Optimization the solid-state electrolytes for dye-sensitized solar cells
Dongmei
Li
,
Da
Qin
,
Minghui
Deng
,
Yanhong
Luo
and
Qingbo
Meng
*
Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, P. O. Box 603, 100190, Beijing, China. E-mail: qbmeng@aphy.iphy.ac.cn; Fax: +86 10-82649242; Tel: +86 10-82649242
First published on 10th November 2008
Abstract
The dye-sensitized solar cell (DSC) as a low price photovoltaic technology has attracted widespread attention in recent years. During its progress to practical application, replacement of the liquid electrolytes by solid-state ones has been found to be necessary. Alternative solid-state electrolytes have thus been developed. This review deals with the recent progress of different solid-state electrolytes on DSCs. In particular, representative examples are highlighted with the results of our solid-state composite electrolytes based on addition compounds.
 Dongmei Li Dongmei Li | Dr Dongmei Li got her PhD from Jilin University in 1999. From July 2000 to Jan. 2001, she worked as a postdoc in Nihon University, Japan. From 2001 to 2003 she worked as a postdoc in Cardiff University, UK. Then, she worked as an associate professor in College of Chemistry, Jilin University. Since 2005, she has moved to the Institute of Physics, Chinese Academy of Sciences (CAS). Her current interests include the fabrication and characterization of solid-state dye-sensitized solar cells (DSCs). |
 Da Qin Da Qin | Da Qin got his BSc from the Department of Chemistry, University of Science and Technology of China in 2006. Currently he is a PhD student at the Institute of Physics, CAS under the supervision of Prof. Qingbo Meng. His research interest focuses on the preparation of solid-state electrolytes and their application on DSCs. |
 Minghui Deng Minghui Deng | Minghui Deng got his BS degree from Fudan University in 2005. Currently he is a PhD student at the Institute of Physics, CAS under the supervision of Prof. Qingbo Meng. His research interest focuses on the fabrication of nanoscale photoanode materials for DSCs and surface property investigation of these semiconductor nanoporous film. |
 Yanhong Luo Yanhong Luo | Dr Yanhong Luo got her PhD from the Institute of Chemistry, CAS in 2003. From 2003 to 2005, she worked as a postdoc in the National Institute for Materials Science (NIMS), Japan. Currently, she is an associate professor in the Institute of Physics, CAS. Her current research interest focuses on design, controlled synthesis and characterization of key materials for low price, flexible dye-sensitized solar cells (DSCs). |
 Qingbo Meng Qingbo Meng | Prof. Dr Qingbo Meng got his PhD from the Changchun Institute of Applied Chemistry, CAS in 1997. In 2001, he was selected as ‘Hundreds of Talents of CAS’. He joined the Institute of Physics, CAS in 2002 and set up an independent solar energy materials and device group. He has been a full professor since 2004. He was awarded ‘Excellent Hundreds of Talents’ from CAS in 2005 and ‘Distinguished Young Scholars’ from National Nature Sciences Foundation of China in 2007, respectively. His current interest focuses on solar energy materials, including DSCs, photocatalytic materials and Photonic Crystal materials. |
Broader contextSolar energy is a clean, renewable and inexhaustible energy source. Photovoltaics have been considered as an ideal energy transformational technology for conversion from solar energy into electricity. As a representative of new generation thin film photovoltaics, dye-sensitized solar cells (DSCs) offer the prospect of low-cost and easy-handling fabrication together with other attractive advantages, i.e. flexibility. At present, those with high conversion efficiencies over 10% are all based on toxic organic solvents, which will easily lead to long-term instability of the device and environmental problems. Solid-state DSCs have been supposed a potential candidate that can overcome these problems and promote its practical application in the future. This paper describes alternative solid-state DSCs including: (1) quasi-solid DSCs based on ionic liquids and polymer electrolytes; (2) solid-state DSCs based on p-type inorganic semiconductor materials, organic hole-transport materials and solid-state composite electrolytes. In different systems, the working principles and optimal fabrication methods are discussed in detail. And a cheap, environmentally friendly solid-state DSC is suggested. |
1. Introduction
Increasing energy demands and shortage of fossil energy have forced people to seek highly effective, renewable energy sources in recent years. Undoubtedly, conversion from solar energy to electrical power is one of the most favorable ways in the future. In comparison with commercial Si-based solar cells, dye-sensitized solar cell (DSC) is a potential photovoltaic alternative owing to its low price, easy fabrication and suitability for large area production.1–4 It mimics photosynthetic process, in which the light absorption and charge separation occur at different mediums. In DSCs, nanocrystalline semiconductor film as a photoanode can provide enormous surface area for adsorbing the dye to ensure enough photon absorption, high electron injection as well as high conversion efficiency.1,5 An outline of the operation of DSC is depicted in Fig. 1. The dye molecule is excited from ground state (D0) to excited state (D*) by sunlight, rapidly injects an electron into the conduction band (CB) of semiconductor porous film. The electrons in CB arrive at the counter electrode through the external circuit, and reduce the I3− ions to I− ions with the aid of Pt. Then, I− ions donate electrons to dye cations to complete the cycle of dye regeneration.6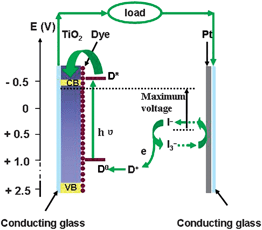 | ||
| Fig. 1 Schematic illustration of the working principle of dye-sensitized solar cells. | ||
Following the initial exploration on DSCs by Prof. Gratzel's group, extensive investigation has been carried out in all aspects of DSCs,7–12 and many reviews and reports on DSCs have been published.13–16 Up to now, the most efficient DSCs have been based on nanocrystalline TiO2 porous film, ruthenium pyridyl complexes as dyes, Pt counter electrode and those electrolytes consisting of I3−/I−redox couple in volatile organic solvents.17 However, several key issues have been revealed to hinder the practical application of DSCs: (1) ruthenium-polypyridine dyes are expensive; (2) the I3−/I−redox couple is corrosive toward most high conductivity metals, easily leading to poor long-term stability and technical complexity; (3) sealing problems and poor durability resulting from the liquid electrolytes. In this context, organic dyes have emerged as competitive substitutes, which exhibit high absorption efficiency, chemical stability, tunable absorption spectral response from the visible to near infrared (NIR) region and low cost. Impressive conversion efficiencies up to 9% have been achieved on indoline, thiophene, hemicyanine, p-conjugated oligo-phenylenevinylene unit dyeset al.18–21 The amount of Pt on the counter electrode is getting less (below 50 mg/m2), however, future industrial DSC systems need abundantly available catalytic materials. Moreover, the corrosion of iodine on Pt always obsesses scientists. Thus, corrosion-inert carbon materials with good catalytic activity become ideal replacements.22,23 So far, 9.1% of efficiency based on carbon black has already been attained.24
The electrolyte containing I3−/I−redox couple is still the most efficient charge mediator, and regarded as a key element to realize ‘low-tech’ fabrication process. In the meantime, it has substantial influence on the dynamics and photovoltaic performance of the cell. The acetonitrile-based electrolyte could give the maximum efficiency of 11.1%, however, its drawbacks hinder the cell's practical development.17,25 In particular, when a high-speed roll-to-roll continuous manufacturing process is introduced to the industrial production of DSCs, the use of liquid electrolytes will strongly retard large-scale application of DSCs.26 As a consequence, this glass-to-plastic conversion also promotes intensive investigation of solid-state electrolytes. In this present review, we will mainly address the application of solid-state electrolytes on DSCs. And representative examples are highlighted with the results of our addition compound-based composite solid-state electrolytes.
2. General design of solid-state electrolytes
Solid-state electrolytes are one of ideal replacements of liquid electrolytes in order to accelerate the future industrial production. Generally, the solid-state electrolyte should primarily conform to the following essential requirements: (1) high ionic conductivity or hole transfer rate to maintain regular regeneration of the dye; (2) good stability; (3) no strong absorption in visible spectrum region; (4) good physical contact at TiO2/dye/electrolyte interface.Alternative solid-state electrolytes have been designed over a decade. The development of solid-state electrolytes is generally divided into two main parts: quasi-solid-state electrolyte and solid-state electrolyte. Polymeric ionic gels, ionic liquids and plastic crystal systems et al. constitute quasi-solid-state electrolytes.27–30 Aiming at solving the sealing and long-term stability of liquid electrolyte-based DSCs, polymer or nanoparticles-solidified electrolytes called physically cross-linked gel electrolytes were first developed. The characteristics of this kind of electrolyte are that the organic solvent is entrapped in the nanoparticle (SiO2, TiO2et al.) or polymeric network to slow the evaporation of volatile components. Several polymer matrices are commonly used, such as poly(ethylene oxide), poly(acrylonitrile), poly(vinyl pyrrolidinone), poly(vinylidene carbonate) and so on.31 A uniform gel with host matrix exhibited high ionic conductivity, however, its mechanical property was not good, leading to electrical contact failure and lower device performance.32 P. Lianos et al. used the sol-gel method and SiO2 as a gelling agent to improve this method and obtained conversion efficiency of ∼4% at 96 mW cm−2 (AM 1.5).33 More recently, some interesting results on quasi-solid-state DSCs assembled with ‘click polymers’ or ‘glass transition polymers’ have been reported, the efficiencies were remarkably increased up to 7.1%.34,35 At present, more chemically cross-linked gel electrolytes have been applied on DSCs, which mainly makes use of the various viscosities before and after gelation to ameliorate the interfacial connectivity between the gel electrolyte and electrodes.36 Initial viscosity for gel electrolyte precursors was not very high, so the precursors could directly impregnate into the nanoporous film. When the cell was heated, gelation took place in the cell giving rise to a 3D covalent polymer network. This method is simple and easy handling. However, the reaction rate was sometimes too fast to complete total wetting of the TiO2 film for the large area DSC. Hayase et al. adopted latent gel electrolyte precursors (L-Gel-Pre) to improve this situation, in which the gelation abilities of the gelators were hidden at room temperature, and the solidification occurred at a certain higher temperature. L-Gel-Pre system involving SiO2 nano-particles, polyvinylpyridine (PVP), hexadecanedioic acid and ionic liquid electrolytes could present overall conversion efficiency of 6.8%.37 Fang et al. developed a novel necklace-like polymer gel electrolyte consisting of polypyridyl-pendant poly(amidoamine) dendritic derivatives (PPDD) and I(CH2CH2O)nCH2CH2I as latent chemically cross-linked gel electrolyte precursors. The assembled quasi-solid-state DSC exhibited current density of 17.1 mA cm−2, conversion efficiency of 7.72% with an active area of 0.25 cm2 (AM 1.5, 100 mW cm−2 irradiation).38
Improvement in quasi-solid DSCs can also be achieved by using ionic liquid-based electrolytes. Ionic liquids possess high chemical and thermal stability, non-flammability and wide electrochemical window, thus have been regarded as the ideal components of solid-state electrolytes.39–43 Among different kinds of ionic liquids, imidazolium-based ionic liquids have been widely used in DSCs, as depicted in Fig. 2.28a However, the relatively high viscosity will retard the diffusion rate of the I3−/I−redox couple, leading to poor performance. A binary ionic liquid electrolyte system composed of 1-propyl-3-methylimidazolium iodide (PMII) and 1-ethyl-3-methylimidazolium tricyanomethanide (EMITCM) was suggested by Prof. Gratzel et al., and presented 7.4% of conversion efficiency at full sunlight (AM 1.5, 100 mW cm−2). EMITCM was adopted to reduce the viscosity of PMII.28b, 41 Further solidification of ionic liquids is usually realized by addition of silica nanoparticles, for example, the quasi-solid electrolyte of PMII and SiO2 could present 6.4% conversion efficiency.42 Yanagida et al. employed ionic liquid crystal, 1-dodecyl-3-methylimidazolium iodide to provide a self-assembled pathway for the I3− and I−transport, thus, the conductivity of the electrolyte was significantly enhanced.43 Several research groups detailedly investigated charge transport properties in the cells containing ionic liquid quasi-solid electrolytes.39,44 These results showed that the gelation did not significantly influence I3− and I− diffusion in ionic liquid electrolytes. Further investigation demonstrated that effective I3− and I−transport in ionic liquid-based electrolyte is rationalized by Grotthus-type charge exchange mechanism (polyiodide bond exchange mechanism),44 shown as follows:
| I3− + I− → I−–I2 ⋯ I− → I− ⋯ I2–I− → I− + I3− |
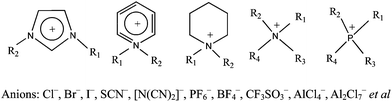 | ||
| Fig. 2 Examples of different ionic liquids. | ||
To quasi-solid-state gel electrolytes, the main problem is still stability, since gel systems are generally thermodynamically unstable. Under long storage or exposure to open air, solvent exudation is unavoidable, leading to low conversion efficiency. Therefore, under high temperatures, quasi-solid-state DSCs are less stable and need a perfect sealing, too. In this regard, all solid-state electrolytes can hold a number of practical advantages over liquid and quasi-solid-state electrolytes.
Solid-state electrolytes mainly cover various hole transporting materials (HTM).45 In principle, if the material with p-type semiconducting behavior can accept holes from the dye cation, it can theoretically replace the liquid electrolyte of DSCs. In HTM-based solid-state DSC, the carrier transport is typically electronic instead of ionic transport in liquid electrolyte-based DSCs.46 Conventional HTMs are inorganic p-type materials (i.e.CuI and CuSCN), which possess higher hole mobility, however, their crystallization rate is too fast. When they were directly applied on DSCs, the control of crystal size and growth rate is very difficult, resulting in incomplete filling into the TiO2pores, as shown in Fig. 3a. Thus, the efficiency was below 1%.47–51 Molten salts (1-methy-3-ethylimidazolium thiocyanate and triethylamine hydrothiocyanate) have been successfully used as crystal growth inhibitors to control the growth of CuI crystals. This CuI/molten salt composite electrolyte took a great advantage of filling into the pore of dyed TiO2 anode, as demonstrated in Fig. 3b.52–54 The light-to-electrical efficiency was significantly improved up to 3.8%.
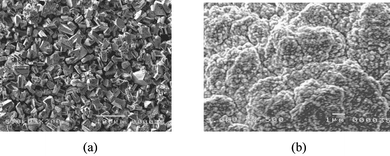 | ||
| Fig. 3 The SEM images of CuI crystallines deposited on the dyed TiO2 porous film: (a) CuI without molten salt; (b) CuI/1-methy-3-ethylimidazolium thiocyanate composite electrolyte. | ||
Comparable to inorganic HTMs, organic molecular solids and polymers display attractive diversity, which can be modified by chemically tailored method to fit different technological purposes. Some conducting polymers, such as polypyrrole, polythiophene, polyaniline et al. have been attempted on DSCs, which chemical structures are depicted in Fig. 4.55–57 These materials exhibit fine electrical, electronic, optical properties of metals or semiconductors as well as good mechanical property of conventional polymer. Some results showed the possibility of their application on solid-state DSCs.58,59 The major drawback concerns the poor connectivity between the electrolyte and nanocrystalline porous film. In order to improve this connectivity, Yanagida and coworkers developed in situ photoelectropolymerization (PEP) method to prepare conductive polymer, poly(3, 4-ethylenedioxythiophene) (PEDOT) on dyed TiO2 porous film, as shown in Fig. 4e. By doping different counteranions (i.e.TFSI−, BF4−, CF3SO3−), the hole conductivity was further improved, and overall cell efficiency has been enhanced to 2.85%.60–64 Another promising organic hole-conductor is spiro-MeOTAD (2, 2′, 7, 7′-tetrakis (N, N-di-p-methoxyphenyl-amine)-9, 9′-spiro-bifluorene), see Fig. 4f. Since it was first successfully applied on DSCs as the replacement of the liquid electrolyte, the efficiency has increased from 0.74% up to 4% by optimization the thickness of TiO2 film, doping with Li[CF3SO2]2N and 4-tert-butylpyridine and usage of an amphiphilic ruthenium dye.65–70 So far, it was revealed that the efficiencies of the devices fabricated with spiro-MeOTAD are much better than other types of organic hole conductors. In general, both high mobility of hole conductors and good pore filling are key issues to get high efficiency value. Durrant et al. have further investigated the factors determining the yield of hole transfer at the dye/HTM interface as well as the correlation with the device performance, and found that the photocurrent is directly proportional to the hole-transfer yield. This hole-transfer yield relies on the electrolyte penetration into the dyed porous film as well as interfacial charge transfer. Thus, for solid-state DSCs assembled with HTM, optimization of the pore-filling property and the control of interfacial energetics should be addressed rather than increasing the hole mobility of materials.71
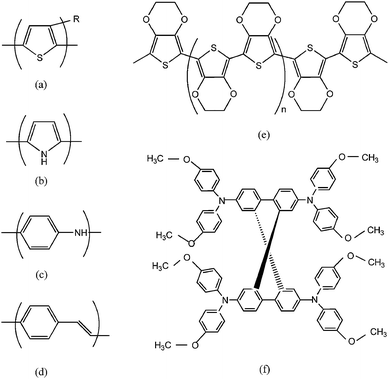 | ||
| Fig. 4 Chemical structures of some conducting polymers with hole transport properties: (a) R-substituted polythiophene (R-substituted PTh); (b) polypyrrole (PPy); (c) polyaniline (PAn); (d) poly(p-phenylenevinylene) (PPV); (e) poly(3,4-ethylenedioxythiophene) (PEDOT); (f) 2,2′,7,7′-tetrakis (N,N-di-p-methoxyphenylamine)-9,9′-spiro-bifluorene) (spiro-MeOTAD). | ||
Despite considerable progress, the poor penetration of solid conductive materials into the semiconductor porous film and low conductivity of organic HTM which limits the diffusion length of charge carriers, always have strong impacts on the conversion efficiency of solid-state DSCs. Recently, our research group has first employed solid-state composite electrolytes based on LiI-addition compounds, such as [Li(HPN)2]I/SiO2 (HPN = 3-hydroxylproponitrile), LiI–CH3OH/THT/SiO2, LiI–C2H5OH/SiO2 systems et al., to replace the liquid electrolytes. As a kind of promising candidate, their solid-state DSCs exhibited higher conversion efficiency (a complete description will be given in a sequence section).72–77 In the next section, we will outline some representative research work and the more recent advances in this field.
3. Solid-state composite electrolytes based on addition compounds
Recently, an alternative approach has been addressed by our group to introduce the addition compounds to the solid-state DSCs called solid-state composite electrolytes. These electrolytes were the combination of nanoparticles and solid-state addition compounds based on the reactions of LiI and small organic molecules (HPN, methanol, ethanolet al.). They have been found to be effective in increasing charge carrier mobility and pore filling into the film, thus directly brought higher photovoltaic performance.The first solid-state addition compound electrolyte was [Li(HPN)x]I/I2 system, which was derived from the reaction between LiI and HPN in different molar ratio. A single compound [Li(HPN)2]I can be obtained when the ratio of LiI/HPN was 1 : 2.72X-ray crystallographic analysis showed that each lithium cation is four-coordinated to two nitrile nitrogen atoms and two hydroxyl oxygen atoms from four different HPN molecules.72 The I⋯H–O hydrogen bonding, ionic clusters [Li+kIl−] (k ≠ 1) as well as their association with the HPN molecules at high salt content constructed a 3D framework in the lattice. This spatial configuration is beneficial to triiodide and iodide ion transportation in the electrolyte.72,73 Thus, a series of solid-state electrolytes based on LiI(HPN)x (2 ≤ x ≤4) addition compounds were obtained. Among them, Li(HPN)4I gives the highest conductivity (σ = 3.1 × 10−3 s cm−1) at room temperature. But solid-state DSCs based on [Li(HPN)4]I only gave 1.8% of efficiency.73 When SiO2 nano-particles were added into the electrolyte, the performance of the DSCs has been dramatically improved. The highest conversion efficiency reached 5.48% by using this [Li(HPN)4]I/SiO2 composite electrolyte (see Fig. 5). A simple test about the long-time stability of DSCs with solid-state composite electrolytes has been carried out, that is, the DSC fabricated with nanocomposite electrolyte LiI(HPN)4/15 wt% SiO2 (15 nm) was stored in a desiccator without sealing. One month later, the conversion efficiency maintained 70% of the initial efficiency.72 Obviously, this solid-state composite electrolyte was superior to the liquid electrolytes. And this result was inspiring for further development of this kind of electrolytes. The detailed experiments concerning the long-term stability and the aging test of sealed DSCs will be carried out in the near future.
![The photocurrent density vs. photovoltage curve of the DSCs assembled with [Li(HPN)4]I/15 wt% SiO2 electrolyte. Inset: the X-ray crystal structure plot for the [Li(HPN)2]I along the c axis.71](/image/article/2009/EE/b813378f/b813378f-f5.gif) | ||
| Fig. 5 The photocurrent density vs. photovoltage curve of the DSCs assembled with [Li(HPN)4]I/15 wt% SiO2 electrolyte. Inset: the X-ray crystal structure plot for the [Li(HPN)2]I along the c axis.71 | ||
Besides, the reactions between LiI and other small organic molecules, i.e.methanol, ethanolet al. have also been investigated. Reaction of LiI and CH3OH afforded Li[(CH3OH)4]I, which crystal structure was reported by Rabenau et al.78 To Li(CH3OH)4I/SiO2 solid-state composite electrolyte, the DSC only gave 2.7% of conversion efficiency. Addition of ionic liquids (THT = triethylamine hydrothiocyanate or MEII = 1-methyl-3-ethyl-imimidazolium iodide) could obviously improve the cell performance. DSCs based on Li(CH3OH)4I/THT/SiO2 electrolyte presented 680 mV of open-circuit voltage, 9.1 mA cm−2 of short-circuit photocurrent density, 0.66 of fill factor and 4.43% of efficiency, respectively.77DSCs assembled with Li(C2H5OH)4I/SiO2 composite electrolyte (see Fig. 6) could exhibit 6.1% of efficiency. Its open-circuit voltage, short-circuit photocurrent density, fill factor were 619 mV, 16.5 mA cm−2, 0.547, respectively. The above two solid-state composite electrolytes were principally derived from the addition reactions between LiI and methanol or ethanol, and exhibited high conductivity.
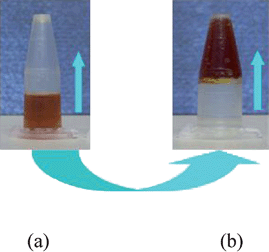 | ||
| Fig. 6 The photographs of the LiI–C2H5OH electrolyte system at room temperature: (a) liquid state without adding SiO2nanoparticles; (b) solid state with SiO2nanoparticles (14 nm, 5 wt%). | ||
On the basis of the ionic conductivity measurements, the charge transport in the addition compound-based solid state composite electrolyte was in charge of I3− and I− diffusion. This is different from ionic liquid or gel-based solid-state electrolytes, which are proposed to obey the Grotthus-type charge carrier exchange mechanism.79,80 In the meantime, ab initio density functional-based total energy calculation could help us to better understand ion diffusion dynamics. To the [Li(HPN)2]I, its ion-conductor type can be deduced by comparison of the energy barriers of Li+ and I− ions, that is, I− displays much lower energy barrier (0.73 eV) than that of Li+ (8.39 eV), shown in Fig. 7. So, this addition compound is actually an I− ion conductor.81
![Energy barriers for the hopping of Li+ and I− ions to their neighboring sites in the crystalline [Li(HPN)2]I along the c axis.](/image/article/2009/EE/b813378f/b813378f-f7.gif) | ||
| Fig. 7 Energy barriers for the hopping of Li+ and I− ions to their neighboring sites in the crystalline [Li(HPN)2]I along the c axis. | ||
For the solid-state composite electrolyte systems, the introduction of silica nanoparticles is crucial to the improvement of photovoltaic performance. In fact, besides silica nanoparticles, other inorganic nanoparticles such as TiO2 (P25), Al2O3etc., have also been used in the solid-state or quasi-solid-state electrolyte systems for DSCs.82 On one hand, silica particles can effectively control the crystalline growth rate of addition compounds and prevent the formation of large crystallites. As a consequence, good filling of the solid-state electrolyte into the pores of the photoanode film and better interfacial contact between the electrolyte and the electrodes will be obtained. On the other hand, the homogeneous dispersion of nanoparticles into the electrolyte can increase the ionic conductivity of the electrolyte.72,80 Generally, ionic conductivity derives from both bulk and interfacial charge carrier transport. In many cases, interfacial contribution even becomes dominant. To the solid-state composite electrolytes, this enhancement of ionic conductivity is mainly attributed to the interfacial defect concentration, which is derived from the space charge layer effect as well as the inhibiting of the crystallization of solid-state electrolyte.72,83,84 That is to say, the interaction between silica and the electrolyte weakened the electrostatic force felt by [Li(HPN)4]+ and I− ions, resulting in the increasing of I− ion mobility. As we know, to the [Li(HPN)4]I/SiO2 electrolyte system, only I− ions can move in the crystal lattice. As a result of the silica effect, I− ions tend to transport at the interface rather than in the crystal lattice, the ionic conductivity of the electrolyte system was thus significantly increased.
Succinonitrile is a kind of molecular plastic crystal, and its plastic phase is in the range −40 °C and 58 °C in a body-centered cubic structure. High polarity ensures its good solvent property. Grätzel and Dai et al. have investigated succinonitrile-based solid-state electrolytes for DSCs, separately.30,85 All these electrolytes were derived from the mixtures of succinonitrile and ionic liquids. Under the illumination of 51.9 mW cm−2 sunlight (AM 1.5), a conversion efficiency of 6.7% was obtained.85 In our lab, we obtained a LiI-succinonitrile electrolyte by mixing LiI, I2 and succinonitrile in a molar ratio of 1 : 10 : 10. The mixture was heated at 60 °C to afford a homogeneous solution. When the mixture was cooled down to room temperature, a waxy solid was obtained. At 28 °C, the electrolyte has conductivity of 1.31 × 10−2 S cm−1, which is higher than the LiI-MPN based electrolyte. Besides, its SiO2-solidified analogue also gives high conductivity (4.79 × 10−3 S cm−1), as shown in Table 1. This result is in agreement with the studies in lithium battery, namely, doping with Li+ cation will remarkably increase the diffusivity and conductivity of the electrolytes, thus leading to fast ionic conduction.17 Prof. M. Armand et al. ascribed the remarkable increase of conductivity to interaction of the cations (Li+, Na+, Pb2+, Ca2+, La3+et al.) with the succinonitrile. It is likely that this interaction is strongly influenced by the charge, van der Waals radii and hardness of the cations.86 In LiI-succinonitrile based electrolytes for DSCs, it is suggested that this interaction will still have an impact on the conductivity although no obvious stretching vibration shift of nitrile group was observed in the infrared spectrum. In the meantime, transportation of I3− and I− ions is expected to be promoted through the weak interaction between Li+ and the nitriles of succinonitrile.
| Electrolytes | LiI in MPNb | Electrolyte Ac | Solid-state electrolyte Bd |
|---|---|---|---|
| a The conductivities of electrolytes A and B were recorded on impedance measuring unit (IM 6) from Zahner using symmetric Pt-activated TCO electrodes (distance between two electrodes is 25 µm, the active area is 0.25 cm2) at a temperature of 28 °C. The conductivity values were determined by the value of the real part of the impedance at the frequency that gave the minimum value of the imaginary part.40 b 0.6 M LiI, 2 M pyridine and 0.06M I2 in MPN (3-methoxy-proponitrile).87 c Electrolyte A = 0.5 M LiI, 0.05 M I2, 0.5 M 4-tert-butylpyridine in succinonitrile. d Electrolyte B = electrolyte A + 5 wt% SiO2 (14 nm, Degussa). | |||
| Conductivities/S cm−1 | 5.8 × 10−3 | 1.31 × 10−2 | 4.79 × 10−3 |
DSCs were fabricated by following the literature.88Fig. 8 presents the photocurrent–photovoltage curves of the DSCs using LiI/I2/succinonitrile-based electrolytes with and without SiO2nanoparticles. Under full sunlight (100 mW cm−2, AM 1.5), to DSCs based on LiI/I2/succinonitrile electrolyte, the short-circuit photocurrent density (Jsc), the open-circuit photovoltage (Voc) and the fill factor (ff) are 8.76 mA cm−2, 0.611 V, 0.73, respectively, yielding the light-to-electricity efficiency of 3.92%. After solidification with SiO2nanoparticles, the performance of DSCs slightly changed, its short-circuit photocurrent density, open-circuit photovoltage, fill factor and efficiency were 8.51 mA cm−2, 0.633 V, 0.71 and 3.81%, respectively. Initial results, without optimization, confirmed that the LiI/succinonitrile-based composite systems are effective electrolytes for the DSCs.
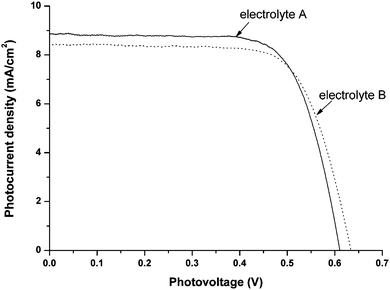 | ||
| Fig. 8 The photocurrent density vs. photovoltage curves for the DSCs with LiI/I2/succinonitrile electrolytes: LiI/I2/succinonitrile electrolyte A (solid line), its SiO2 solidified electrolyte B (dot line), under AM 1.5 illumination (100 mW cm−2). Active area: 0.15 cm2. | ||
The solid-state electrolytes based on addition compounds have several advantages comparable to other solid-state electrolytes: firstly, they induce a degree of high order in the crystalline phase, which provide transportation channels of iodide and triiodide ions, thus increasing the free space of charge carriers in the system. Secondly, addition compound electrolytes could give high ionic conductivities approaching 10−3 S cm−1 at 25 °C. This constitutes a class of inorganic coordination polymers in which the cations are attached to each other viahydrogen bonds, thus the transportation properties are assigned to the mobile I− anions. Thirdly, inorganic nanoparticles, such as SiO2, play a crucial role in the cell performance. They could well restrain the crystalline rate of addition compounds in the process of obtaining the uniform solid-state electrolyte. On the other hand, such small size nanoparticles are supposed to increase the conductivity of the electrolyte. Fourthly, the synthetic method is easy-to-handle. More importantly, low-cost and environmentally friendly solid-state composite electrolytes could be prepared by careful selection of the small organic molecules.88 Thus, this kind of solid-state composite electrolytes has highly potential application in solid-state DSCs.
4. Summary and outlook
Dye-sensitized solar cells can efficiently convert solar energy into electricity by fine combination of several materials (TiO2 nanoporous film, dye molecule, electrolyte containing I3−/I−redox couple, Ptet al.). In order to promote its large scale application, people are attempting to solve some technical problems. Recently, much effort has been directed towards the optimization of all aspects of solid-state DSCs. Poor pore filling of solid conductive materials into the semiconductor porous film is the biggest obstacle for highly efficient solid-state DSCs. To organic HTMs, low conductivity also limits the diffusion length of charge carriers. Some organic HTMs have already exhibited promising device performances through optimization of the porous films, electrolytes and the dyes. Light-to-electricity conversion efficiency has been increased step by step. In the meantime, other unconventional solid-state electrolytes have been proposed and applied on DSCs. For example, the high order of addition compounds has been introduced into the solid-state composite electrolytes, which can remarkably enhance the diffusion of triiodide and iodide ions, thus leading to high light-to-electricity conversion efficiency. More attractively, the diversity of solid-state composite electrolytes can provide the possibility to obtain the environmentally benign solid-state electrolytes for DSCs.The future development of this exciting field in DSCs will need to combine novel approaches to solid-state electrolyte systems' design with appropriate modelling of the various cell ingredients. Key challenges will include further improvement of the overall conversion efficiency, device stability and scale-up of fabrication. Consequently, more work will focus on identifying and optimizing alternative charge carrier materials that are cheap, stable and suitable for large scale screen-printing if possible.
Acknowledgements
We gratefully thank the support of the National Science Fund for Distinguished Young Scholars under Grant No. 20725311, the National Natural Science Foundation of China under Grant No. 20673141, 20703063 and 20873178, China-Japan (NSFC-JST) Joint Research Program under Grant No. 20721140647, the National Basic Research Program of China (973) under Grant No. 2006CB202606, the National High Technology Research and Development Program (863) under Grant No. 2006AA03Z341 and the 100-Talents Project of Chinese Academy of Sciences.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222 CrossRef CAS.
- J. Drechsel, B. Mannig, F. Kozlowski, M. Pfeiffer, K. Leo and H. Hoppe, Appl. Phys. Lett., 2005, 86, 244102 CrossRef.
- M. Murayama and T. Mori, Thin Solid Films, 2008, 516, 2716 CrossRef CAS.
- C. J. Barbe, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grazel, J. Am. Ceram. Soc., 1997, 80, 3157 CAS.
- M. Gratzel, J. Photochem. Photobiol., A, 2004, 164, 3 CrossRef CAS.
- (a) M. Durr, A. Schmid, M. Obermaier, S. Rosselli, A. Yasuda and G. Nelles, Nat. Mater., 2005, 4, 607 CrossRef; (b) X. Fan, Z. Chu, F. Wang, C. Zhang, L. Chen, Y. Tang and D. Zou, Adv. Mater., 2008, 20, 592 CrossRef CAS; (c) A. B. F. Martinson, T. W. Hamann, M. J. Pellin and J. T. Hupp, Chem.–Eur. J., 2008, 14, 4458 CrossRef CAS.
- J. M. Kroon, N. J. Bakker, H. J. P. Smit, P. Liska, K. R. Thampi, P. Wang, S. M. Zakeeruddin, M. Gratzel, A. Hinsch, S. Hore, U. Wurfel, R. Sastrawan, J. R. Durrant, E. Palomares, H. Pettersson, T. Gruszecki, J. Walter, K. Skupien and G. E. Tullo, Prog. Photovoltaics, 2007, 15, 1 CAS.
- (a) X. Liu, Z. Huang, K. Li, H. Li, D. Li, L. Chen and Q. Meng, Chin. Phys. Lett., 2006, 23, 2606 CrossRef CAS; (b) L. Schmidt-Mende, U. Bach, R. Humphry-Baker, T. Horiuchi, H. Miura, S. Ito, S. Uchida and M. Gratzel, Adv. Mater., 2005, 17, 813 CrossRef.
- H. Nusbaumer, S. M. Zakeeruddin, J.-E. Moser and M. Gratzel, Chem.–Eur. J., 2004, 9, 3576.
- (a) S. Cazzanti, S. Caramori, R. Argazzi, C. M. Elliott and C. A. Bignozzi, J. Am. Chem. Soc., 2006, 128, 9996 CrossRef CAS; (b) S. A. Sapp, M. Elliott, C. Contado, S. Caramori and C. A. Bignozzi, J. Am. Chem. Soc., 2002, 124, 11215 CrossRef CAS.
- (a) E. Ramasamy, W. J. Lee, D. Y. Lee and J. S. Song, J. Power Sources, 2007, 165, 446 CrossRef CAS; (b) A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2008, 130, 4007 CrossRef CAS.
- (a) J. R. Durrant, S. A. Haque and E. Palomares, Chem. Commun., 2006, 3279 RSC; (b) L. Peter, Phys. Chem. Chem. Phys., 2007, 9, 2630 RSC.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338 CrossRef CAS.
- (a) M. Gratzel, Philos. Trans. R. Soc. London, Ser. A, 2007, 365, 993 CrossRef CAS; (b) M. Gratzel, Nature, 2001, 414, 338 CrossRef CAS.
- (a) H. Tributsch, Coord. Chem. Rev., 2004, 248, 1511 CrossRef CAS; (b) D. Cahen, G. Hodes, M. Gratzel, J. F. Guillemoles and I. Riess, J. Phys. Chem. B, 2000, 104, 2053 CrossRef CAS.
- (a) Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS; (b) M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Gratzel, J. Am. Chem. Soc., 2005, 127, 16385.
- D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Gratzel, Angew. Chem., Int. Ed., 2008, 47, 1 CrossRef.
- S. Hwang, J. H. Lee, C. Park, H. Lee, C. Kim, C. Park, M.-H. Lee, W. Lee, J. Park, K. Kim, N. -G. Park and C. Kim, Chem. Commun., 2007, 4887 RSC.
- S. Kim, J. K. Lee, S. O. Kang, J. Ko, J.-H. Yum, S. Fantacci, F. De Angelis, D. Di Censo, M. K. Nazeeruddin and M. Gratzel, J. Am. Chem. Soc., 2006, 128, 16701 CrossRef CAS.
- S. Ito, S. M. Zakeeruddin, R. Humphry-Baker, P. Liska, R. Charvet, P. Comte, M. K. Nazeeruddin, P. Pechy, M. Takata, H. Miura, S. Uchida and M. Gratzel, Adv. Mater., 2006, 18, 1202 CrossRef CAS.
- A. Kay and M. Gratzel, Sol. Energy Mater. Sol. Cells, 1996, 44, 99 CrossRef CAS.
- (a) E. Ramasamy, W. J. Lee, D. Y. Lee and J. S. Song, Appl. Phys. Lett., 2007, 90, 173103 CrossRef; (b) Z. Huang, X. Liu, K. Li, D. Li, Y. Luo, H. Li, W. Song, L. Chen and Q. Meng, Electrochem. Commun., 2007, 9, 596 CrossRef CAS.
- T. N. Murakami, S. Ito, Q. Wang, M. K. Nazeeruddin, T. Bessho, I. Cesar, P. Liska, R. Humphry-Baker, P. Comte, P. Pechy and M. Gratzel, J. Electrochem. Soc., 2006, 153, A2255 CrossRef CAS.
- M. Gratzel, Inorg. Chem., 2005, 44, 6841 CrossRef.
- A. F. Nogueira, C. Longo and M.-A. De Paoli, Coord. Chem. Rev., 2004, 248, 1455 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, I. Exnar and M. Gratzel, Chem. Commun., 2002, 2972 RSC.
- (a) M. Gorlov and L. Kloo, Dalton Trans., 2008, 2655 RSC; (b) P. Wang, B. Wenger, R. Humphry-Baker, J.-E. Moser, J. Teuscher, W. Kantlehner, J. Mezger, E. V. Stoyanov, S. M. Zakeeruddin and M. Gratzel, J. Am. Chem. Soc., 2005, 127, 6850 CrossRef.
- Y. Bai, Y. Cao, J. Zhang, N. Wang, R. Li, P. Wang, S. M. Zakeeruddin and M. Gratzel, Adv. Mater., 2008, 7, 626 CAS.
- Q. Dai, D. R. MacFarlane, P. C. Howlett and M. Forsyth, Angew. Chem., Int. Ed., 2005, 44, 313 CrossRef CAS.
- (a) G. Nazmutdinova, S. Sensfuss, M. Schrodner, A. Hinsch, R. Sastrawan, D. Gerhard, S. Himmler and P. Wasserscheid, Solid State Ionics, 2006, 177, 3141 CrossRef CAS; (b) J. H. Kima, M.-S. Kanga, Y. J. Kima, J. Wonb and Y. S. Kang, Solid State Ionics, 2005, 176, 579 CrossRef CAS.
- (a) F. Cao, G. Oskam and P. C. Searson, J. Phys. Chem., 1995, 99, 17071 CrossRef CAS; (b) P. Judeinstein and C. Sanchez, J. Mater. Chem., 1996, 6, 511 RSC; (c) Y. Ren, Z. Zhang, S. Fang, M. Yang and S. Cai, Sol. Energy Mater. Sol. Cells, 2002, 71, 253 CrossRef CAS.
- E. Stathatos, P. Lianos, U. Lavrencic-Stangar and B. Orel, Adv. Mater., 2002, 14, 354 CrossRef CAS.
- M. A. Karim, Y.-R. Cho, J. S. Park, S. C. Kim, H. J. Kim, J. W. Lee, Y.-S. Gal and S.-H. Jin, Chem. Commun., 2008, 16, 1929 RSC.
- C.-W. Tu, A.-T. Chien, C. H. Lee, K.-C. Ho and K.-F. Lin, Eur. Polym. J., 2008, 44, 608 CrossRef CAS.
- (a) S. Murai, S. Mikoshiba, H. Sumino, T. Kato and S. Hayase, Chem. Commun., 2003, 1534 RSC; (b) T. Kato, A. Okazaki and S. Hayase, Chem. Commun., 2005, 363 RSC; (c) S. Murai, S. Mikoshiba, H. Suminoa and S. Hayase, J. Photochem. Photobiol., A, 2002, 148, 33 CrossRef CAS.
- T. Kato, A. Okazaki and S. Hayase, J. Photochem. Photobiol., A, 2006, 179, 42 CrossRef CAS.
- L. Wang, S. Fang, Y. Lin, X. Zhou and M. Li, Chem. Commun., 2005, 5687 RSC.
- N. Papageorgiou, Y. Athanassov, M. Armand, P. BonhGte, H. Pettersson, A. Azam and M. Gratzel, J. Electrochem. Soc., 1996, 143, 3099 CAS.
- N. Yamanaka, R. Kawano, W. Kubo, N. Masaki, T. Kitamura, Y. Wada, M. Watanabe and S. Yanagida, J. Phys. Chem. B, 2007, 111, 4763 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, R. Humphry-Baker and M. Gratzel, Chem. Mater., 2004, 16, 2694 CrossRef CAS.
- (a) P. Wang, S. M. Zakeer uddin, P. Comte, I. Exnar and M. Gratzel, J. Am. Chem. Soc., 2003, 125, 1166 CrossRef CAS; (b) P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Gratzel, Nat. Mater., 2003, 2, 402 CrossRef CAS.
- N. Yamanaka, R. Kawano, W. Kubo, T. Kitamura, Y. Wada, M. Watanabe and S. Yanagida, Chem. Commun., 2005, 740 RSC.
- (a) J. E. Kroeze, N. Hirata, S. Koops, M. K. Nazeeruddin, L. Schmidt-Mende, M. Gratzel and J. R. Durrant, J. Am. Chem. Soc., 2006, 128, 16376 CrossRef CAS; (b) T. Odaa, S. Tanakab and S. Hayase, Sol. Energy Mater. Sol. Cells, 2006, 90, 2696 CrossRef; (c) W. Kubo, S. Kambe, S. Nakade, T. Kitamura, K. Hanabusa, Wada and S. Yanagida, J. Phys. Chem. B, 2003, 107, 4374 CrossRef CAS.
- Y. Saito, T. Azechi, T. Kitamura, Y. Hasegawa, Y. Wada and S. Yanagida, Coord. Chem. Rev., 2004, 248, 1469 CrossRef CAS.
- S. A. Haque, T. Park, C. Xu, S. Koops, N. Schulte, R. J. Potter, A. B. Holmes and J. R. Durrant, Adv. Funct. Mater., 2004, 14, 435 CrossRef CAS.
- K. Tennakone, G. R. R. A. Kumara, A. R. Kumarasinghe, K. G. U. Wijayantha and P. M. Sirimanne, Semicond. Sci. Technol., 1995, 10, 1689 CrossRef.
- B. O'Regan and D. T. Schwartz, J. Appl. Phys., 1996, 80, 4749 CrossRef CAS.
- B. O'Regan and D. T. Schwartz, Chem. Mater., 1998, 10, 1501 CrossRef CAS.
- K. Tennakone, G. R. R. A. Kumara, I. R. M. Kottegoda, K. G. U. Wijayantha and V. P. S. Perera, J. Phys. D: Appl. Phys., 1998, 31, 1492 CrossRef CAS.
- K. Tennakone, G. K. R. Senadeera, V. P. S. Perera, I. R. M. Kottegoda and L. A. A. De Silva, Chem. Mater., 1999, 11, 2474 CrossRef CAS.
- G. R. A. Kumara, S. Kaneko, M. Okuya and K. Tennakone, Langmuir, 2002, 18, 10493 CrossRef CAS.
- B. O'Reagan, F. Lenzmann, R. Muis and J. Wienke, Chem. Mater., 2002, 14, 5023 CrossRef CAS.
- Q.-B. Meng, K. Takahashi, X.-T. Zhang, I. Sutanto, T. N. Rao, O. Sato and A. Fujishima, Langmuir, 2003, 19, 3572 CrossRef CAS.
- G. K. R. Senadeera, T. Kitamura, Y. Wada and S. Yanagida, J. Photochem. Photobiol., A, 2006, 184, 234 CrossRef CAS.
- X. Tan, J. Zhai, M. X. Wan, Q. B. Meng, Y. L. Li, L. Jiang and D. B. Zhu, J. Phys. Chem. B, 2004, 108, 18693 CrossRef CAS.
- Y. P. Wang, K. Yang, S. C. Kim, R. Nagarajan, L. A. Samuelson and J. Kumar, Chem. Mater., 2006, 18, 4215 CrossRef CAS.
- E. Lancelle-Beltran, P. Prene, C. Boscher, P. Belleville, P. Buvat and C. Sanchez, Adv. Mater., 2006, 18, 2579 CrossRef CAS.
- E. Lancelle-Beltran, P. Prene, C. Boscher, P. Belleville, P. Buvat, S. Lambert, F. Guillet, C. Marcel and C. Sanchez, Eur. J. Inorg. Chem., 2008, 6, 903 CrossRef.
- G. K. R. Senadeera, T. Kitamura, Y. Wada and S. Yanagida, J. Photochem. Photobiol., A, 2006, 184, 234 CrossRef CAS.
- R. Senadeera, N. Fukuri, Y. Saito, T. Kitamura, Y. Wada and S. Yanagida, Chem. Commun., 2005, 2259 RSC.
- N. Fukuri, Y. Saito, W. Kubo, G. K. R. Senadeera, T. Kitamura, Y. Wada and S. Yanagida, J. Electrochem. Soc., 2004, 151, A1745 CrossRef CAS.
- A. J. Mozer, K. J. Jiang, Y. Wada, N. Masaki, S. N. Mori and S. Yanagida, Appl. Phys. Lett., 2006, 89, 043509 CrossRef.
- J. Xia, N. Masaki, M. Lira-Cantu, Y. Kim, K. Jiang and S. Yanagida, J. Am. Chem. Soc., 2008, 130, 1258 CrossRef CAS.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Gratzel, Nature, 1998, 395, 583 CrossRef.
- J. Kruger, R. Plass, M. Gratzel and H. J. Matthieu, Appl. Phys. Lett., 2002, 81, 367 CrossRef CAS.
- J. Kruger, R. Plass, L. Cevey, M. Piccirelli, M. Gratzel and U. Bach, Appl. Phys. Lett., 2001, 79, 2085 CrossRef CAS.
- L. Schmidt-Mende, S. M. Zakeeruddin and M. Gratzel, Appl. Phys. Lett., 2005, 86, 013504 CrossRef.
- D. Poplavskyy and J. Nelson, J. Appl. Phys., 2003, 93, 341 CrossRef CAS.
- H. J. Snaith and M. Gratzel, Adv. Mater., 2007, 19, 3643 CrossRef CAS.
- J. E. Kroeze, N. Hirata, L. Schmidt-Mende, C. Orizu, S. D. Ogier, K. Carr, M. Gratzel and J. R. Durrant, Adv. Funct. Mater., 2006, 16, 1832 CrossRef CAS.
- H. Wang, H. Li, B. Xue, Z. Wang, Q. Meng and L. Chen, J. Am. Chem. Soc., 2005, 127, 6394 CrossRef CAS.
- H. Wang, Z. Wang, B. Xue, Q. Meng, X. Huang and L. Chen, Chem. Commun., 2004, 2186 RSC.
- H. L. An, B. F. Xue, D. M. Li, H. Li, Q. B. Meng, L. Guo and L. Q. Chen, Electrochem. Commun., 2006, 8, 170 CrossRef CAS.
- B. F. Xue, H. X. Wang, Y. S. Hu, H. Li, Z. X. Wang, Q. B. Meng, X. J. Huang, L. Q. Chen, O. Sato and A. Fujishima, C. R. Chim., 2006, 9, 627 CrossRef CAS.
- B. F. Xue, H. X. Wang, Y. S. Hu, H. Li, Z. X. Wang, Q. B. Meng, X. J. Huang, L. Q. Chen, O. Sato and A. Fujishima, Chin. Phys. Lett., 2004, 21, 1828 CrossRef.
- B. F. Xue, H. X. Wang, Y. S. Hu, H. Li, Z. X. Wang, Q. B. Meng, X. J. Huang, O. Sato, L. Q. Chen and A. Fujishima, Photochem. Photobiol. Sci., 2004, 3, 918 RSC.
- W. Weppner, W. Welzel, R. Kniep and A. Rabenau, Angew. Chem., Int. Ed. Engl., 1986, 25, 1087 CrossRef.
- H. X. Wang, B. F. Xue, Y. S. Hu, Z. X. Wang, Q. B. Meng, X. J. Huang and L. Q. Chen, Electrochem. Solid-State Lett., 2004, 7, A302.
- H. Wang, X. Liu, Z. Wang, H. Li, D. Li, Q. Meng and L. Chen, J. Phys. Chem. B, 2006, 110, 5970 CrossRef CAS.
- S. Shi, L. Xu, C. Ouyang, Z. Wang and L. Chen, Ionics, 2006, 12, 343 CAS.
- (a) H. X. Wang, Preparation, structural study of novel solid-state electrolytes and their application on dye-sensitized solar cell, PhD thesis, Institute of Physics, Chinese Academy of Science, Beijing, China, 2005; (b) S. Yanagida, C.R. Chimie, 2006, 9, 597 Search PubMed; (c) B. I. Ito, J. N. de Freitas, M.-A. De Paoli and A. F. Nogueira, J. Braz. Chem. Soc., 2008, 19, 688 CAS; (d) C. C. Liang, J. Electrochem. Soc., 1973, 120, 1289 CrossRef CAS.
- J. Maier, Prog. Solid State Chem., 1995, 23, 171 CrossRef CAS.
- (a) F. Croce, G. B. Appetecchi, L. Persi and B. Scrosati, Nature, 1998, 394, 456 CrossRef CAS; (b) K.-S. Ji, J. -S. Moon, J. -W. Kim and J. -W. Park, J. Power Sources, 2003, 117, 124 CrossRef CAS.
- (a) P. Wang, Q. Dai, S. M. Zakeeruddin, M. Forsyth, D. R. MacFarlane and M. Gratzel, J. Am. Chem. Soc., 2004, 126, 13590 CrossRef CAS; (b) Z. Chen, H. Yang, X. Li, F. Li, T. Yi and C. Huang, J. Mater. Chem., 2007, 17, 1602 RSC.
- (a) P.-J. Alarco, Y. Abu-Lebdeh, A. Abouimrane and M. Armand, Nat. Mater., 2004, 3, 476 CrossRef CAS; (b) S. Long, D. R. MacFarlane and M. Forsyth, Solid State Ionics, 2003, 161, 105 CrossRef CAS.
- X. Liu, D. Qin, Y. Fan, K. Li, D. Li and Q. Meng, Electrochem. Commun., 2007, 9, 1735 CrossRef CAS.
- B. F. Xue, Z. W. Fu, H. Li, X. Z. Liu, S. C. Cheng, J. Yao, D. M. Li, L. Q. Chen and Q. B. Meng, J. Am. Chem. Soc., 2006, 128, 8720 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
