Quo vadis biofuels?
Oliver R.
Inderwildi
* and
David A.
King
Smith School of Enterprise and the Environment, University of Oxford, Hayes House, 75 George Street, Oxford, OX1 2BQ, UK. E-mail: oliver.inderwildi@smithschool.ox.ac.uk
First published on 5th March 2009
 Oliver R. Inderwildi Oliver R. Inderwildi | Dr Oliver Inderwildi is a research fellow at the Smith School of Enterprise and the Environment at the University of Oxford, where he coordinates the School's work on transport and transport fuel. He is also an associated researcher at the Department of Chemical Engineering and Trinity Hall, both at the University of Cambridge. |
 David A. King David A. King | Sir David King is the Director of the Smith School of Enterprise and the Environment at the University of Oxford. He was the UK Government's Chief Scientific Adviser and Head of the Government Office of Science from 2000 to 2007. He is also Director of Research in Physical Chemistry at Cambridge University, President of the British Science Association and Senior Science Adviser to the investment bank, UBS. |
Since the beginning of industrialisation in the north of England in the 19th century, copious amounts of fossil fuels have been combusted. In the earlier period this was mainly coal, but later included fuels such as diesel, petrol or kerosene which are derived from crude oil. The energy provided has enabled economies to grow continuously for almost 200 years. This growth in economies and in turn population demands more energy, and consequently more fossil fuel combustion.1 The main product of the combustion process, carbon dioxide (CO2), is responsible for the enhanced green-house effect and the associated warming of the planet, particularly over the last 50 years.2–4
In order to mitigate climate change, CO2 emissions have to be drastically reduced.5,6 Currently with a global population of 6.7 billion we emit 5.4 tons of CO2 per person and annum; current climate science indicates the need to reduce this to 2 tons per person and annum by 2050.7 In the EU more than 20% of net CO2 emissions originate from the combustion of transportation fuels: petrol, diesel and kerosene.8 Carbon-neutral fuels, i.e. fuels which do not produce CO2 at all or only as is consumed in production, are needed to mitigate emissions in this sector. Cars powered by fuel cells operated with hydrogen, where the hydrogen is produced by zero-carbon energy sources, remain a possible future option.9 Plug-in hybrid cars, based on the development of rechargeable batteries, are the option that is closest to market and may well provide competitive efficiency and potentially low CO2 production when grid energy is decarbonised.10
Biofuels, produced from plants,11,12 are a readily available energy alternative that can be used in conventional combustion engines with simple adjustments, and distributed through the existing petrol infrastructure. There is currently much debate around the economic and ecological benefits of biofuels as there is considerable uncertainty regarding their contribution to overall green-house gas emissions.13–18 Here, we outline some of the problems associated with biofuels and demonstrate how they can be produced to potentially provide a fuel for the mid-term that meets the twin requirements of economy and ecological benefit.
Plants convert CO2 and water to sugars and sugar polymers by photosynthesis utilising sunlight as the energy source. If these plants or portions of them are converted into fuels such as alcohols or hydrocarbons using biochemical or chemical procedures, atmospheric CO2 is technically converted to fuel. To harvest the plants – the biofuel feedstock – harvesters and lorries are used which consume energy. Most plants need fertilisers and other additives to grow and the production of these commodities requires energy. The conversion of plants into fuels also use processes that need energy. Technically biofuels are not carbon neutral but they are potentially low-carbon fuels. Moreover, (bio)fuels have to satisfy a variety of other technical prerequisites. They have to be combustible in petrol or diesel engines of cars, as well as turbines of aeroplanes. The reliability of the fuel is of paramount importance, particularly for air travel where the fuel has to be stable and combustible under extreme conditions. Overcoming these technical issues is the first challenge for biofuels.
There is another set of issues, which must be accounted for. While ethanol derived from the fermentation of sugar cane is viable for countries such as Brazil, bioethanol derived from other sugar- or starch-rich plants invokes other environmental problems. Corn is one of the most water intensive crops, and demands large quantities of nitrogen-based fertilisers.19 In the United States, the remnants of these fertilisers are swept into the Gulf of Mexico creating dead zones so devoid of oxygen that most sea life cannot exist.20 Moreover, nitrogen-based fertilisers generate nitrogen oxides, which are also important green-house gases.21 In Malaysia, palm trees are grown and the derived palm oil is blended into diesel fuel. While the carbon footprint of palm oil may be lower than that of fossil fuels, many new palm tree plantations are created by deforestation of rain forest. Deforestation not only threatens biodiversity, but it also removes a carbon sink. This land-use change creates a so-called carbon debt, which has to be paid back by the biofuel; in the case of palm oil this would take an estimated 423 years.22 Hence, palm oil cannot be a major component of the solution. In Europe diesel is blended with rapeseed oil, which is grown and manufactured locally, again rapeseed monocultures are under suspicion of emitting vast amounts of particulates and the green-house gas N2O. When N2O emissions are converted into CO2-equivalents, the CO2 savings from rapeseed biodiesel are outweighed by the N2O emissions from rapeseed farming.23 Negating these environmental costs is the second challenge for biofuels.
And, there is a third problem associated with biofuels. The US and the EU sponsor this conversion of crop food into fuel with massive subsidies,24 while the global demand for food is expected to increase by 50% by 2030!25,26 Global demand for transportation fuels is expected to increase at an even faster rate.1 Food-based biofuels, the so-called first-generation biofuels, can only ever provide a fraction of the fuel required to meet demand and is clearly detrimental to the need to complement the world's food demands. According to a World Bank report on biofuels,27 the disturbingly large increase in food prices in 2008 is to a large extent (ca. 75%) due to the generation of biofuels from food. The continued diversion of grain away from food to fuel production, and the incentive to set aside agricultural land for biofuel production,27 puts pressure on the global food market as well as on global land use. Due to this increased pressure, food price spikes such as the one encountered last year are likely to get more frequent. This in turn can only continue to exacerbate food security issues in the developing world. During such food price spikes the poor – those living on less than $2 per day28 – are pushed into malnutrition, while the extremely poor – those living on less than $1 per day – are already malnourished and hence more likely to starve.29,30 Thus the conversion of food into fuel on a large scale has to be avoided.
Biofuels of the future must have a low carbon footprint, and for other environmental consequences to be minimised, their feedstock should not be edible biomass. Non-food-based biofuels such as synthetic fuel and fuel ethanol produced from inedible cellulosic material – leafy materials, stems, stalks etc. – referred to as second-generation biofuels could fulfil these prerequisites. Such a concept would combine farming for food and fuel.
Fuel ethanol can be blended into conventional petrol. In Europe and the USA mixtures with up to 85% ethanol (E85) can be used for petrol engines. In Brazil flexi-fuel vehicles are able to cope with pure ethanol† (E100) and pure petrol and any composite between the two. The conversion of the inedible part of a plant, on the other hand, is not as developed as sugar/starch fermentation.11,16,31 There are several issues that have to be resolved or optimised. For instance, the protective shield of hemicellulose and lignin that surrounds cellulose has to be broken down. The chemical route to do this (hydrolysation) is expensive, since it requires heat, while the biological route (enzymatic) gives suboptimal yields and is not cost competitive due to the price of the enzymes.32 The unique wood-degrading abilities of termites have drawn considerable attention recently, since the bacterial genomics and mechanism of lignocellulose degradation has been determined.33 Mimicking the action of enzymes from termites could provide a cheap system to degrade cellulosic biomass to sugars.
However, the conversion of cellulose into sugar using cellulases—enzymes derived from bacteria or fungi is rather slow and the process has still to be optimised.34,35 The hurdle that has to be overcome in the conversion of sugar into ethanol step, is that the yeasts that are active in this conversion cannot cope with the remnants from the two previous steps and are sensitive towards high alcohol concentrations. Hence, novel microorganisms that break down corn-stover or straw and convert the generated sugars to ethanol efficiently have to be engineered.36
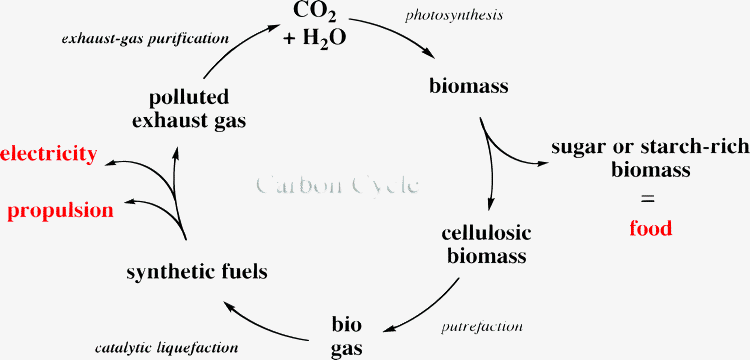
In an alternative chemical route, biomass is gasified to yield synthesis gas, a mixture of CO and hydrogen, which can be converted into ethanol using catalysts such as metallic rhodium.37 Neither route to ethanol – biological or chemical – is yet feasible, i.e. the fuel produced is not yet cost competitive with fossil fuels. Ethanol also suffers from certain other specific limitations. It cannot be used as fuel for aircraft mainly due to its hygroscopic nature and its tendency to corrode the fuel system. Also, it is not well suited for heavy-duty vehicles or ships that cover long distances because its energy density is much lower than that of the diesel used at present. This gap could, however, be filled by synthetic fuels derived from cellulose. An illustration of the carbon cycle for the production of such a fuel is provided.
Biomass can also be converted to biogas, mainly methane, using anaerobic digestion. This process has four key biological and chemical stages, hydrolysis, acidogenesis, acetogenesis, methanogenesis.38 First, complex, long-chain sugars have to be broken down into simple sugars (analogous to the route to ethanol) by hydrolysis and subsequently, these sugars are digested to carboxylic acid, CO2 and by-products in the acidogenesis step. In the next step, acetogenesis, actetic acid, hydrogen and CO2 are produced. In the final step, methanogenesis, the product of the preceding three steps are converted to methane, CO2 and water, the main components of biogas. The digestate contains all the phosphorus and nitrogen compounds and can be used as fertiliser for the feedstock farming. Biogas can then be converted into syngas, a mixture of CO and hydrogen using catalytic processes; catalytic partial oxidation (CPO), steam-reforming (SR) in conjunction with water–gas shift (WGS) reaction usually catalysed by platinum group metals.39–42 Both CPO and WGS reactions are exothermic and hence, this step is the first in which energy in the form of heat is retrieved, and is available to heat the next step, the so-called Fischer–Tropsch synthesis.43 Here, the syngas is pressurised and heated ready to be converted to hydrocarbons, i.e. liquid synthetic fuel and gaseous hydrocarbons using catalysts such as cobalt, ruthenium or iron.44,45 The composition of the synthetic fuels can then be tuned so that they are compatible for diesel or petroleum engines and even aeroplane turbines. Already, jets at Johannesburg airport are fuelled with synthetic kerosene supplied by Sasol.46 Unfortunately, this fuel is made from coal47 and not from biomass.
Portions of biofuel left-over from its synthesis (CO, hydrogen and gaseous hydrocarbons) can be burnt in gas turbines to create electricity. This electricity could then be used to pressurise syngas for the synthesis of fuel and for export to electricity networks.48 Pollutants created during biofuel combustion such as CO, hydrocarbons, nitrous oxides and particulates have to be removed from exhaust gases to complete the carbon cycle.49 The ability to produce food, fuels and electricity simultaneously is sensible but to be viable, the processes must be evaluated by calculating both the net energy gained during the lifecycle of such a fuel, and its carbon footprint – the net amount of CO2 generated during the lifecycle. A novel process called catalytic fast pyrolysis is notable because it converts cellulosic biomass into a range of gasoline aromatics in a batch reactor. This method, although interesting, is still undergoing laboratory testing.50
Converting agricultural by-products into fuel will not meet all the world's fuel demand, but they could be important as feedstocks. Especially important will be feedstocks such as switchgrass, which is grown on agriculturally marginal land with minimal fertiliser and pesticide input and which can be converted completely into fuel. Research has shown that low-input, high-diversity grassland perennials actually sequester more than thirty times as much CO2 into the soil than monocultures51—fuels derived from biomass in this way could potentially be carbon negative.
Additionally, oils from algae or jatropha via transeseterification can become a source of biodiesel or jet fuel following hydrotreatment52,53, the so-called hydrotreated renewable jet (HRJ) fuels. Just like switchgrass, jatropha gives high yields per acre and hence minimises the pressure on land use; algae on the other hand can be grown in bags filled with wastewater also eliminating the pressure on land use.54 Moreover, cellulosic waste from the extraction process can be used again in the synthetic fuels cycle described above or used in biomass electricity plants.
In addition to the other advantages mentioned, the last concept of using biomaterials from marginal agricultural land to create fuel would provide poorer countries and rural areas in developed countries with tremendous development opportunities. Jobs in farming, transportation and fuel synthesis would be generated. Ideally, to minimise transportation costs and emissions the fuel should be produced locally in small biorefineries. The infrastructure required for small-scale, local biorefineries would additionally enhance the quality of life in rural areas. Combining farming for food and fuel and might help to alleviate the growing demand for food and fuel if incentivised by appropriate policy levers.
We conclude that the fuels of the future should be chosen only after in-depth scientific analyses of the short, medium and long-term impacts of feedstock farming, fuel production and fuel combustion are undertaken. Full account must be taken of the impact on climate change, food production and prices, and the economics of the developing world. Unintended consequences can only be avoided by research collaborations like the Commercial Aviation Alternative Fuels Initiative (CAAFI) in the United States55 which is an excellent example of a robust approach that addresses all of the key issues.
References
- Energy Information Administration, International Energy Outlook, U.S. Department of Energy, Washington D.C., 2008, DOE/EIA-0484 Search PubMed.
- T. Flannery, The Weather Makers: The History and Future Impact of Climate Change, Text Publishing, Melbourne, 2005 Search PubMed.
- G. Walker and D. A. King, The Hot Topic: What We Can Do about Global WarmingHarvest Books, London, 2008 Search PubMed.
- N. Nakicenovic and R. Swart, Special Report on Emissions Scenarios, 2007, IPCC, Geneva Search PubMed.
- B. K. Moon, A Climate Culprit in Darfur, Washington Post, Washington D.C., 2008, A15 Search PubMed.
- D. A. King, Science, 2004, 303, 176–177 CrossRef CAS.
- G8 Summit Environment and Climate Change, 2008, http://www.g8summit.go.jp.
- European Environment Agency, Greenhouse gas emission trends and projections in Europe 2008, European Environment Agency, Copenhagen Denmark, 2008, (No. 5) Search PubMed.
- P. P. Edwards, V. L. Kuznetsov, W. I. F. David and N. P. Brandon, Energy Policy, 2008, 2008, 4356–4362.
- J. King (Ed.), The King Review on Low-Carbon Cars, H.M. Treasury, London U.K., 2007 Search PubMed.
- The Gallagher Review of Biofuels, ed. E. Gallagher, Renewable Fuels Agency, St Leonards-on-Sea, UK, 2008 Search PubMed.
- Sustainable Biofuels: Prospects and Challenges, ed. J. Pickett, Royal Society Policy Document, Royal Society, London, 2008, 01/08 Search PubMed.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. X. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T. H. Yu, Science, 2008, 319, 1238–1240 CrossRef CAS.
- T. D. Searchinger, Science, 2008, 321, 200–201 CAS.
- T. D. Searchinger and R. A. Houghton, Science, 2008, 322, 372–374 CAS.
- A. E. Farrell, R. J. Plevin, B. T. Turner, A. D. Jones, M. O'Hare and D. M. Kammen, Science, 2006, 311, 506–508 CrossRef CAS.
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Science, 2008, 321, 199–200 CrossRef CAS.
- T. Searchinger, The Impacts of Biofuels on Greenhouse Gases: How Land Use Change Alters the Equation, Policy Brief, The German Marshall Fund of the United States, Washington D.C., 2008 Search PubMed.
- Water Implications of Biofuels Production in the United States, ed. W. S. Logan, The National Academy Press, Washington D.C., 2008 Search PubMed.
- N. N. Rabalais, R. E. Turner and W. J. Wiseman, Annu. Rev. Ecol. Syst., 2002, 33, 235–263 Search PubMed.
- J. G. J. Olivier, A. F. Bouwman, K. W. Van der Hoek and J. J. M. Berdowski, 1st International Nitrogen Conference 1998, Noordwijkerhout, Netherlands, 1998 Search PubMed.
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Science, 2008, 319, 1235–1238 CrossRef CAS.
- P. J. Crutzen, A. R. Mosier, K. A. Smith and W. Winiwarter, Atmos. Chem. Phys., 2008, 8, 389–395 CAS.
- C. Lynch, U.N. Chief to Prod Nations On Food Crisis, Washington Post, Washington D.C, 2 June 2008 See http://www.washingtonpost.com/wp-dyn/content/article/2008/06/01/AR2008060101963_pf.html Search PubMed.
- N. V. Fedoroff and J. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5903–5907 CrossRef CAS.
- R. Owen, World Food Supply must Rise 50%, Ban Ki Moon tells Rome Summit, The Times, London U.K., 2008, http://www.timesonline.co.uk/tol/news/world/article4056801.ece Search PubMed.
- D. Mitchell, A Note on Rising Food Prices, Policy Research Working Paper, World Bank, 2008, 4682 Search PubMed.
- Policies and Actions for Achieving the Millennium Development Goals and Related Outcomes, World Bank, Washington D.C., 2006 Search PubMed.
- D. Dawe, Have Recent Increases in International Cereal Prices Been Transmitted to Domestic Economies?, ESA Working Paper, The Food and Agriculture Organization of the United Nations Rome, 2008, No. 08–03 Search PubMed.
- M. Ivanic and W. Martin, Implications of Higher Global Food Prices for Poverty in Low-Income Countries, Policy Research Working Paper, World Bank, Washington D.C., 2008, 4594 Search PubMed.
- L. R. Lynd, J. H. Cushman, R. J. Nichols and C. E. Wyman, Science, 1991, 251, 1318–1323 CrossRef CAS.
- Y. Sun and J. Y. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS.
- F. Warnecke, P. Luginbuhl, N. Ivanova, M. Ghassemian, T. H. Richardson, J. T. Stege, M. Cayouette, A. C. McHardy, G. Djordjevic, N. Aboushadi, R. Sorek, S. G. Tringe, M. Podar, H. G. Martin, V. Kunin, D. Dalevi, J. Madejska, E. Kirton, D. Platt, E. Szeto, A. Salamov, K. Barry, N. Mikhailova, N. C. Kyrpides, E. G. Matson, E. A. Ottesen, X. N. Zhang, M. Hernandez, C. Murillo, L. G. Acosta, I. Rigoutsos, G. Tamayo, B. D. Green, C. Chang, E. M. Rubin, E. J. Mathur, D. E. Robertson, P. Hugenholtz and J. R. Leadbetter, Nature, 2007, 450, 560–U517 CrossRef CAS.
- Y. Lin and S. Tanaka, Appl. Microbiol. Biotechnol., 2006, 69, 627–642 CrossRef CAS.
- L. R. Lynd, W. H. van Zyl, J. E. McBride and M. Laser, Curr. Opin. Biotechnol., 2005, 16, 577–583 CrossRef CAS.
- B. S. Dien, M. A. Cotta and T. W. Jeffries, Appl. Microbiol. Biotechnol., 2003, 63, 258–266 CrossRef CAS.
- J. J. Spivey and A. Egbebi, Chem. Soc. Rev., 2007, 36, 1514–1528 RSC.
- S. G. Pavlostathis and E. Giraldogomez, Crit. Rev. Environ. Control, 1991, 21, 411–490 Search PubMed.
- B. C. Enger, R. Lodeng and A. Holmen, Appl. Catal., A: Gen., 2008, 346, 1–27 CrossRef CAS.
- Y. H. Hu and E. Ruckenstein, in Advances in Catalysis, ed. B. C. Gates and H. Knoezinger, Academic Press, 2004, vol. 48, pp. 297–345 Search PubMed.
- R. M. Navarro, M. A. Pena and J. L. G. Fierro, Chem. Rev., 2007, 107, 3952–3991 CrossRef CAS.
- M. A. Pena, J. P. Gomez and J. L. G. Fierro, Appl. Catal., A: Gen., 1996, 144, 7–57 CrossRef CAS.
- H. Schulz, Appl. Catal., A, 1999, 186, 3–12 CrossRef CAS.
- O. R. Inderwildi and S. J. Jenkins, Chem. Soc. Rev., 2008, 37, 2274–2309 RSC.
- O. R. Inderwildi, S. J. Jenkins and D. A. King, Angew. Chem., Int. Ed., 2008, 47, 5253 CrossRef CAS.
- M. Bosch, Sasol says its synthetic fuel approved for jet use, Reuters, 2008 Search PubMed.
- D. Lamprecht and P. N. J. Roets, Proceedings Jet Fuel Symposium, 2004, 49, 426–430 Search PubMed.
- M. J. A. Tijmensen, A. P. C. Faaij, C. N. Hamelinck and M. R. M. van Hardeveld, Biomass Bioenergy, 2002, 23, 129–152 CrossRef CAS.
- R. J. Farrauto and R. M. Heck, Catal. Today, 2000, 55, 179–187 CrossRef CAS.
- T. R. Carlson, T. R. Vispute and G. W. Huber, Chemsuschem, 2008, 1, 397–400 CrossRef CAS.
- D. Tilman, J. Hill and C. Lehman, Science, 2006, 314, 1598–1600 CrossRef CAS.
- N. Foidl, G. Foidl, M. Sanchez, M. Mittelbach and S. Hackel, Bioresour. Technol., 1996, 58, 77–82 CrossRef CAS.
- A. K. Tiwari, A. Kumar and H. Raheman, Biomass Bioenergy, 2007, 31, 569–575 CrossRef.
- J. Gressel, Plant Sci., 2008, 174, 246–263 CrossRef CAS.
- Commercial Alternative Aviation Fuels Initiative, http://www.caafi.org.
Footnote |
| † They merely require minor amounts of fossil fuels for cold start. |
| This journal is © The Royal Society of Chemistry 2009 |