Hydride-induced cleavage of C–O bond in pyran ring of 2-pyranylidene carbene complexes: mechanism and synthetic application†
Qifeng Wanga, Lantao Liua, Chen Linb, Hui Sunb, Wen-Xiong Zhangb and Zhenfeng Xi*abc
aBeijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. Fax: 86-(0)10 62759728; Tel: 86-(0)10 62759728
bBeijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China. E-mail: zfxi@pku.edu.cn; Fax: 86-(0)10 62759728; Tel: 86-(0)10 62759728
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 8th October 2009
Abstract
Hydride-induced cleavage of the C–O bond in the pyran ring followed by ring closure of unsaturated acylmetalates and reductive elimination was achieved.
The demetalation reaction of Fischer carbene complexes (Cr, Mo, W) has been a topic of significant importance both for fundamental research in organometallic chemistry and for synthetic chemistry.1 Among various methods reported so far,1 reductive demetalation with metal hydrides has been shown to be an efficient method.2 In principal, hydride attack will afford M
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond reduction, reduction/isomerization or reduction/M(CO)5-transfer products.1,2 In this manuscript, we wish to report, as the first example of this type, hydride-induced cleavage of the C–O bond in the pyran ring of 2-pyranylidene carbene (Cr, W) complexes followed by ring closure and reductive elimination. We also report, based on understanding the reaction mechanism mentioned above, a highly efficient synthesis of a new type of unsaturated reactive acylmetalates, which are of general interest as reactive and useful organometallic compounds.3–5
C bond reduction, reduction/isomerization or reduction/M(CO)5-transfer products.1,2 In this manuscript, we wish to report, as the first example of this type, hydride-induced cleavage of the C–O bond in the pyran ring of 2-pyranylidene carbene (Cr, W) complexes followed by ring closure and reductive elimination. We also report, based on understanding the reaction mechanism mentioned above, a highly efficient synthesis of a new type of unsaturated reactive acylmetalates, which are of general interest as reactive and useful organometallic compounds.3–52-Pyranylidene is an interesting and useful Fischer carbene complex,6 and has been widely utilized in organic synthesis.7–9 Recently, we reported the first examples of fully alkyl substituted 2-pyranylidene carbene complexes.10 Therefore, we started this project to investigate the reactivity of these complexes. Carbene complexes 1 were treated with 0.5 equivalents of LiAlH4 at room temperature affording 3-cyclopentenones 2 in high yields as a mixture of trans/cis isomers upon hydrolysis with H2O (Scheme 1). 2-Cyclopentenones 3 were obtained in high yields when the above reaction was quenched with 3 N HCl. Experimental results demonstrated that products 2 could be quantitatively converted into products 3 under acidic conditions. It is noteworthy that when we treated analogous carbene complexes with electron-withdrawing substituents reported by the groups of Aumann and Wulff,8 a mixture of unidentified products were obtained under the same conditions, demonstrating that substituents on the pyran skeleton play a very important role in its reactivity.
 | ||
| Scheme 1 Reductive demetalation of 2-pyranylidene carbene complexes with LiAlH4. | ||
The above unprecedented reductive demetalation reaction, which should be very important for the synthetic application of carbene complexes in general, was investigated by deuterium labelling experiments using different deuterium reagents. As summarized in Scheme 2, these results strongly suggest that a hydride might first attack the 6 position of the pyran ring, similar to that proposed previously by Iwasawa on the reaction of hydride with benzopyranylidene tungsten(0) complexes.9a During our attempts to investigate the intermediates, a large amount of a solid complex was observed in situ and was characterized by NMR spectroscopy to have a carbonyl group, which indicated that carbonyl metal complexes are “released” during the reductive demetalation process.
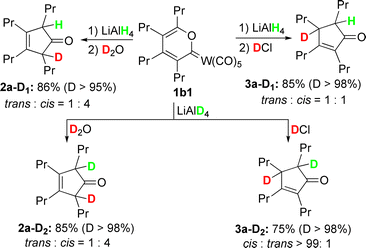 | ||
| Scheme 2 Deuterium labelling experiments for reductive demetalation of 1b1 under different conditions. | ||
In addition to the deuterium labelling experiments, benzoyl chloride was used to trap the intermediate with the aim of providing more evidence for the reaction mechanism and further application of this reductive demetalation reaction. α-Acylated 3-cyclopentenones 4 in perfect cis form were obtained in high isolated yields (eqn (1)).
 | (1) |
Based on the above results, a reaction mechanism is proposed and shown in Scheme 3. Hydride attack on 1 leads to ring opening to give intermediate A, an unsaturated reactive acylmetalate.3–5,7 The resulting intermediate A undergoes electrocyclic ring closure to form the six-membered metallacycle B,5 which, via reductive elimination of a carbonyl complex,11 generates cyclopenta-1,3-dienolate C. Trapping of C with electrophiles gives α-protonated or α-acylated products.12
 | ||
| Scheme 3 A proposed mechanism for the reductive demetalation process. | ||
Acylmetalate, the ring-opening intermediate A in the above proposed reaction mechanism, is the key intermediate in this reaction, and is of general interest as an active and useful organometallic compound.3–5,7 We then envisioned that the reaction of 1-lithio-1,3-butadienes 5 with M(CO)6 would give intermediate D, which might be converted into acylmetalate A (Scheme 4). If this is true, such a reaction will provide direct evidence for the reaction mechanism shown in Scheme 3, and more significantly, provides a novel method for the generation of unsaturated acylmetalates.
 | ||
| Scheme 4 A novel approach to generate unsaturated acylmetalates. | ||
In Scheme 5 are representative results obtained from the reaction of 1-lithio-1,3-butadienes 5 with M(CO)6 (M = Cr, W). Obviously, reactions of 1-lithio-1,3-butadienes 5 with M(CO)6 proceed via intermediates D and A as shown in Scheme 4, providing a novel method for the generation of unsaturated acylmetalates. All these results obtained are based on understanding the reaction mechanisms shown in Schemes 3 and 4 respectively, and are very informative and important, particularly for the generation and application of unsaturated acylmetalates.
 | ||
| Scheme 5 Generation and synthetic application of unsaturated acylmetalates from 1-lithio-1,3-diene with M(CO)6. | ||
In conclusion, we have developed a novel reductive demetalation reaction of 2-pyranylidene carbene complexes with LiAlH4, and a novel synthetic approach to unsaturated acylmetalates based on understanding the hydride-induced reductive demetalation reaction mechanism. Further applications of these useful methodologies, especially the method for the generation of unsaturated acylmetalates, are in progress.
Acknowledgements
This work was supported by the Natural Science Foundation of China and the Major State Basic Research Development Program (2006CB806105). BASF, Dow Corning Corporation, and Eli Lilly China are gratefully acknowledged.Notes and references
- For selected reviews: (a) J. Barluenga, M. A. Fernández-Rodríguez and E. Aguilar, J. Organomet. Chem., 2005, 690, 539–587 CrossRef CAS; (b) M. Gómez-Gallego, M. J. Mancheño and M. A. Sierra, Acc. Chem. Res., 2005, 38, 44–53 CrossRef CAS; (c) J. Barluenga, J. Santamaría and M. Tomás, Chem. Rev., 2004, 104, 2259–2283 CrossRef CAS; (d) K. H. Dötz, C. Jäkel and W.-C. Haase, J. Organomet. Chem., 2001, 617–618, 119–132 CrossRef CAS; (e) A. de Meijere, H. Schirmer and M. Duetsch, Angew. Chem., Int. Ed., 2000, 39, 3964–4002 CrossRef CAS; (f) R. Aumann, Eur. J. Org. Chem., 2000, 17–31 CrossRef CAS; For selected examples: (g) J. Chen, Z. Yu, Z. Zheng, K. Gu, S. Wu, F. Zeng, W. Tan, X. Wu and W. Xiao, Organometallics, 2005, 24, 302–308 CrossRef CAS; (h) L. S. Hegedus, G. de Weck and S. D'Andrea, J. Am. Chem. Soc., 1988, 110, 2122–2126 CrossRef CAS; (i) W. D. Wulff and D. C. Yang, J. Am. Chem. Soc., 1983, 105, 6726–6728 CrossRef CAS.
- (a) P. Ramírz-López, M. A. Sierra, M. Gómez-Gallego, M. J. Mancheño and H. Gornitzka, Organometallics, 2003, 22, 5092–5099 CrossRef CAS; (b) J. Barluenga, A. Granados, F. Rodríguez, J. Vadecard and F. J. Fañanás, Tetrahedron Lett., 1997, 38, 6465–6466 CrossRef CAS.
- M. A. Sierra, Chem. Rev., 2000, 100, 3591–3637 CrossRef CAS and references therein.
- (a) N. Faux, F. Robin-Le Guen, P. Le Poul, B. Caro, K. Nakatani, E. Ishow and S. Golhen, Eur. J. Inorg. Chem., 2006, 3489–3497 CrossRef CAS; (b) Y. Lian and W. D. Wulff, J. Am. Chem. Soc., 2005, 127, 17162–17163 CrossRef CAS; (c) J. Barluenga, J. Alonso, F. J. Fañanás, J. Borge and S. García-Granda, Angew. Chem., Int. Ed., 2004, 43, 5510–5513 CrossRef CAS.
- (a) L. Gong, Y. Li, T. Wen, H. Zhang, B. Zeng and H. Xia, Organometallics, 2008, 27, 2584–2589 CrossRef CAS; (b) N. Xiao, S. Zhang, Z. Qiu, R. Li, B. Wang, Q. Xu, J. Sun and J. Chen, Organometallics, 2002, 21, 3709–3715 CrossRef CAS; (c) Y. T. Wu, H. Schirmer, M. Noltemeyer and A. de Meijere, Eur. J. Org. Chem., 2001, 2501–2506 CrossRef CAS; (d) R. Ferede and N. T. Allison, Organometallics, 1983, 2, 463–465 CrossRef CAS.
- C. W. Rees and E. von Angerer, J. Chem. Soc., Chem. Commun., 1972, 420–420 RSC.
- (a) Z. Yu, R. Aumann, R. Fröhlich, K. Roths and J. Hecht, J. Organomet. Chem., 1997, 541, 187–198 CrossRef CAS; (b) R. Aumann, K. Roths, B. Jasper and R. Fröhlich, Organometallics, 1996, 15, 1257–1264 CrossRef CAS.
- (a) R. Aumann, A. G. Meyer and R. Fröhlich, J. Am. Chem. Soc., 1996, 118, 10853–10861 CrossRef CAS; (b) S. L. B. Wang and W. D. Wulff, J. Am. Chem. Soc., 1990, 112, 4550–4552 CrossRef CAS.
- (a) H. Kusama, T. Sawada, A. Okita, F. Shiozawa and N. Iwasawa, Org. Lett., 2006, 8, 1077–1079 CrossRef CAS; (b) H. Kusama, F. Shiozawa, M. Shido and N. Iwasawa, Chem. Lett., 2002, 124–125 CrossRef CAS; (c) N. Iwasawa, M. Shido, K. Maeyama and H. Kusama, J. Am. Chem. Soc., 2000, 122, 10226–10227 CrossRef CAS; (d) K. Ohe, K. Miki, T. Yokoi, F. Nishino and S. Uemura, Organometallics, 2000, 19, 5525–5528 CrossRef CAS.
- Q. Wang, W.-X. Zhang and Z. Xi, Organometallics, 2008, 27, 3627–3629 CrossRef CAS.
- M. Yeh, L. Chuang, S. Chang, M. Lai and C. Chou, Organometallics, 1997, 16, 4435–4444 CrossRef CAS.
- (a) Q. Song, Z. Li, J. Chen, C. Y. Wang and Z. Xi, Org. Lett., 2002, 4, 4627–4629 CrossRef CAS; (b) Q. Song, J. Chen, X. Jin and Z. Xi, J. Am. Chem. Soc., 2001, 123, 10419–10420 CrossRef CAS; (c) P. Baierweck, D. Hoell and K. Mullen, Angew. Chem., Int. Ed. Engl., 1985, 24, 972–973 CrossRef; (d) M. Koreeda and S. G. Mislankar, J. Am. Chem. Soc., 1983, 105, 7203–7205 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, scanned NMR spectra of all products and X-ray crystal structural data for 4c. CCDC reference number 734642. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b918679b |
| This journal is © The Royal Society of Chemistry 2009 |
