values based on the consensus of multiple laboratory analyses. The limitation of both the NIST and ELPAT RMs is that they are powdered and therefore do not challenge the paint grinding performance of the tissue homogenizer. NIST does offer one intact real-world RM, NIST RM 8680.16 Each sample is one section of fiberboard from an old house; the value for each piece has been determined by NIST, and the values range from approximately 1.2 mg lead/cm2–1.9 mg lead/cm2. Though the true values would not be known, real-world paint chips offer a range of concentrations and physical/chemical composition; the limitation is that the performance assessment of the rotor/stator-based grinding/extraction method must be based on aggressive analysis of residue remaining after applying the method.In the first experiment, 10% (v/v) HNO3 was tested using two real-world paints. The paint chips for these two materials were relatively thin and brittle; they were crushed by hand before the extraction. Extraction efficiency was determined by using inductively coupled plasma atomic emission spectroscopy (ICP-AES)24,29 to separately analyze the extract solution and any remaining residue; these two phases were separated using centrifugation. The microwave/aqua regia digestion method, which is used for preparing ELPAT paint materials,21 was used to digest the solid residue remaining in the 15-mL centrifuge tube following extraction and centrifugation of the rotor/stator paint extract. The recovery in the extract, which was calculated as the amount measured in the extract divided by the total of the amounts in the extract and the residue, was found to be in the range of 92%–96%.
An added test was to use a standard electric drill running at approximately 2,000 rpm to perform the grinding using the Omni rotor/stator. The recovery with the drill was only approximately 70% and more variable than that achieved with the Omni homogenizer/grinder at 30,000 rpm. Another set of extractions was performed with the Omni homogenizer/grinder in 25% (v/v) HNO3 to see if recovery could be improved. Using 25% HNO3 raised the recovery to above 95%, and the repeatability was excellent. Total grinding times of 2, 3, and 4 minutes were used to determine the minimum time needed to achieve above 95% recovery: each of these yielded recoveries above this value. As noted earlier, 2 minutes of total grinding time (in cycles of 30 seconds on, 15 seconds off) appeared sufficient to achieve this goal. However, 3 minutes total grinding time was used in subsequent work to provide a high margin of confidence.
Because the Omni motor is costly, and the relatively inexpensive electric drill did not yield good extraction recovery, another search for other grinding motors was made. It was determined that a Dremel tool, though having high speeds, did not have drill chucks large enough to hold the Omni-produced rotor/stator probe. The search revealed the availability of a grinding and cutting tool called the RotoZip, which is manufactured by Robert Bosch Tool Corp. in Stuttgart, Germany. This tool spins to 30,000 rpm, has a sufficiently large chuck for the Omni rotor, and costs significantly less that the Omni drive motor. The only change that was made was that part of the upper portion of the stator had to be cut off so that the rotor and stator could be properly aligned. Two paint chip materials from the same repository sources used in earlier experiments were crushed by hand and then extracted using the RotoZip to power the rotor/stator. For these experiments, rotation speed and grinding time were varied. The samples (extracts and residues) were analyzed, and the results are presented in Table 1. This tool yielded a recovery in excess of 96% for all samples, except for one, for rotation speeds of 15,000 rpm–30,000 rpm and total grinding times of 3 or 4 minutes. Thus, the sample recovery was excellent. The RotoZip electric tool was further tested with different paints using both 10% and 25% HNO3 to determine if the weaker acid provided acceptable recovery with this power source. The results of this test are shown in Table 2. Recoveries of <90% using 10% HNO3 with one of the paints that was considered the easiest to grind was a surprise; the reasons for this outcome remain unknown. Results showed that the 10% HNO3 yielded >90% with all other samples; most values were at approximately 95%. The 25% HNO3 always yielded >95% recovery. As a quality control measure, NIST SRM 2580 powdered paint was placed in the 15-mL centrifuge tube and treated just like the paint chips. Results showed that the recoveries were 94%–95% for this SRM; these values imply that the values for the “unknown” paint chips are essentially 100% recovery.
| RTI Paint Repository Material Identifier | Rotation Speed (rpm) | Grinding Time, (Minutes) with 30 Seconds on, 15 Seconds off | Sample Mass (mg) | Liquid Extract, Lead (µg) | Solid Residue Extract, Lead (µg) | Lead Recovery in Liquid Extract (%) |
|---|---|---|---|---|---|---|
| 3 | 30,000 | 4:00 | 53 | 166 | 4.99 | 97.1 |
| 3 | 30,000 | 3:00 | 47 | 133 | 5.00 | 96.4 |
| 3 | 25,000 | 4:00 | 56 | 172 | 4.52 | 97.4 |
| 3 | 25,000 | 3:00 | 58 | 181 | 6.08 | 96.8 |
| 3 | 15,000 | 4:00 | 55 | 172 | 5.66 | 96.8 |
| 3 | 15,000 | 3:00 | 48 | 147 | 4.56 | 97.0 |
| 4 | 30,000 | 4:00 | 72 | 599 | 23.6 | 96.2 |
| 4 | 30,000 | 3:00 | 68 | 402 | 15.7 | 96.2 |
| 4 | 25,000 | 4:00 | 63 | 284 | 11.0 | 96.3 |
| 4 | 25,000 | 3:00 | 89 | 430 | 18.3 | 95.9 |
| 4 | 15,000 | 4:00 | 71 | 405 | 15.4 | 96.3 |
| 4 | 15,000 | 3:00 | 56 | 415 | 15.8 | 96.3 |
| RTI Paint Repository Material Identifier | Preparation of Chips Before Grinding | HNO3 Conc. (%) | Grinding Time (Minutes) with 30 Seconds On, 15 Seconds Off | Sample Mass (mg) | Liquid Extract, Lead (µg) | Solid Residue Extract, Lead (µg) | Lead Recovery in Liquid Extract (%) |
|---|---|---|---|---|---|---|---|
| a NIST SRM 2580, nominally 4.34% in lead.26 | |||||||
| 3 | Chips already broken | 10 | 3 | 63 | 167 | 19.6 | 89.5 |
| 3 | 10 | 3 | 60 | 149 | 19.9 | 88.2 | |
| 3 | 25 | 3 | 59 | 173 | 5.01 | 97.2 | |
| 3 | 25 | 3 | 59 | 179 | 5.38 | 97.1 | |
| 4 | Chips crushed by hand in weighing paper | 10 | 3 | 77 | 332 | 19.3 | 94.5 |
| 4 | 10 | 3 | 85 | 272 | 24.0 | 91.9 | |
| 4 | 25 | 3 | 57 | 325 | 14.5 | 95.7 | |
| 4 | 25 | 3 | 69 | 392 | 16.8 | 95.9 | |
| 5 | Chips cut on glass with a scalpel; some brittle some pliable | 10 | 3 | 25 | 159 | 4.67 | 97.2 |
| 5 | 10 | 3 | 82 | 766 | 34.9 | 95.6 | |
| 5 | 25 | 3 | 35 | 211 | 4.67 | 97.8 | |
| 5 | 25 | 3 | 12.5 | 585 | 19.2 | 96.8 | |
| P926 (ELPAT) | Chips crushed by hand in weighing paper | 10 | 3 | 37 | 52.4 | 1.39 | 97.4 |
| P926 (ELPAT) | 10 | 3 | 40 | 48.6 | 1.47 | 97.1 | |
| P926 (ELPAT) | 25 | 3 | 65 | 72.5 | 1.65 | 97.8 | |
| P926 (ELPAT) | 25 | 3 | 70 | 104 | 2.72 | 97.5 | |
| NIST SRM 2580a | NIST SRM already in powdered form | 10 | 3 | 50 | 1,860 | 94.4 | 95.2 |
| NIST SRM 2580a | 10 | 3 | 67 | 2,420 | 154 | 94.0 | |
| NIST SRM 2580a | 25 | 3 | 58 | 1,870 | 74.5 | 96.2 | |
| NIST SRM 2580a | 25 | 3 | 63 | 2,580 | 84.1 | 96.8 |
The potential users of the method might have some hesitancy to use a kit that includes “strong acid”. An alternative considered was acetic acid (i.e., strong vinegar). Therefore, tests were made with 10% and 25% acetic acid using the RotoZip tool at varying speeds. The recoveries with two different paints and the two acid strengths averaged approximately 50% (data not shown). To further address this issue, an experiment was conducted to determine the minimum concentration of HNO3 that yielded 95% recovery or better. Four different paints were tested using 10%, 15%, 20%, and 25% HNO3; the results are presented in Table 3. As noted, the 10% and 15% both yielded <95% recovery. The 20% HNO3 yielded an average recovery of 95.7 ± 2.4%, and the 25% HNO3 yielded an average recovery of 97.0 ± 1.3%. Thus, the 25% HNO3 continued to be the extractant of choice.
| Paint Material Identifiers | Sample Weight Range (mg) | HNO3 Conc. (%, v/v) | Lead Recovery in Liquid Extract, (%), n = 4 |
|---|---|---|---|
| Field Paint Chip Samples | 55–87 | 10 | 84.2 ± 4.8 (5.7%) |
| 50–82 | 15 | 90.8 ± 1.9 (2.1%) | |
| 75–93 | 20 | 94.1 ± 1.3 (1.4%) | |
| 51–65 | 25 | 96.1 ± 1.1 (1.1%) | |
| ELPAT Samples | 57–68 | 10 | 95.8 ± 2.4 (2.5%) |
| 42–112 | 15 | 95.8 ± 4.6 (4.8%) | |
| 44–91 | 20 | 97.4 ± 2.1 (2.2%) | |
| 61–91 | 25 | 97.9 ± 0.7 (0.7%) |
At this point, it appeared that the optimum procedure for sample preparation was using the Omni rotor/stator probe with the RotoZip electric tool at 15,000 rpm–25,000 rpm for 3 minutes total grinding time (in cycles of 30 seconds on, 15 seconds off) using 2 mL of 25% (v/v) HNO3.
Several thick paint chip samples were acquired from the ELPAT repository to further challenge the new method. Some that were approximately 1 cm2 weighed an average of approximately 0.25 g compared to the light chips tested earlier, which weighed approximately 0.05 g. When rotor/stator grinding/extraction was performed on these paints, a layer of oily, sticky mass sometimes formed, along with the loose particles; the reason for the gooey material formation remains unknown. One could speculate that this material is a result of the breakdown of the linseed soil and/or other binders used in the manufacturing of paint. This mass tended to clog the rotor/stator. It was first thought that these relatively large amounts of paint may be too much for the 2 mL of 25% HNO3. Therefore, experiments were performed to determine if more acid would eliminate the formation of the gooey material; these included using more 25% HNO3 and smaller paint samples. These changes did not alleviate the problem, and the gooey material still formed. It was thought that although this gooey material does form, the majority of the lead has still dissolved and is in the liquid phase, which is separate from the gooey material and loose particles. To check this, several paint samples that form gooey materials were ground/extracted using the rotor/stator method. Because the material sticks to the rotor/stator, the gooey material from each sample was carefully washed from the rotor/stator using a small brush and soap and water, which break up and apparently dissolve the goo. The wash water was quantitatively collected in a beaker. The solutions that were collected in this manner were then digested using microwave/aqua regia digestion and analyzed for lead by ICP-AES. The results of these tests are shown in Table 4. As noted, the recovery was still excellent, being above 95% for these samples, and it is apparent that the material does not negatively affect the recovery of these real-world paints. A simplified cleaning method for the rotor/stator has been developed to remove the gooey material and prepare the rotor/stator for reuse: the rotor/stator assembly is dipped into an ∼8-cm deep bath of a 2% trisodium phosphate, phosphate-free soap solution (Savogran Co., Norwood, MA), and the rotor is operated at 30,000 rpm for 1 minute, then rinsed in water.
As final tests of this new method, available RMs were analyzed. Included were six samples of certified NIST RM 8680 Paint on Fiberboard16 (actually collected by RTI for EPA/NIST in the 1990s) and four standard reference paint films prepared by RTI for EPA that consist of uniform sheets of lead nitrate-spiked, water-based paint characterized by sampling and acid extraction/ICP-AES measurement.30,31 The paint samples were removed from the fiberboard pieces using the modified drill bit method described earlier in this paper, and these samples were then ground/extracted using the rotor/stator method. The RTI reference paint films were rubbery when made, but they became brittle over approximately 10 years of storage. When these “imbrittled” samples were put through the rotor/stator process, well-ground samples were produced. These results are shown in Table 5, along with results for several ELPAT materials and samples of NIST SRM 2581 powdered paint.27 The average recovery always exceeded 95%. Residue analysis was also performed with the RTI-prepared paint films, and the recovery based on this analysis was 97.3 ± 0.53 (0.54%).
| NIST Fiber Board, RM 8680 | ICP-AES Lead Conc. (mg/cm2) | NIST Value (mg/cm2) | Recovery Based on Expected Value (%) |
|---|---|---|---|
| KB2 | 1.20 ± 0.12 | 1.25 ± 0.35 | 96.0 |
| TD5 | 1.48 ± 0.05 | 1.21 ± 0.38 | 122 |
| DG2 | 1.13 ± 0.07 | 1.14 ± 0.32 | 99.1 |
| HA3 | 1.28 ± 0.36 | 1.31 ± 0.34 | 97.7 |
| MD2 | 1.09 ± 0.09 | 1.10 ± 0.30 | 99.1 |
| JH1 | 1.57 ± 0.09 | 1.29 ± 0.40 | 122 |
| Avg. 106 ± 12 (11%) |
| RTI-Made Reference Film | ICP-AES Lead Conc. (mg/cm2) | Expected Value, Lead (mg/cm2) | Recovery Based on Expected Value (%) |
|---|---|---|---|
| RTI-49-A1-B | 0.36 | 0.30 | 120 |
| RTI-49-A1-B | 0.36 | 0.30 | 120 |
| RTI-35-A6-T | 0.57 | 0.60 | 95.0 |
| RTI-35-A6-T | 0.52 | 0.60 | 86.7 |
| Avg. 105 ± 17 (16%) |
| Reference Materials | ICP-AES Lead Conc. (%) | Expected Value, Lead (%) | Recovery Based on Expected Value (%) |
|---|---|---|---|
| ELPAT 51P1 | 2.18 | 2.22 ± 0.13 (5.9%) | 98.2 |
| ELPAT 51P2 | 1.47 | 1.51 ± 0.105 (7.3%) | 101 |
| ELPAT 39P3 | 0.53 | 0.558 ± 0.039 (7.0%) | 95.0 |
| ELPAT 40P2 | 0.50 | 0.506 ± 0.032 (6.3%) | 98.8 |
| ELPAT 51P3 | 0.48 | 0.461 ± 0.035 (7.6%) | 104 |
| SRM 2581 | 0.43 | 0.449 ± 0.011 | 95.6 |
| SRM 2581 | 0.44 | 0.449 ± 0.011 | 98.0 |
| Avg. 99.5 ± 4.7 (4.7%) |
Conclusions
Collecting the paint samples from wood and fiber wallboard substrates by using the modified drill bit method reduces the time, labor, and potential lead exposure from hand crushing or grinding, and it reduces the potential loss of sample before using the new high-rpm rotor with the stator extraction step. For metal and masonry substrates, a chisel-based collection procedure works best. Pieces of paint collected by this method will need to be broken into smaller pieces with a blade or by hand crushing, so that they will fit into the rotor/stator grinder tube. On the basis of all of the tests performed by using both real-world field samples and all RMs, it appears clear that the rotor/stator method of grinding and extracting lead in paint consistently leads to >95% recovery of lead in real-world paints. As shown in Fig. 5, there is very good agreement between expected lead values and values determined with the new method for a variety of sample types. The method is fast, taking approximately 10 minutes per sample. The plastic rotor/stator units cost approximately $12 each, and the high-speed grinder motor costs approximately $100 (based on 2008 costs). The rotor/stator units and the test tubes can be cleaned for reuse. The next challenges of this work are to evaluate paint collection from plaster substrates using the new method described in this paper, so that it can be used in the field by renovation and painting staff. The principle challenges will be to develop apparatus and procedures that will allow for the safe handling of a small amount of HNO3 needed for this procedure and any follow-on procedure to measure the dissolved lead.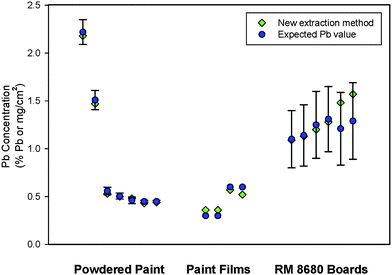 | ||
| Fig. 5 Summary of performance of new extraction method with various paint sources. | ||
Disclaimer
This work has been reviewed in accordance with EPA's peer and administrative review process and approved for presentation and publication. It has been subjected to the EPA's peer and administrative review. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.Acknowledgements
This research was funded by the EPA under EPA Contract Number EP-D-05-065 to Alion Science and Technology, Inc., and RTI Subcontract Number SUB1174861RB. Special acknowledgement is given to Dr. Hunter Daughtrey of Alion Science and Technology for his support of this effort and careful review of this document.References
- D. E. Jacobs, R. P. Clickner, J. Y. Zhou, S. M. Viet, D. A. Marker, J. W. Rodgers, D. C. Zeldin, P. Broene and W. Friedman, Environmental Health Perspectives, 2002, 110(10), A599–A606 Search PubMed.
- Title X of the Housing & Community Development Act, Residential Lead-Based Paint Hazard Reduction Program Act of 1992, Public Law 102–550.
- U.S. EPA (Environmental Protection Agency), Lead: Renovation, Repair, and Painting Program; Final Rule, Federal Register, U.S. EPA, 40 CFR Part 745, EPA-HQ-2005-0049, April 22, 2008, pp. 21691–21769 Search PubMed.
- ASTM (American Society for Testing and Materials). 2051-01, Standard Practice for the Determination of Lead in Paint, Settled Dust, Soil, and Air Particulate by Field Portable Electroanalysis. ASTM International, West Conshohocken, PA, 2001 Search PubMed.
- K. Ashley, R. Song, C. A. Eschey, P. A. B. Schlecht and T. J. Wise, J. Environ. Monit., 1999, 1, 457–464 Search PubMed.
- ASTM (American Society for Testing and Materials). E1775-07, Standard Guide for Evaluating Performance of On-Site Extraction and Field Portable Electrochemical Analysis for Lead. ASTM International, West Conshohocken, PA, 2007 Search PubMed.
- E. E. Williams, C. C. Van Hise, and W. F. Gutknecht, Evaluation of the Performance of Reflectance and Electrochemical Technologies for the Measurement of Lead in Characterized Paints, Bulk Dusts, and Soils. EPA 600/R-95/093, U.S. Environmental Protection Agency, Research Triangle Park, NC, 1996 Search PubMed.
- H. Sussell and K. Ashley, J. Environ. Monit., 2002, 4, 156–161 RSC.
- M. E. McKnight, W. E. Byrd, W. E. Roberts, and E. S. Lagergren, Methods for Measuring Lead Concentrations in Paint Films. NIST Report NIST IR 89-4209. National Institute of Standards and Technology, 1989 Search PubMed.
- R. L. Schmehl, D. C. Cox and F. G. DeWalt, American Industrial Hygiene Association Journal, 1999, 60(4), 444–451 Search PubMed.
- E. E. Williams, C. A. Clayton, E. D. Hardison, and W. F. Gutknecht, Laboratory Evaluation of Six New/Modified Portable X-ray Fluorescence Spectrometers for the Measurement of Lead in Characterized Paint Films and Research Material Boards. EPA 600/R-97/100a,b. U.S. Environmental Protection Agency, Research Triangle Park, NC, 1999 Search PubMed.
- EPA Method 6200. Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment. U.S. Environmental Protection Agency, Washington, DC, 1997 Search PubMed.
- K. Y. Yamamoto, D. A. Cremers, L. E. Foster and M. J. Ferris, Appl. Spectrosc., 1996, 50, 222–233 CAS.
- ASTM (American Society for Testing and Materials). E 1729-05. Standard Practice for Field Collection of Dried Paint Samples for Subsequent Lead Determination. ASTM International, West Conshohocken, PA, 2005 Search PubMed.
- K. Ashley, M. Hunter, L. H. Tait, J. Dozier, J. L. Seaman and P. F. Berry, Field Analytical Chemistry and Technology, 1998, 2, 39–50 Search PubMed.
- NIST, Certificate of Analysis: Research Material 8680 Lead-Based Paint on Fiberboard, National Institute of Standards and Technology, Gaithersburg, MD, 1997 Search PubMed.
- L. L. Hodson, E. D. Hardison, W. F. Gutknecht, D. A. Binstock, M. J. Messner, and A. A. Leinbach, An Intercomparison of Grinding Techniques Used for the Preparation of Lead-in-Paint Samples, EPA 600/R-95/112, U.S. Environmental Protection Agency, Research Triangle Park, NC, 1997, Available from NTIS (NTIS #PB98-131881) Search PubMed.
- S. L. Harper and W. F. Gutknecht, J. Environ. Monit., 2001, 3, 335–340 RSC.
- U.S. Environmental Protection Agency: A Field Test of Lead-Based Paint Testing Technologies: Summary Report. (EPA 747-R-950-002a). Washington, DC: U.S. Environmental Protection Agency/Office of Prevention, Pesticides, and Toxic Substances, 1995 Search PubMed.
- U.S. Environmental Protection Agency: A Field Test of Lead-Based Paint Testing Technologies: Technical Report. (EPA 747-R-95-002b). Washington, DC: U.S. Environmental Protection Agency/Office of Prevention, Pesticides, and Toxic Substances, 1995 Search PubMed.
- ELPAT, Web site, Environmental Lead Proficiency Analytical Testing (ELPAT) Program, www.aiha.org/Content/LQAP/PT/ELPAT.htm. American Industrial Hygiene Association, Fairfax, VA, 2007, Accessed 09/21/2007 Search PubMed.
- ASTM (American Society for Testing and Materials). E 1979-04. Standard Practice for Ultrasonic Extraction of Paint, Dust, Soil, and Air Samples for Subsequent Determination of Lead. ASTM International, West Conshohocken, PA, 2005 Search PubMed.
- ASTM (American Society for Testing and Materials). E 1645-01. Standard Practices for Preparation of Dried Paint Samples by Hot Plate or Microwave Digestion for Subsequent Lead Analysis. ASTM International, West Conshohocken, PA, 2007 Search PubMed.
- D. A. Binstock, D. L. Hardison, P. M. Grohse and W. F. Gutknecht. Standard Operating Procedures for Lead in Paint by Hotplate- or Microwave-Based Acid Digestion and Atomic Absorption or Inductively Coupled Plasma Emission Spectrometry, EPA 600/8-91/213, U.S. EPA, Research Triangle Park, NC, 1991, Available from NTIS (NTIS #PB92-114172) Search PubMed.
- K. K. Luk, P. M. Grohse, L. L. Hodson, D. A. Binstock, C. C. Van Hise and W. F. Gutknecht. Standard Operating Procedure for the Field Analysis of Lead in Paint, Bulk Dust, and Soil by Ultrasonic, Acid Digestion, and Colorimetric Measurement. EPA 600/R-93/200, U.S. Environmental Protection Agency, Research Triangle Park, NC, 1993 Search PubMed.
- NIST, Certificate of Analysis: Standard Reference Materials 2580, Powdered Paint, Nominal 4% Lead, National Institute of Standards and Technology, Gaithersburg, MD, 1996 Search PubMed.
- NIST, Certificate of Analysis: Standard Reference Materials 2581, Powdered Paint, Nominal 0.5% Lead, National Institute of Standards and Technology, Gaithersburg, MD, 1997 Search PubMed.
- NIST, Certificate of Analysis: Standard Reference Materials 2582, Powdered Paint, Nominal 200 mg/Kg Lead, National Institute of Standards and Technology, Gaithersburg, MD, 1997 Search PubMed.
- ASTM (American Society for Testing and Materials). E 1613-04. Standard Test Method for Determination of Lead by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES), Flame Atomic Absorption Spectrometry (FAAS), or Graphite Furnace Atomic Absorption Spectrometry (GFAAS) Techniques. ASTM International, West Conshohocken, PA, 2004 Search PubMed.
- E. E. Williams, D. A. Binstock and W. F. Gutknecht. Preparation of Lead-Containing Paint and Dust Method Evaluation Materials and Verification of the Preparation Protocol by Round-Robin Analysis, EPA 600/R-93/235, U.S. EPA, Research Triangle Park, NC, 1993, Available from NTIS (NTIS #PB94-141165) Search PubMed.
- D. A. Binstock, E. E. Williams, J. D. Neefus and W. F. Gutknecht. Standard Operating Procedure for Preparation of Method Evaluation Materials for Lead in Paint, EPA 600/R-94/132, U.S. EPA, Research Triangle Park, NC, 1994, Available from NTIS (NTIS #PB95-174090) Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |
