Long-term stability of organic–dye-sensitized solar cells based on an alkyl-functionalized carbazole dye
Kohjiro
Hara
*,
Zhong-Sheng
Wang
,
Yan
Cui
,
Akihiro
Furube
and
Nagatoshi
Koumura
National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: k-hara@aist.go.jp; Fax: +81-29-861-4638; Tel: +81-29-861-4638
First published on 23rd July 2009
Abstract
We investigated the long-term stability of performance for dye-sensitized solar cells (DSSCs) based on an alkyl-functionalized carbazole dye (MK-2) and used in conjunction with ionic liquid-based electrolytes. We observed good long-term stability of the performance of the DSSCs during 60 days under visible-light irradiation at ca. 50 °C. The performance of the DSSC decreased gradually under white-light irradiation including UV light or at 80 °C under dark conditions. However, no decomposition or detachment of the dye molecule from the TiO2electrode was observed after these measurements. These results indicate that the MK-2dye molecule in the cell was stable even under white-light irradiation and at 80 °C under dark conditions.
Broader contextFor commercialization of dye-sensitized solar cells (DSSCs), long-term stability is one of the most important factors in addition to possessing high conversion efficiencies. Generally, however, high-performance DSSCs are not stable over extended periods of time, because volatile solvents such as acetonitrile are used in the electrolyte, leading to difficulty in sealing the solar cells. We have designed and synthesized alkyl-functionalized carbazole dyes (MK dyes) to improve both solar cell performance and long-term stability of the solar cells. We investigated long-term stability of DSSCs based on an alkyl-functionalized carbazole dye (MK-2) and ionic liquid (IL)-based electrolytes in detail. We found that DSSCs with MK-2 and IL electrolytes showed good long-term stability under continuous simulated solar-light irradiation. In addition, the MK-2 molecule itself showed good stability even under white-light irradiation including UV light or at high temperature (80 °C) under dark conditions in the solar cells. |
1. Introduction
Over the last decade, dye-sensitized solar cells (DSSCs) have attracted much attention because these unconventional solar cells exhibit high performance and have the potential for low-cost production.1–5 Solar energy-to-electricity conversion efficiencies as high as 11% under AM 1.5 G irradiation have been attained with DSSCs.6–8 In addition to possessing high conversion efficiencies, solar cells composed of DSSCs must also keep long-term stability. Generally, however, high-performance DSSCs are not stable over extended periods of time, because volatile solvents such as acetonitrile are used in the supporting electrolyte, leading to difficulty in sealing the solar cells especially at high temperatures (e.g., >80 °C). Alternatively, the long-term stability of small cells and large-area modules of DSSCs under simulated solar light at relatively lower temperatures (<60 °C) is currently being investigated.9–13 Conventional DSSCs based on Ru complexes such as cis-dithiocyanato bis(4,4′-dicarboxy-2,2′-bipyridine)ruthenium(II) (known commonly as N3 or N719 dyes) have shown good long-term stability under continuous irradiation of up to 10,000 h, especially in the absence of UV light.9,10Organic dyes have also been used instead of Ru complexes as sensitizers in DSSCs, and the photovoltaic performance of DSSCs based on organic-dye sensitizers has been improved relative to earlier studies of DSSCs with organic dyes.14–37 The long-term stability of organic dye DSSCs under visible-light irradiation has also been investigated and reported.26,33 For example, we have studied the stability of a DSSC based on a coumarin dye (NKX-2883) under continuous AM 1.5 G irradiation through a UV (<420 nm) cut-off filter (100 mW cm−2, 50–55 °C). No signs of dye degradation and no decrease in solar-cell performance are observed over a period of 1000 h.33 These results indicate that DSSCs based on organic dyes also should show sufficient long-term stability during visible-light irradiation, although the stability of cell performance under white-light irradiation including UV light and at high temperature must be investigated in further detail to confirm.
Furthermore, alkyl-functionalized Ru complexes38,39 and organic dyes15,40–44 have been designed and used in DSSCs to improve both performance and stability. In addition, we have designed and synthesized alkyl-functionalized carbazole dyes (MK dyes) to improve both solar-cell performance (especially electron lifetime and consequently open-circuit voltage) and long-term stability of the solar cells.35–37 In the present study, we investigated the long-term stability of DSSCs based on an alkyl-functionalized-carbazole dye (MK-2) and ionic-liquid-based electrolytes in detail. Here, we report good long-term stability of the performance of DSSCs based on MK-2 under continuous visible-light irradiation. In addition, the effects of UV-light irradiation and high temperature on the stability of the performance are investigated and discussed.
2. Experimental
2.1. Materials
Reagent chemicals and materials, toluene (Kanto Chemicals, dehydrated), acetonitrile (AN, Wako Pure Chemicals), tert-butanol (Tomiyama Pure Chemical Industries Ltd.), 1-methyl-3-n-propylimidazolium iodide (MPImI, Tomiyama), LiI (Tomiyama), I2 (Tomiyama), 4-tert-butylpyridine (TBP, Tomiyama), were used without further purification. The molecular structure of MK-2 is shown in Fig. 1. The detailed synthesis procedure for MK-2 is described elsewhere.35,36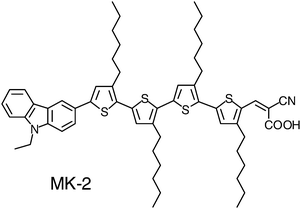 | ||
| Fig. 1 Molecular structure of MK-2. | ||
2.2. Solar-cell fabrication
Nanocrystalline TiO2 photoelectrodes were prepared by a screen-printing technique. TiO2nanoparticles and an organic TiO2 paste for screen-printing were prepared by methods reported previously.45 The TiO2 paste consisted of TiO2nanoparticles, large TiO2 particles (av. 100 nm) as scattering centers, ethyl cellulose as a binder, and α-terpineol as a solvent. The TiO2 paste was printed on a glass substrate coated with transparent conducting oxide (TCO, F-doped SnO2, Nippon Sheet Glass Co.) and subsequently sintered at 500 °C in air for 1 h. The size of the TiO2 films was 0.5 mm × 0.5 mm. The thickness of the TiO2 thin films, measured with an Alpha-Step 300 profiler (Tencor Instruments), was ca. 6 µm. MK-2 was dissolved at a concentration of 0.3 mM in toluene. The TiO2 films were immersed in dye solution and then kept at 25 °C for at least 12 h to allow the dye to adsorb to the TiO2 surface.The sealed electrochemical cell used for photovoltaic measurements consisted of a dye-sensitized TiO2electrode, a Pt-coated TCO electrode as a counter electrode, a spacer (surlyn film, 30 µm thick), and an electrolyte. The counter electrode consisted of a Pt film (ca. 200 nm thick) sputtered onto a TCO-coated glass plate. We used two electrolytes: 0.1 M LiI-0.4 M I2-0.1 M TBP in MPImI, referred to herein as electrolyte A, and 0.4 M I2 in MPImI, referred to herein as electrolyte B.
2.2. Photovoltaic measurements
The photovoltaic performance of the solar cells was measured with a source meter (Advantest, R6243). We employed an AM 1.5 G solar simulator (Wacom Co., WXS-80C-3 with a 300-W Xe lamp and an AM filter) as the light source. The incident light intensity was calibrated by using a standard solar cell composed of a crystalline silicon solar cell and an IR-cut off filter (Schott, KG-5), giving the photoresponse range of an amorphous silicon solar cell (produced and calibrated by the Japan Quality Assurance Organization). To avoid the penetration of diffuse light into the active dye-sensitized film, a black mask with an aperture area of 0.2354 cm2 was employed to measure the photovoltaic performance. The long-term stability of the performance of the DSSCs was measured with an AM 1.5 G solar simulator (Bunkoh-keiki Co., Ltd., OTENTO-SUN5) with and without a UV cut-off filter (<420 nm). Action spectra of the monochromatic incident photon-to-current conversion efficiency (IPCE) of the DSSC were measured with a CEP-99W system (Bunkoh-keiki).2.4. Transient absorption spectroscopy measurements
To observe the generation of the MK-2 cation by electron injection from the photo-excited MK-2 into the TiO2electrode in the completely fabricated DSSC, the femtosecond diffuse reflectance transient absorption technique was used. This technique allows the measurement of light scattering materials by detecting diffusely reflected probe light after the incidence of pump and probe laser beams onto the samples, instead of detecting transmitted light associated with conventional transmittance-mode transient absorption spectroscopy.46 The light source for the experiment was a regenerative amplifier system consisting of a Ti:sapphire laser (800 nm wavelength, 150 fs FWHM pulse width, 1.0 mJ pulse intensity, 1 kHz repetition, Spectra Physics, Hurricane) combined with an optical parametric amplifier (OPA; TOPAS, Quantronix). For a pump pulse, the OPA output at 532 nm with an intensity of about 200 nJ per pulse was focused on a ∼300 µm diameter area of a dye-sensitized TiO2electrode; for a probe pulse, the fundamental beam at 800 nm was focused at the center of the pumped area. The probe beam diffusely reflected from the transparent electrode side of a DSSC was collected by a lens with a 50 mm diameter and an 80 mm focal length and was detected with an InGaAs photodetector after passing through a monochromator (Acton Research, SpectraPro-150). The transient absorption intensity was evaluated from the following equation: %absorption = 100 × (1–I/I0), where I and I0 are diffuse reflected probe light intensities with and without the pump pulse, respectively.3. Results and discussion
3.1. Long-term stability under visible light irradiation
Fig. 2 shows long-term stability data of a DSSC based on MK-2 and electrolyte A under continuous AM 1.5 G irradiation through a UV (<420 nm) cut-off filter (ca. 85 mW cm−2 and at ca. 50 °C) under open-circuit conditions. No decrease in the solar-to-electricity conversion efficiency (η) was observed over a period of 60 days (Fig. 2a). In the long-term stability tests for this DSSC, the short-circuit photocurrent density, JSC, slightly decreased and the open-circuit voltage, VOC, increased with increasing time (Fig. 2b). Neither dye degradation nor detachment of dye from the TiO2electrode was observed. This result clearly indicates that the DSSC based on MK-2 was stable under visible-light irradiation at a relatively low temperature.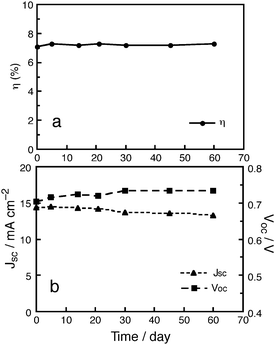 | ||
| Fig. 2 Long-term stability of a DSSC based on MK-2 with electrolyte A under visible-light irradiation: (a) (●) η and (b) (▲) JSC and (■)VOC. | ||
We reported stability of organic dyes adsorbed on nanocrystalline TiO2electrode under visible light irradiation.47 We observed good stability of MK-2, which has oligothiophene moiety, under visible light irradiation, while coumarin dyes, NKX-2311 and NKX-2587, were decomposed. The transient absorption data suggested that delocalization of holes on the oligothiophene moiety of MK-2 leads to high stability of dye cation after photoexcitation.47 This result also indicates that MK-2 and its cation are relatively stable due to its oligothiophene moiety under visible light irradiation.
3.2. Long-term stability under white-light irradiation
The normalized long-term stability data for a DSSC with MK-2 and electrolyte B under continuous white-light irradiation (100 mW cm−2), including UV light (<420 nm, ca. 15 mW cm−2), is shown in Fig. 3. The value of VOC gradually decreased from its initial value to 83% after irradiation for 45 days. The value of JSC increased for the first 10 days and then gradually decreased to 92% of its initial value after 45 days. The value of η decreased to 68% of its initial value under white-light irradiation after 45 days. | ||
| Fig. 3 Long-term stability of a DSSC based on MK-2 with electrolyte B under white-light irradiation including UV light: (●) η, (▲) JSC, and (■)VOC. | ||
Hagfeldt et al. and Ferrere et al. reported that the conduction band (ECB) of TiO2electrodes was shifted positively under UV-light irradiation, and that this shift is caused by trapping of photogenerated holes in surface states.48,49 Considering their results, we concluded that the decreased VOC (Fig. 3) was probably caused by the positive shift of ECB of the TiO2electrode under UV-light irradiation. Hagfeldt et al. and Ferrere et al. also reported that the IPCE performance of DSSCs were improved under UV-light irradiation, because the positive shift of ECB of the TiO2electrode leads to increasing electron-injection yield from the dye to the TiO2electrode.48,49 For the first 10 days of our experiments, we also observed an improved JSC for the DSSC based on MK-2 under white-light irradiation (Fig. 3a).
Fig. 4 shows absorption spectra of MK-2 adsorbed on the TiO2electrode before and after white-light irradiation with electrolyte B. Decreasing the absorption peak and broadening of the spectrum were observed after 14 days of white-light irradiation. If the dye molecules were decomposed or detached from the TiO2electrode, the absorption spectrum would decrease in whole wavelength region, as has been observed in the cases of other dyes.47 These results suggest that the MK-2 molecules were stable even under white-light irradiation including UV light in the solar cell. The change in the absorption spectrum would be caused by changing the adsorption structure of the dye molecules, as discussed later.
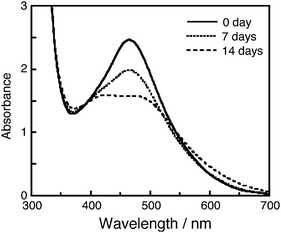 | ||
Fig. 4 The absorption spectrum of MK-2 adsorbed on TiO2electrode before and after white-light irradiation: ( ) 0 day; ( ) 0 day; ( ) after 7 days; ( ) after 7 days; ( ) after 14 days. ) after 14 days. | ||
Considering the reason why the dye was not decomposed on the TiO2electrode under white-light irradiation including UV light, we believe that holes, which are formed in the valence band (EVB) of the TiO2electrode under UV-light irradiation, reacted with iodide ions (I−) before the holes could oxidize the dye molecules on the surface. To support this conclusion, we fabricated a solar cell with an iodine redox electrolyte and an unsensitized TiO2electrode. A photocurrent generated by the band-gap excitation of the TiO2electrode under UV-light absorption was observed, suggesting that electron transfer from I− to the EVB of the TiO2electrode occurred effectively. In addition, UV-light absorption owing to the high concentration of I3− ion in the electrolytes might have stabilized the MK-2 molecules.
The IPCE spectra for DSSCs with MK-2 before and after white-light irradiation are shown in Fig. 5a and 5b for electrolytes A and B, respectively. For the DSSC with electrolyte A, we observed two different changes in the IPCE spectrum as a result of the white-light irradiation (cell 1 and 2 were fabricated with the same materials condition). For the cell 1, the IPCE performance increased and the spectrum red-shifted toward the long-wavelength region after 34 days of irradiation. For the cell 2, the IPCE performance decreased and the spectrum red-shifted after 34 days (Fig. 5a). In the case of electrolyte B, only a red-shift of the IPCE spectrum was observed after 60 days of white-light irradiation (Fig. 5b); no substantial decrease in IPCE performance was observed.
 | ||
Fig. 5 The IPCE spectra for DSSCs based on MK-2 before and after white-light irradiation. (a) electrolyte A: ( ) before; ( ) before; ( ) cell 1 after 34 days; ( ) cell 1 after 34 days; ( ) cell 2 after 34 days; (b) electrolyte B: ( ) cell 2 after 34 days; (b) electrolyte B: ( ) before; ( ) before; ( ) after 30 days; ( ) after 30 days; ( ) after 60 days. ) after 60 days. | ||
The IPCE spectra after white-light irradiation also clearly indicate that MK-2 was stable under white-light irradiation, because the absorption at longer wavelengths remained unchanged. In contrast, if the π-conjugated structure of the dye had decomposed, the absorption at longer wavelengths would have disappeared. The broadening of the absorption spectra (Fig. 4) and red-shifts of the IPCE spectra (Fig. 5) suggest that the white light including UV light changed the adsorption structure of the dye molecules on the TiO2 surface in terms of increasing π–π stacking interactions between MK-2 molecules. Similar red-shifts in IPCE spectra are also observed in DSSCs based on coumarin dyes when the number of thiophene moieties in the dye molecules increases.32 This result suggests that increased π–π stacking of coumarin dyes aroused by the increasing number of thiophene moieties in turn arouses the observed red-shift of the IPCE spectra. The increased π–π stacking of coumarin dyes causes a positive shift of the lowest unoccupied molecular orbital (LUMO) level of the dye, which leads to a decreasing electron injection yield and consequently a lower IPCE performance. Considering these results for DSSCs with coumarin dyes, we concluded that the broadening of the absorption spectrum (Fig. 4) and the red-shift of the IPCE spectra for DSSCs based on MK-2 (Fig. 5a and 5b) were probably caused by increasing π–π stacking interactions between MK-2 molecules (e.g., dimerization or aggregation), whereas the detailed mechanism is unclear.
3.3. Thermal stability of the solar cells
We investigated the long-term stability of DSSCs based on MK-2 at 80 °C under dark conditions. The efficiency of the solar cells decreased gradually while both JSC and VOC decreased with increasing time. Fig. 6a and 6b show the IPCE spectra for DSSCs with MK-2 before and after heating at 80 °C with electrolytes A and B, respectively. After heating for 14 days, the IPCE performance markedly decreased. However, no change in the color of the dye-sensitized TiO2electrode was observed after 14 days, again indicating that dye degradation or detachment of dye from the electrode did not occur. This observation indicates that MK-2 molecules were stable at 80 °C in the solar cell. Tanaka et al. have reported long-term stabilities for a DSSC based on an indoline dye (D131) having a cyanoacrylic acid moiety (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(–CN)COOH) as the acceptor, similar in structure to MK-2.50 They observed degradation of the dye caused by decarboxylation in the presence of iodine, amine or both in the electrolyte, especially at high temperature. In our study, no degradation of MK-2 caused by decarboxylation was observed. These results strongly suggest that the stability of a given dye molecule depends on the whole molecular structure of dye including the donor and linkage, not only the structure of the acceptor.
C(–CN)COOH) as the acceptor, similar in structure to MK-2.50 They observed degradation of the dye caused by decarboxylation in the presence of iodine, amine or both in the electrolyte, especially at high temperature. In our study, no degradation of MK-2 caused by decarboxylation was observed. These results strongly suggest that the stability of a given dye molecule depends on the whole molecular structure of dye including the donor and linkage, not only the structure of the acceptor.
 | ||
Fig. 6 The IPCE spectra for DSSCs based on MK-2 before and after heating at 80 °C under dark conditions. (a) electrolyte A: ( ) before; ( ) before; ( ) after 2 days; ( ) after 2 days; ( ) after 5 days; ( ) after 5 days; ( ) after 14 days; (b) electrolyte B: ( ) after 14 days; (b) electrolyte B: ( ) before; ( ) before; ( ) after 2 days; ( ) after 2 days; ( ) after 5 days; ( ) after 5 days; ( ) after 14 days. ) after 14 days. | ||
Fig. 7 shows the transient absorption profile of MK-2 cations measured directly in a cell with electrolyte A, for which the IPCE value at 500 nm after heating at 80 °C for 14 days was ca. 20% of its initial value. The transient absorption profile of a cell before heating is also shown. The pump and probe wavelengths were 532 nm and 800 nm, respectively. In Fig. 7, the absorption value after photoexcitation corresponds to the amount of dye cations formed after electron injection from the dye to the TiO2electrode, because the characteristic peak of the MK-2 cation absorption band appears around 800 nm as determined by prior measurement with our conventional transient absorption spectrometer for MK-2-sensitized TiO2 films (data not shown). The observed prompt rise and slow decay for both kinetic profiles are mostly due to fast electron transfer on a sub-picosecond time scale and to relaxation processes of the generated cation state, although the details of these processes are now under investigation and will be published elsewhere. The absorption value decreased only by about 15% after heating compared to the value observed before heating. In other words, 85% of the electron injection yield still remained after heating (Fig. 7). This result obviously indicates that electron transfer occurred effectively even when the cell was subjected to heating, whereas the IPCE performance decreased markedly. In other words, the decrease in electron injection yield from the dye to TiO2electrode, which would be caused by decomposition or detachment of the dye molecule from the TiO2electrode, was not the factor determining the decreased IPCE performance.
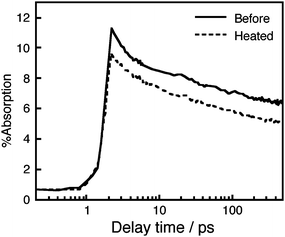 | ||
Fig. 7 Transient absorption of MK-2 cations measured using a cell containing electrolyte A. ( ) before heating and ( ) before heating and ( ) after the heating at 80 °C under dark condition for 14 days. The pump and probe wavelengths were 532 nm and 800 nm, respectively. ) after the heating at 80 °C under dark condition for 14 days. The pump and probe wavelengths were 532 nm and 800 nm, respectively. | ||
The IPCE value near 380 nm, where absorption due to I3− ion is observed, increased after heating for 2 days (Fig. 6a and 6b). This result suggests that the concentration of I3− ion in the electrolyte decreased after 2 days of heating. Then, IPCE values in the range from 400 to 600 nm, where photon absorption due to the dye is relatively strong, decreased markedly after 14 days of heating (Fig. 6a and 6b). Therefore, the concentration of I− ions, which reduce MK-2 cations to their ground state, might also have decreased in the solar cells, consequently decreasing the IPCE performance. We observed recovery of the decreased IPCE performance after keeping the cell at room temperature for several days (but not all samples). Kato et al. reported that concentration of I3− ion in the electrolyte decreased after outdoor exposure test of DSSC module.51
In order to clarify the degradation mechanism by heating, only electrolyte B was kept at 80 °C for 11 days, and then we made solar-cell devices and measured their performance. As a result, similar performance compared to those of devices with non-heating electrolyte was obtained, indicating that electrolyte B itself was not changed and not degraded at 80 °C. On the other hand, a change in the surface of the Pt counter electrode from mirror condition to black was observed after heating test. From these results, we conclude that degradation of the Pt electrode by the iodine redox electrolyte at 80 °C changed the concentration of redox ions, and consequently decreased the solar-cell performance, although the detailed reaction mechanism is unclear.
4. Conclusions
DSSCs based on MK-2, an alkyl-functionalized carbazole dye, and used in conjunction with ionic liquid-based electrolytes showed good long-term stability under continuous irradiation of visible light for 60 days, although the DSSCs' photovoltaic performance decreased gradually under white-light irradiation including UV light. The broadening of the UV-vis absorption spectrum of MK-2 adsorbed on the TiO2electrode and the red-shift of IPCE spectra of the DSSC based on MK-2 were observed after white-light irradiation, suggesting that π–π stacking interactions between dye molecules on the TiO2electrode increased. Further modification of the molecular structure of MK-2, for control of adsorption conditions of the dye on the TiO2electrode, are necessary to improve the long-term stability of the cells under white-light irradiation. The IPCE performance decreased remarkably after heating the DSSCs at 80 °C under dark conditions for 14 days, though no degradation or detachment of the dye from the electrode was observed. Transient absorption profiles indicated that the electron injection yield decreased by only 15% after heating. Degradation of the Pt electrode by the iodine redox electrolyte at 80 °C was suggested to change the concentration of redox ions, and consequently decreased the solar-cell performance.Acknowledgements
We acknowledge financial support from the Industrial Technology Research Grant Program in 2005 from the New Energy and Industrial Technology Development Organization (NEDO) of Japan. We thank Mr. Takaya Shida and Dr. Ryuzi Katoh for their experimental assistance.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–739 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- G. Smestad, C. Bignozzi and R. Argazzi, Sol. Energy Mater. Sol. Cells, 1994, 32, 259–272 CrossRef CAS.
- A. Kay and M. Grätzel, Sol. Energy. Mater. Sol. Cells, 1996, 44, 99–117 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 2000, 33, 269–277 CrossRef CAS.
- M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835–16847 CrossRef CAS.
- Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638–L640 CrossRef CAS.
- Y. Cao, Y. Bai, Q. Yu, Y. Cheng, S. Liu, D. Shi, F. Gao and P. Wang, J. Phys. Chem. C, 2009, 113, 6290–6297 CrossRef CAS.
- O. Kohle, M. Grätzel, A. F. Meyer and T. B. Meyer, Adv. Mater., 1997, 9, 904–906 CrossRef CAS.
- R. Kern, N. van der Burg, G. Chmiel, J. Ferber, G. Hasenhindl, A. Hinsch, R. Kinderman, J. Kroon, A. Meyer, T. Meyer, R. Niepmann, J. van Roosmalen, C. Schill, M. Sommeling, M. Späth and I. Uhlendorf, Opto–Electron. Rev., 2000, 8, 284–288 Search PubMed.
- M. Sommeling, M. Späth, H. J. P. Smit, N. J. Bakker and J. M. Kroon, J. Photochem. Photobiol., A, 2004, 164, 137–144 CrossRef CAS.
- T. Toyoda, T. Sano, J. Nakajima, S. Doi, S. Fukumoto, A. Ito, T. Tohyama, M. Yoshida, T. Kanagawa, T. Motohiro, T. Shiga, K. Higuchi, H. Tanaka, Y. Takeda, T. Fukano, N. Katoh, A. Takeichi, K. Takechi and M. Shiozawa, J. Photochem. Photobiol., A, 2004, 164, 203–207 CrossRef CAS.
- D. Kuang, S. Ito, B. Wenger, C. Klein, J. E. Moser, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 4146–4154 CrossRef CAS.
- T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, J. Am. Chem. Soc., 2004, 126, 12218–12219 CrossRef CAS.
- S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Péchy and M. Grätzel, Chem. Commun., 2008, 5194–5196 RSC.
- M. Matsui, K. Nagasaka, S. Tokunaga, K. Funabiki, T. Yoshida and H. Minoura, Dyes Pigm., 2003, 58, 219–226 CrossRef CAS.
- M. Matsui, A. Ito, M. Kotani, Y. Kubota, K. Funabiki, J. Jin, T. Yoshida, H. Minoura and H. Miura, Dyes Pigm., 2008, 80, 233–238.
- T. Kitamura, M. Ikeda, K. Shigaki, T. Inoue, N. A. Anderson, X. Ai, T. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806–1812 CrossRef CAS.
- H. Otaka, M. Kira, K. Yano, S. Ito, H. Mitekura, T. Kawata and F. Matsui, J. Photochem. Photobiol., A, 2004, 164, 67–73 CrossRef CAS.
- Y. Chen, C. Li, Z. Zeng, W. Wang, X. Wang and B. Zhang, Chem. Lett., 2005, 34, 762–763 CrossRef CAS.
- Velusamy, K. R. Justin Thomas, J. T. Lin, Y.-C. -Hsu and K.-C. Ho, Org. Lett., 2005, 7, 1899–1902 CrossRef CAS.
- R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt and L. Sun, Chem. Mater., 2007, 19, 4007–4015 CrossRef CAS.
- P. Qin, X. Yang, R. Chen, L. Sun, T. Marinado, T. Edvinsson, G. Boschloo and A. Hagfeldt, J. Phys. Chem. C, 2007, 111, 1853–1860 CrossRef CAS.
- D. P. Hagberg, J.-H. Yum, H. Lee, F. De Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt, M. Grätzel and Md. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259–6266 CrossRef CAS.
- S.-L. Li, K.-J. Jiang, K.-F. Shao and L.-M. Yang, Chem. Commun., 2006, 2792–2794 RSC.
- S. Kim, J. K. Lee, S. O. Kang, J. Ko, J.-H. Yum, S. Fantacci, F. De Angelis, D. Di Censo, Md. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 16701–16707 CrossRef CAS.
- X.-H. Zhang, C. Li, W.-B. Wang, X.-X. Cheng, X.-S. Wang and B.-W. Zhang, J. Mater. Chem., 2007, 17, 642–649 RSC.
- S. Hwang, J. H. Lee, C. Park, H. Lee, C. Kim, C. Park, M.-H. Lee, W. Lee, J. Park, K. Kim, N.-G. Park and C. Kim, Chem. Commun., 2007, 4887–4889 RSC.
- Y. Ooyama, Y. Shimada, Y. Kagawa, Y. Yamada, I. Imae, K. Komaguchi and Y. Harima, Tetrahedron Lett., 2007, 48, 9167–9170 CrossRef.
- M. Liang, W. Xu, F. Cai, P. Chen, B. Peng, J. Chen and Z. Li, J. Phys. Chem. C, 2007, 111, 4465–4472 CrossRef CAS.
- M. Wang, M. Xu, D. Shi, R. Li, F. Gao, G. Zhang, Z. Yi, R. Humphry-Baker, P. Wang, S. M. Zakkeeruddin and M. Grätzel, Adv. Mater., 2008, 20, 1–4.
- K. Hara, Z.-S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo and S. Suga, J. Phys. Chem. B, 2005, 109, 15476–15482 CrossRef CAS.
- Z.-S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138–1141 CrossRef CAS.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224–7230 CrossRef CAS.
- N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256–14257 CrossRef CAS (Addition and correction, 2008, 130, 4202).
- Z.-S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993–4003 CrossRef CAS.
- Z.-S. Wang, N. Koumura, Y. Cui, M. Miyashita, A. Furube, S. Mori and K. Hara, Chem. Mater., 2009, 21, 2810–2816 CrossRef CAS.
- S. M. Zakeeruddin, Md. K. Nazeeruddin, R. Humphry-Baker, P. Péchy, P. Quagliotto, C. Barolo, G. Viscardi and M. Grätzel, Langmuir, 2002, 18, 952–954 CrossRef CAS.
- P. Wang, C. Klein, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 808–809 CrossRef CAS.
- D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed, 2008, 47, 1923–1927 CrossRef CAS.
- H. Choi, C. Baik, S. O. Kang, J. Ko, M.-S. Kang, Md. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed, 2008, 47, 327–330 CrossRef CAS.
- S. Kim, D. Kim, H. Choi, M.-S. Kang, K. Song, S. O. Kang and J. Ko, Chem. Commun., 2008, 4951–4953 RSC.
- A. Forneli, M. Planells, M. A. Sarmentero, E. Martinez-Ferrero, B. C. O'Regan, P. Ballester and E. Palomares, J. Mater. Chem., 2008, 18, 1652–1658 RSC.
- G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 2198–2200 RSC.
- Z.-S. Wang, H. Kawauchi, T. Kashima and H. Arakawa, Coord. Chem. Rev., 2004, 248, 1381–1389 CrossRef CAS.
- T. Asahi, A. Furube, H. Fukumura, M. Ichikawa and H. Masuhara, Rev. Sci. Instrum., 1998, 69, 361–371 CrossRef CAS.
- R. Katoh, A. Furube, S. Mori, M. Miyashita, K. Sunahara, N. Koumura and K. Hara, Energy Environ. Sci., 2009, 2, 542–546 RSC.
- A. Hagfeldt, U. Bjorksten and M. Grätzel, J. Phys. Chem., 1996, 100, 8045–8048 CrossRef CAS.
- S. Ferrere and B. A. Gregg, J. Phys. Chem. B, 2001, 105, 7602–7605 CrossRef CAS.
- H. Tanaka, A. Takeichi, K. Higuchi, T. Motohiro, M. Takata, N. Hirota, J. Nakajima and T. Toyoda, in Abstr., 17th International Photovoltaic Science and Engineering Conference, 3–7 December 2007, Fukuoka, Japan, pp 149 Search PubMed.
- N. Kato, Y. Takeda, K. Higuchi, A. Takeichi, E. Sudo, H. Tanaka, T. Motohiro, T. Sano and T. Toyoda, Sol. Energy Mater. Sol. Cells, 2009, 93, 893–897 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
