Optically active uniform potassium and lithium rare earth fluoride nanocrystals derived from metal trifluroacetate precursors†
Ya-Ping Du, Ya-Wen Zhang*, Ling-Dong Sun and Chun-Hua Yan*
Beijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, PKU-HKU Joint Laboratory in Rare Earth Materials and Bioinorganic Chemistry, Peking University, Beijing, 100871, China. E-mail: yan@pku.edu.cn; ywzhang@pku.edu.cn; Fax: +86-10-6275-4179; Tel: +86-10-6275-4179
First published on 2nd September 2009
Abstract
This paper reports the first systematical synthesis of near-monodisperse potassium and lithium rare earth (RE) fluoride (K(Li)REF4) nanocrystals with diverse shapes (cubic KLaF4 and KCeF4 wormlike nanowires, nanocubes and nanopolyhedra; cubic LiREF4 (RE = Pr to Gd, Y) nanopolyhedra; tetragonal LiREF4 (RE = Tb to Lu, Y) rhombic nanoplates) via co-thermolysis of Li(CF3COO) or K(CF3COO) and RE(CF3COO)3 in a hot oleic acid/oleylamine/1-octadecene solution. The effects of the solvent composition, reaction temperature and time on the crystal phase purity, shape, and size of the as-prepared nanocrystals have been investigated in detail. The formation of monodisperse nanocrystals is found to strongly depend upon the nature of both alkali metals from Li to K, and the rare earth series from La to Lu and Y. Based on the series of experimental results, a controlled-growth mechanism has also been proposed. In addition, the ease of doping of these as-synthesized host nanocrystals for designed luminescence properties is assessed. For example, monodisperse and single-crystalline Eu3+ doped KGdF4, Yb3+ and Er3+ co-doped LiYF4 nanocrystals redispersed in cyclohexane exhibit visible room-temperature red and green emissions under ultraviolet (UV) excitation and near infrared (NIR) 980 nm laser excitation, respectively.
Introduction
Studies on monodisperse colloidal inorganic nanocrystals have advanced dramatically in the past few years. The colloidal nanocrystals with controlled shape, size, composition, phase, and surface endow such materials with unique optical, electronic, magnetic, and catalytic properties, which are fundamentally important and technologically useful.1 Precisely controlled synthesis allows manipulation of the properties of nanocrystals as desired. Therefore, up to present, various wet chemical strategies have been designed and developed for obtaining such nanocrystals, which not only are recognized for their unique material properties, but also have already acted as the building blocks for the advanced nanodevices composed of 2D (two dimensional) and 3D (three dimensional) assemblies.2 Amongst these, the relatively successful synthetic methods are based on the nonhydrolytic approach and its alternatives, with which nanocrystalline products can be grown with controlled-size, shape, phase, and composition for a given inorganic compound.1e,g2a–fColloidal rare earth (RE) compound nanocrystals such as oxides, phosphates, fluorides, vanadates, sulfides, and oxyhalides have become a new focus of research, due to their extraordinary properties arising from the 4f electron configuration and the potential applications in solid-state lasers, optical amplifies, lighting and displays, and biolabels.3 Among them, the rare earth doped fluorides based on AREF4 (A = alkali metal, RE = rare earth) have been investigated for several decades due to their unique luminescent, ferromagnetic, insulating/magnetic, and piezoelectric properties and wide applications in laser devices, three-dimensional flat-panel displays, and low-intensity infrared imaging.4 For example, the hexagonal phase of NaYF4 is considered as the most efficient host material for green and blue upconversion (UC) phosphors until now,4e LiGdF4 is an outstanding host for downconversion luminescence, for which the quantum efficiency is close to 200%.5b Rare earth doped single crystals of KYF4 are excellent solid-state laser materials, and laser action has been observed for Yb3+, Tm3+, Ho3+ ions in the KYF4 lattice.5a,c
As an important category of rare earth fluoride compounds of AREF4 material, KREF4 and LiREF4 are particularly suitable luminescence hosts for doping lanthanide ions due to their low phonon energies, which reduce the thermal disturbance effects under strong pumping conditions.5 There have already some reports on the solution phase synthesis and optical characterization of nanosized NaREF4.6 To our knowledge, the systematic study of the synthesis and material properties of monodisperse colloidal KREF4 and LiREF4 nanocrystals has never been described until now.7 The current synthesis methods available for nanosized KREF4 and LiREF4 are mainly based on the liquid coprecipitation reaction between soluble rare earth salts and alkali fluorides.7a,df–h
In this article, we demonstrate a general one-step synthesis of near-monodisperse KREF4 (RE = La to Gd, Y) and LiREF4 (RE = Tb to Lu, and Y) colloidal nanocrystals via the co-thermolysis of K(CF3COO) or Li(CF3COO) and RE(CF3COO)3 precursors in oleic acid/oleylamine/1-octadecene. The effects of different experimental conditions on the crystal phase purity, shape and size of the KREF4 and LiREF4 nanocrystals have been investigated. The formation mechanisms of the nanocrystals have also been proposed. With the developed synthesis method, monodisperse KGdF4:Eu, LiYF4:Yb,Er nanocrystals have also been obtained, which show interesting optical properties under ultraviolet (UV) and near infrared (NIR) laser (980 nm) excitations.
Experimental
Materials
The synthesis was carried out using standard oxygen-free procedures and commercially available reagents. Rare earth oxides, oleic acid (OA; 90%, Alpha), oleylamine (OM; >80%, Acros), 1-octadecene (ODE; >90%, Acros), trifluoroacetic acid (99%, Acros), K(CF3COO) (>97%, Acros), absolute ethanol, cyclohexane were used as received. RE(CF3COO)3 and Li(CF3COO) were prepared with the literature method.8 All the chemicals were of analytical grade and used as received without further purification.Synthesis of KREF4 (RE = La to Gd, Y) nanocrystals
A given amount of K(CF3COO) and RE(CF3COO)3 (1 mmol) were added into 40 mmol of OA/OM/ODE (Table S1, ESI†) in a three-necked flask at room temperature. Then the slurry was heated to 120 °C to remove water and oxygen, with vigorous magnetic stirring under vacuum for 30 min in a temperature-controlled electromantle, and thus forming a transparent solution. The solution was then heated to a certain temperature in the range of 250–330 °C at a rate of 22 K min−1 and maintained at the given temperature for 15–120 min under an Ar atmosphere. When the reaction was completed, an excessive amount of ethanol was poured into the solution at room temperature. The resultant mixture was centrifugally separated and the products were collected. The as-precipitated nanocrystals were washed several times with ethanol and dried in air at 80 °C overnight. The yields of all the obtained nanocrystals without size-selection were 60–70%. All these as-prepared nanocrystals could be easily dispersed in various nonpolar organic solvents (e.g., cyclohexane).Synthesis of LiREF4 (RE = Tb to Lu, Y) nanocrystals
The synthetic procedure was the same as that used to synthesize KREF4 nanocrystals, except that a certain amount of as-prepared Li(CF3COO) and RE(CF3COO)3 were added into a mixture of 40 mmol of OA/ODE (Table S1, ESI†) in a three-necked flask at room temperature.Synthesis of KGdF4:5%Eu and LiYF4:20%Yb,2%Er nanocrystals
The synthetic procedure was the same as that used to synthesize KREF4 nanocrystals, except that 1 mmol K(CF3COO) or Li(CF3COO) and a suitable proportion of Gd(CF3COO)3, Eu(CF3COO)3, Y(CF3COO)3, Yb(CF3COO)3, and Er(CF3COO)3 were added into a mixture of OM and/or OA and ODE (total: 40 mmol) in a three-necked flask at room temperature.Instrumentation
Powder X-ray diffraction (PXRD) patterns of the dried powders were recorded on a Rigaku D/MAX-2000 diffractometer (Japan) with a slit of 1/2° at a scanning rate of 4° min−1, using Cu-Kα radiation (λ = 1.5418 Å). The lattice parameters were calculated with the least-squares method. Samples for transmission electron microscopy (TEM) analysis were prepared by drying a drop of nanocrystal dispersion in cyclohexane on amorphous carbon-coated copper grids. Particle sizes and shapes were examined by a JEOL 200CX-TEM (Japan) operated at 160 kV. High-resolution TEM (HRTEM) characterization was performed with a Philips Tecnai F30 FEG-TEM (USA) operated at 300 kV. FTIR spectra were obtained on a Bruker Vector22 spectrophotometer (German). Room-temperature fluorescence spectrum of KGdF4:Eu nanocrystals were recorded on a Hitachi F-4500 spectrophotometer (Japan) equipped with a 150 W Xe arc lamp at a fixed bandpass of 0.2 nm with the same instrument parameters (10.0 nm for excitation slit, 10.0 nm for emission slit, and 700 V for PMT voltage), with the as-dried nanocrystals redispersed in cyclohexane at a concentration of about 2 wt%. The photoluminescence quantum yields of the KGdF4:Eu nanocrystals were determined by comparing the integral emission intensity of the dilutions of the concentrated nanocrystal suspension in cyclohexane with that of rhodamine B in absolute ethanol at an excitation of 275 nm with collection between 550 and 700 nm at room temperature.3l Upconversion emission spectra of LiYF4:Yb,Er nanocrystals were obtained on a modified Hitachi F-4500 spectrophotometer with an external tunable 2 W 980 nm laser diode as the excitation source, with the as-dried nanocrystals redispersed in cyclohexane at a concentration of about 2 wt%.Results and discussion
Characterizations of KREF4 and LiREF4 nanocrystals
 | ||
| Fig. 1 (a) XRD patterns of cubic KLaF4 and KCeF4 wormlike nanowires. (b) TEM and HRTEM (inset) images of KLaF4 wormlike nanowires. | ||
 | ||
| Fig. 2 (a) XRD patterns of as-obtained cubic KREF4 nanopolyhedra. TEM and HRTEM (inset) images of (b) KPrF4, (c) KEuF4, and (d) KYF4 nanopolyhedra. | ||
The nanocrystals are of polyhedron shape, and have a near-monodisperse size of 18.2 ± 3.6 nm for KPrF4, 16.4 ± 4.2 nm for KNdF4, 18.9 ± 3.8 nm for KSmF4, 19.5 ± 4.1 nm for KEuF4, 21.2 ± 6.1nm for KGdF4, except for KYF4 in a size of 13.2–25.9 nm. The HRTEM images insets in Fig. 2b–d and Fig. S2b–d (see ESI†) show that all the KREF4 nanocrystals are of single-crystalline nature, and are mainly enclosed by the (111) and (200) planes.
 | ||
| Fig. 3 (a) XRD patterns of as-obtained tetragonal LiREF4 nanoplates. TEM and HRTEM (inset) images of (b) LiTbF4, (c) LiErF4, and (d) LiYF4 nanoplates. | ||
The surfactant bound on the nanocrystals surfaces was confirmed by FTIR spectroscopy, the FTIR spectrum of the as-synthesized LiYF4 nanocrystals is shown in Fig. S5 (see ESI†), the presence of strong acyclic C–H stretching at 2931 cm−1 and weak carbonyl peaks at 1712 cm−1 indicated the existence of a large amount of free oleic acid in solution, the broad peaks at 1449 and 1645cm−1 could be assigned to a carboxylate (COO−) stretch, implying a chemical bonding of OA molecules on the nanocrystal surfaces.9g
Synthesis of KREF4 and LiREF4 nanocrystals
Normally, KREF4 has two polymorphs at room temperature and ambient pressure: orthorhombic and hexagonal, except that KLaF4 and KCeF4 possess a cubic phase. Light and middle KREF4 (RE = Ce to Gd) appear in orthorhombic form, whereas, the heavy one (RE = Tb to Lu, Y) exists in only hexagonal form.7a,bd,h On the other hand, LiREF4 presents only one tetragonal phase, which is stable in bulk at room temperature.7c,e In addition for most of the AREF4 (A = alkali metal), a high-temperature modification exists, which can be seen as the cubic CaF2 type structure with statistical distribution of the cations A+ and RE3+ on the Ca2+ sites.7d,fg In our work, however, all the as-prepared nanosized KREF4 from La to Gd including Y only take on a cubic structure, while those from Tb to Lu prefer to form KF and REF3 instead of producing KREF4. The stoichiometric LiREF4 nanocrystals from Dy to Lu including Y only display the tetragonal phase, and LiF and REOF are formed in terms of lighter LiREF4 ones.In this work, for a certain compound, the controlled synthesis of monodisperse KREF4 and LiREF4 nanocrystals have been carefully conducted. Experimentally, we found that the crystal phase purity, shape and size of the as-prepared KREF4 and LiREF4 nanocrystals are mainly affected by conditions, such as solvent composition, reaction temperature and time (Table S1, ESI†), the optimal synthetic parameters are screened so as to achieve the appropriate balance between the nucleation and growth stages of the nanocrystals to obtain high-quality products.
 | ||
| Fig. 4 TEM images of KLaF4 nanocrystals synthesized in (a) OA/ODE = 1:1 and (b) OA/OM/ODE = 1:4:5 for 60 min at 280 °C. TEM images of KYF4 nanocrystals synthesized in (c) OA/ODE = 1:1 and (d) OA/OM/ODE = 1:4:5 for 30 min at 300 °C. | ||
Different from the KREF4 (RE = La to Gd, Y) nanocrystals, as for LiREF4 (RE = Tb to Lu, Y), the presence of a mixed solvent of OA/ODE is a prerequisite to achieve phase-pure and monodisperse LiREF4 nanocrystals. The presence of OM ligands in the mixed solvent might significantly hinder the growth of LiREF4 nanocrystals, by producing LiF impurity in the nanocrystal growth. Here, we take LiLuF4 as an example, using 1 mmol Li(CF3COO) and 1 mmol Lu(CF3COO)3 as the precursors, the reaction under OA/OM/ODE = 4:1:5 at 280 °C for 60 min, produced non-dispersed LiF (space group: Fm-3m) impurities (Fig. S6, ESI†). The synthesis of the other monodisperse LiREF4 nanocrystals was found to resemble that of LiLuF4 nanocrystals in OA/ODE (1:1) solvent, such as LiYF4 nanocrystals (Table S1 and Fig. S6, ESI†).
 | ||
| Fig. 5 TEM images of KLaF4 nanocrystals synthesized from co-thermolysis of 1 mmol K(CF3COO) and 1 mmol La(CF3COO)3 in OA/OM/ODE (1:1:2) for 60 min at different reaction temperatures: (a) 290 °C, (b) 300 °C and (c) 320 °C. | ||
As for LiREF4, the influences of reaction temperature on the composition and shape evolution of as-synthesized nanocrystals were observed. Here, taking LiErF4 as an example, at reaction temperatures ranging from 280 °C to 300 °C, the co-thermolysis of 1 mmol Li(CF3COO) and 1 mmol Er(CF3COO)3 under OA/ODE = 1:1 for 60 min produced monodisperse ErOF nanocrystals (23.2 ± 0.4) with few LiF impurities (Fig. S8a,b, ESI†), indicating the relative low temperature (< 300 °C) induced the formation of LiF and monodisperse REOF nanocrystals, instead of stoichiometric LiREF4 ones. As the reaction temperature was increased to 320 °C, the monodisperse LiErF4 nanoplates were harvested (Fig. 3c), further elevation of the reaction temperature to 330 °C resulted in highly aggregated LiErF4 nanocrystals (Fig. S8c, ESI†). For another example of LiLuF4, at 280 °C, the co-thermolysis of 1 mmol Li(CF3COO) and 1 mmol Lu(CF3COO)3 under OA/ODE = 1:1 for 60 min produced monodisperse LiLuF4 nanoplates (Fig. S4e, ESI†). As the reaction temperature was increased to 300 °C, the relatively near-monodisperse LiLuF4 nanoplates with a much broader size distribution (41.6 ± 11.8) nm × (39.8 ± 9.9) nm were formed (Fig. S9a, ESI†). When the reaction temperature was further raised to 320 °C, polygonal aggregated LiLuF4 nanocrystals in the size range of 16.1–28.2 nm instead of nanoplates were formed (Fig. S9b, ESI†).
Under a fixed reaction temperature of 280 °C, as the reaction time was shortened from 60 min to 30 min, the co-thermolysis of 1 mmol K(CF3COO) and La(CF3COO)3 in OA/OM/ODE (1:1:2) resulted in poorly separated wormlike nanowires (Fig. 6a). When the reaction went on to 120 min, some KLaF4 wormlike nanowires coexisted with nanocubes (Fig. 6b). The same tendency was observed for the KCeF4 wormlike nanowires. As for KYF4, when the reaction time was 15 min, polydisperse KYF4 nanocrystals (38.5 ± 5.1) nm with relatively low yields were formed (Fig. S10a, ESI†). When the reaction time was prolonged to 45 min, the well-crystallized but poorly separated nanocrystals in improved yields were harvested (Fig. S10b, ESI†). However, as the reaction time reached 60 min, aggregated KYF4 nanoparticles were formed (Fig. S10c, ESI†). For the LiREF4 nanocrystals, such as the synthesis of LiErF4, under a fixed reaction temperature of 330 °C, as the reaction time was shortened from 60 min to 30 min and 15 min, the LiErF4 products appeared as a mixture of nanoplates and nanowires (Fig. S11a,b, ESI†). When the reaction went on to 120 min, the LiErF4 aggregated polydisperse nanoplates were formed (Fig. S11c, ESI†).
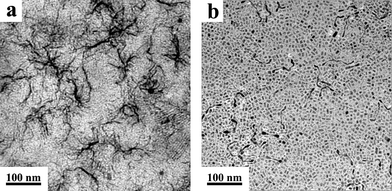 | ||
| Fig. 6 TEM images of KLaF4 nanocrystals obtained from the thermolysis of 1 mmol K(CF3COO) and 1 mmol La(CF3COO)3 in OA/OM/ODE (1:1:2) at 280 °C for different reaction times: (a) 30 min, (b) 120 min. | ||
To sum up, our pure-phase monodisperse KREF4 (RE = La to Gd, Y) nanocrystals could be obtained in mixed solvents of OA/OM/ODE (1:1:2), and LiREF4 (RE = Tb to Lu, Y) nanocrystals could be obtained at a high temperature in OA/ODE (1:1) with a relatively long reaction time.
Formation mechansim of KREF4 and LiREF4 nanocrystals
Just like the case of co-thermolysis of Na(CF3COO) and RE(CF3COO)3 in mixed solvents of OA/OM/ODE,6d as the reaction solutions containing K(CF3COO) or Li(CF3COO) and RE(CF3COO)3 were rapidly heated to their thermolysis temperatures under an Ar atmosphere, tiny gas bubbles formed instantly from the reaction system, indicating a decomposition of the precursors and a simultaneous formation of KREF4 or LiREF4 nuclei (Scheme 1). As indicated by the experimental results, only LiREF4 nuclei were generated for the rare earths from Tb to Lu and Y, while for La to Gd, the LiF and REOF nuclei coexisted. This result suggests that the tendency for the formation of LiREF4 nuclei was depressed for the light rare earths. On the contrary for the synthesis of KREF4 nanocrystals, only KREF4 nuclei were generated for the rare earths from La to Gd and Y, while KF and REF3 nuclei coexisted for Tb to Lu, indicating the tendency for the formation of KREF4 nuclei was easier for the light rare earths, but more difficult for the heavy rare earths. Our research results on the NaREF4 have demonstrated that the NaREF4 nuclei were generated for the rare earths from Pr to Lu and Y, while NaF and REF3 nuclei coexisted for La and Ce.6d,gh These results suggest that the lighter the alkali metal and rare earth, the easier AF (A = alkali metal) and REF3 or REOF nuclei formed, owing to the fact that the basicity of the alkali metal oxide gradually increases along the alkali metal series, while the basicity of the rare earth oxide gradually decreases along the rare earth series. | ||
| Scheme 1 Schematic illustration for a controlled growth mechanism of near-monodisperse KREF4 (RE = La to Gd, Y) and LiREF4 (RE = Tb to Lu, Y) nanocrystals with diverse shapes. | ||
As is well-known, the crystalline phase of the nuclei, the selective adsorption of the surfactant onto specific crystal planes, and the balance between the kinetic and the thermodynamic growth regimes are the three important factors affecting the shape of nanocrystals.1e,9 For the crystalline seeds with an isotropic unit cell structure (e.g., cubic), they often experience an isotropic growth, which results in 0D (zero dimensional) nanostructures. In our synthesis, KREF4 (RE = La to Gd, Y) have a cubic structure (Fig. 7a); therefore, as expected, we obtained 0D-growth shape (nanopolyhedra) of nanocrystals at high temperatures of 300 °C (Scheme 1). The production of wire-like KLaF4 and KCeF4 nanocrystals at reduced temperatures (260–280 °C) may be caused by the oriented aggregation of small particles.9h The wire-like shape was considered to be kinetically stable, since it was observed that the metastable wormlike nanowires were transformed to the thermodynamically stable nanocubes and further nanopolyhedra with increasing reaction temperature and reaction time. As for LiREF4 (RE = Tb to Lu, Y) nanocrystals, the as-observed 2D growth modes for the rhombic nanoplates were probably originated from the crystal structure effect of the anistropic tetrgonal phase (c/a = 2.1) (Fig. 7b).1e,7f,9
 | ||
| Fig. 7 Crystal structures of (a) cubic KREF4 and (b) tetragonal LiREF4 built by CERIUS2 software (ref.: http://www.accelrys.com/cerius2). | ||
Luminescence properties of lanthanide doped KREF4 and LiREF4 nanocrystals
In order to study the luminescence properties of lanthanide doped KREF4 and LiREF4 nanocrystals, monodisperse and single-crystalline (27.2 ± 1.8) nm KGdF4:5%Eu and (14.9 ± 1.4) nm LiYF4:20%Yb,2%Er, nanocrystals were also prepared using the current method (Fig. 8a,b).Fig. 9a describes the emission spectrum of KGdF4:Eu3+ nanocrystals. The emission peaks at 589, 613, 649, and 696 nm can be assigned to the typical 5D0→7FJ emissions (J = 0, 1, 2, 3, 4) of the Eu3+ ions, respectively. The peak located at 589 nm (corresponding to 5D0→7F1 magnetic dipole transition) is weaker than the peak located at 613 nm (corresponding to 5D0→7F2 electric dipole transition), suggesting the presence of a strong surface effect on the red emission of those oleate capped nanocrystals, as compared with the bulk counterpart.3k,l,11 The photoluminescence quantum yields (QYs) of KGdF4:5%Eu nanopolyhedra were determined to be 18%. Fig. 9b exhibits the luminescence decay curves of 5D0→7F2 emission at 613 nm for the KGdF4:5%Eu nanocrystals. These decay curves can be fitted well into a single exponential function: I = I0 exp(−t/τ) (τ is the lifetime of Eu3+). The lifetime of the 5D0→7F2 emission of Eu3+ ions (613 nm) is 1.71 ms for the KGdF4:5%Eu nanopolyhedra, which is close to that (1.72 ms) of 6.3 nm GdOF:5%Eu.3k
 | ||
| Fig. 8 TEM images of lanthanide doped KREF4 and LiREF4 nanocrystals: (a) KGdF4:5%Eu nanopolyhedra (top right is the HRTEM image, bottom right is the eye-visible luminescence photography of its dispersion) and (b) LiYF4:20%Yb,2%Er nanopolyhedra (top right is the HRTEM image, bottom right inset is the eye-visible upconversion luminescence photography of its dispersion). The luminescent nanocrystal dispersions are excited by the 254 nm hand-held UV lamp at room temperature. | ||
 | ||
| Fig. 9 (a) Excitation and emission spectra of KGdF4:5%Eu nanopolyhedra dispersed in cyclohexane (2 wt%). (b) Luminescence decay curve of the 613 nm emission of Eu3+ ions in KGdF4:5%Eu nanopolyhedra (λex = 275 nm). (c) Upconversion spectra of LiYF4:20%Yb,2%Er nanocrystal dispersion in cyclohexane (2 wt%). (d) Power density dependence of the upconversion emission intensity for LiYF4:20%Yb,2%Er nanocrystals under λex = 980 nm. | ||
Fig. 9c shows the UC fluorescence spectrum of as-obtained LiYF4:20%Yb,2%Er nanopolyhedra dispersed in cyclohexane under 980 nm excitation. The green emissions at 521, 541 and 550 nm are attributable to the transitions from 2H11/2 to 4I15/2 and from 4S3/2 to 4I15/2 of Er3+ ions, respectively, and the red emissions at 651 and 667 nm come from the 4F9/2 to 4I15/2 transition of Er3+ ions. To determine the number of photons involved in the UC process of the Yb3+, Er3+ doped LiYF4 nanocrystals, the relationships between laser diode power density and emission intensities for the green and red emission are depicted in Fig. 9d. The slopes of different curves are found to be approximately 2, suggesting the normal two-photon emission processes are involved in the UC mechanism of the present nanocrystals.10 Under the 980 nm laser excitation, the activated Yb3+ ion absorbs one photon and transfers it to the Er3+ ion, the Er3+ ion receives the energy and its ground state 4I15/2 electron is excited to the 4I11/2 level, and the Yb3+ promotes Er3+ to 4F7/2 state via nonradiative energy transfer process. The excited electron decays nonradiatively to 2H11/2, 4S3/2, 4F9/2 levels, and precedently radiative relaxation to the ground state results in the emission of 525 nm, 541 and 550 nm, 651 and 667 nm, respectively. The as-obtained KGdF4:Eu3+ and LiYF4:Yb3+, Er3+ nanocrystals display intense red emission and green emission under UV excitation and 980 nm laser excitation, respectively. (The digital camera images of luminescence from KGdF4:Eu3+ and LiYF4:Yb3+, Er3+ nanocrystals are shown in the insets of Fig. 8a and Fig. 8b, respectively).
Conclusions
Near-monodisperse KREF4 and LiREF4 colloidal nanocrystals with various shapes (cubic KLaF4 and KCeF4 wormlike nanowires, nanocubes and nanopolyhedra; cubic KREF4 (RE = Pr to Gd, Y) nanopolyhedra; LiREF4 (RE = Tb to Lu, Y) rhombic nanoplates) have been synthesized via co-thermolysis of Li(CF3COO) or K(CF3COO) and RE(CF3COO)3 in hot oleic acid/oleylamine/1-octadecene solutions. The crystal phase purity, shape and size of the as-prepared nanocrystals were concluded to be greatly affected by the synthetic parameters such as solvent composition, reaction temperature and time. The controlled-growth of KREF4 and LiREF4 nanocrystals has suggested that the lighter the alkali metal and rare earth, the easier AF (A = K, Li) and REF3 or REOF nuclei formed, owing to the fact that the basicity of the alkali metal oxide gradually increases along the alkali metal series, while the basicity of the rare earth oxide gradually decreases along the rare earth series. Contrarily, AREF4 nuclei were more easily formed. With the developed synthetic strategy, the monodisperse and single-crystalline KGdF4:5%Eu and LiYF4:20%Yb,2%Er nanocrystals have been prepared. The KGdF4:5%Eu nanocrystals display intense visible red emission under UV excitation at room temperature. Under a 980 nm near-IR laser excitation, the LiYF4:20%Yb,2%Er nanocrystals can emit UC green light through a two-photon process. We expect the as-prepared AREF4 nanocrystals to be useful in optical nanodevices and bioprobes in the future.Acknowledgements
We gratefully acknowledge the financial support from the MOST of China (grant no. 2006CB601104), the NSFC (grant nos. 20871006, 20821091, and 20671005).Notes and references
- (a) C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS; (b) A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS; (c) C. C. Chen, A. B. Herhold, C. S. Johnson and A. P. Alivisatos, Science, 1997, 276, 398 CrossRef CAS; (d) G. Markovich, C. P. Collier, S. E. Henrichs, F. Remacle, R. D. Levine and J. R. Heath, Acc. Chem. Res., 1999, 32, 415 CrossRef CAS; (e) C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater. Sci., 2000, 30, 545 CrossRef CAS; (f) E. Rabani, D. R. Reichman, P. L. Geissler and L. E. Brus, Nature, 2003, 426, 271 CrossRef CAS; (g) J. Park, K. An, Y. Hwang, J. G. Park, H. J. Noh, J. Y. Kim, J. H. Park, N. M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS; (h) J. Cheon, J. Park, J. Choi, Y. Jun, S. Kim, M. G. Kim and Y. J. Kim, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 3023 CrossRef CAS; (i) C. N. R. Rao and K. P. Kalyanikutty, Acc. Chem. Res., 2008, 41, 489 CrossRef CAS.
- (a) X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59 CrossRef CAS; (b) W. W. Yu and X. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368 CrossRef CAS; (c) E. V. Shevchenko, D. V. Talapin, H. Schnablegger, A. Kornowski, O. Festin, P. Svedlindh, M. Haase and H. Weller, J. Am. Chem. Soc., 2003, 125, 9090 CrossRef CAS; (d) S. Nayak and L. A. Lyon, Angew. Chem., Int. Ed., 2005, 44, 7686 CrossRef CAS; (e) W. S. Seo, J. H. Shim, S. J. Oh, E. K. Lee, N. H. Hur and J. T. Park, J. Am. Chem. Soc., 2005, 127, 6188 CrossRef CAS; (f) S. C. Glotzer and M. J. Solomon, Nat. Mater., 2007, 6, 557 CrossRef; (g) A. R. Tao, P. Sinsermsuksakul and P. D. Yang, Nat. Nanotechnol., 2007, 2, 435 Search PubMed.
- (a) Y. C. Cao, J. Am. Chem. Soc., 2004, 126, 7456 CrossRef CAS; (b) R. Si, Y. W. Zhang, L. P. You and C. H. Yan, Angew. Chem., Int. Ed., 2005, 44, 3256 CrossRef CAS; (c) T. Yu, J. Joo, Y. I. Park and T. Hyeon, Angew. Chem., Int. Ed., 2005, 44, 7411 CrossRef CAS; (d) K. Kompe, H. Borchert, J. Storz, A. Lobo, S. Adam, T. Moller and M. Haase, Angew. Chem., Int. Ed., 2003, 42, 5513 CrossRef; (e) J. W. Stouwdam and F. C. J. M. van Veggel, Nano Lett., 2002, 2, 733 CrossRef CAS; (f) Y. W. Zhang, X. Sun, R. Si, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2005, 127, 3260 CrossRef CAS; (g) V. Sudarsan, S. Sivakumar, F. C. J. M. van Veggel and M. Raudsepp, Chem. Mater., 2005, 17, 4736 CrossRef CAS; (h) K. Riwotzki and M. Haase, J. Phys. Chem. B, 1998, 102, 10129 CrossRef CAS; (i) T. Mirkovic, M. A. Hines, P. S. Nair and G. D. Scholes, Chem. Mater., 2005, 17, 3451 CrossRef CAS; (j) F. Zhao, M. Yuan, W. Zhang and S. Gao, J. Am. Chem. Soc., 2006, 128, 11758 CrossRef CAS; (k) X. Sun, Y. W. Zhang, Y. P. Du, Z. G. Yan, R. Si, L. P. You and C. H. Yan, Chem.–Eur. J., 2007, 13, 2320 CrossRef CAS; (l) Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2008, 112, 405 CrossRef CAS; (m) F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS; (n) J. Shen, L. D. Sun and C. H. Yan, Dalton Trans., 2008, 5687 RSC; (o) Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 3162 CrossRef CAS; (p) F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- (a) N. Menyuk, K. Dwight and J. W. Pierce, Appl. Phys. Lett., 1972, 21, 159 CAS; (b) J. L. Sommerdijk and A. Bril, Philips Tech. Rev, 1974, 34, 1 Search PubMed; (c) E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CrossRef CAS; (d) T. Sandrock, H. Scheife, E. Heumann and G. Huber, Opt. Lett., 1997, 22, 808 Search PubMed; (e) S. Shionoya, and W. M. Yen, Phosphor Handbook, CRC Press, Roca Raton 1999 Search PubMed; (f) F. van de Rijke, H. Zijlmans, S. Li, T. Vail, A. K. Raap, R. S. Niedbala and H. J. Tanke, Nat. Biotechnol., 2001, 19, 273 CrossRef.
- (a) P. E. A. Mobert, A. Diening, E. Heumann, G. Huber and B. H. T. Chai, Laser Phys., 1998, 8, 210 CAS; (b) R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Science, 1999, 283, 663 CrossRef CAS; (c) A. Braud, S. Girard, J. L. Doualan, M. Thuau, R. Moncorgé and A. M. Tkachuk, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 5280 CrossRef CAS; (d) J. P. R. Wells, M. Yamaga, T. P. J. Han and H. G. Gallagher, J. Phys.: Condens. Matter, 2000, 12, 5297 CrossRef CAS; (e) N. M. Khaidukov, S. K. Lam, D. Lo, V. N. Makhov and N. V. Suetin, Opt. Mater., 2002, 19, 365 CrossRef CAS; (f) M. Marano, G. Galzerano, S. Taccheo, P. Laporta, E. Sani, A. Toncelli and M. Tonelli, Opt. Mater., 2003, 24, 327 CrossRef CAS; (g) G. Galzerano, E. Sani, A. Toncelli, G. Della Valle, S. Taccheo, M. Tonelli and P. Lapo, Opt. Lett., 2004, 29, 715 Search PubMed; (h) G. Galzerano and P. Laporta, Opt. Express, 2007, 15, 3257 CrossRef CAS.
- (a) S. Heer, K. Kömpe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102 CrossRef CAS; (b) G. Yi, H. Lu, S. Zhao, Y. Ge, W. Yang, D. Chen and L. H. Guo, Nano Lett., 2004, 4, 2191 CrossRef CAS; (c) J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119 CrossRef CAS; (d) H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS; (e) J. C. Boyer, F Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444 CrossRef CAS; (f) Z. Li and Y. Zhang, Angew. Chem., Int. Ed., 2006, 45, 7732 CrossRef CAS; (g) H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CrossRef CAS; (h) H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13730 CrossRef CAS; (i) J. C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847 CrossRef CAS.
- (a) N. M. Khaidukov, X. Zhang and J. P. Jouart, J. Lumin., 1997, 72-74, 213 CrossRef; (b) C. Y. Zhao, S. H. Feng, R. R. Xu, C. S. Shi and J. Z. Ni, Chem. Commun., 1997, 945 RSC; (c) A. Sen, S. L. Chaplot and R. Mittal, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 024304 CrossRef; (d) M. Yin, V. N. Makhov, N. M. Khaidukov and J. C. Krupa, J. Alloys Compd., 2002, 341, 362 CrossRef CAS; (e) D. Boyer and R. Mahiou, Chem. Mater., 2004, 16, 2518 CrossRef CAS; (f) M. Karbowiak, A. Mech, A. Bednarkiewicz and W. Stręk, J. Alloys Compd., 2004, 380, 321 CrossRef CAS; (g) M. Karbowiak, A. Mech, L. Kępiński, W. Mielcarek and S. Hubert, J. Alloys Compd., 2005, 400, 67 CrossRef CAS; (h) H. Schäfer, P. Ptacek, O. Zerzouf and M. Haase, Adv. Funct. Mater., 2008, 18, 2913 CrossRef.
- J. E. Roberts, J. Am. Chem. Soc., 1961, 83, 1087 CrossRef CAS.
- (a) V. K. Lamer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS; (b) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS; (c) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2002, 124, 3343 CrossRef CAS; (d) L. Manna, E. C. Scher and A. P. Alivisatos, J. Cluster Sci., 2002, 13, 521 CrossRef CAS; (e) C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS; (f) Y. W. Jun, J. S. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414 CrossRef CAS; (g) Y. P. Du, Y. W. Zhang, Z. G. Yan, L. D. Sun and C. H. Yan, Chem.–Asian J., 2007, 2, 965 CrossRef CAS; (h) A. Narayanaswamy, H. Xu, P. Narayan, K. Myeongseob and X. Peng, J. Am. Chem. Soc., 2006, 128, 10310 CrossRef CAS.
- R. Scheps, Prog. Quantum Electron., 1996, 20, 271 CrossRef CAS.
- (a) Z. G. Wei, L. D. Sun, C. S. Liao and C. H. Yan, Appl. Phys. Lett., 2002, 80, 1447 CrossRef CAS; (b) V. Sudarsan, F. C. J. M. van Veggel, R. A. Herring and M. Raudsepp, J. Mater. Chem., 2005, 15, 1332 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1 and more results obtained by means of TEM, XRD, EDAX, and FTIR for KREF4 and LiREF4 nanocrystals. See DOI: 10.1039/b909145a |
| This journal is © The Royal Society of Chemistry 2009 |
