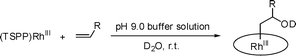
Aerobic oxidation of alkenes mediated by porphyrin rhodium(III) complexes in water†
Jiadi
Zhang
a,
Shan
Li
b,
Xuefeng
Fu
*a and
Bradford B.
Wayland
b
aBeijing National Laboratory for Molecular Sciences, State Key Lab of Rare Earth Materials Chemistry and Applications, Peking University, Beijing, 100871, China. E-mail: fuxf@pku.edu.cn; Fax: +86 10 6275 1708; Tel: +86 10 6275 6035
bDepartment of Chemistry, Temple University, Philadelphia, PA 19122, USA. E-mail: bwayland@temple.edu; Fax: +1 215 204 1532; Tel: +1 215 204 7875
First published on 25th March 2009
Abstract
Selective oxidation of alkenes in water to ketones using molecular oxygen as the oxidant is mediated by rhodium porphyrin complexes through observed β-hydroxy alkyl complexes.
Oxidation of olefins to produce epoxides, ketones, and aldehydes provides valuable routes for oxidative functionalization of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.1–5 An important trend in the oxidation of hydrocarbons is to use dioxygen from air as the terminal oxidant.6–12 Tetra (p-sulfonato phenyl) porphyrin rhodium complexes ((TSPP)RhIII) in water are observed to mediate the selective oxidation of alkenes to ketones by atmospheric molecular oxygen through the intermediacy of β-hydroxy alkyl complexes (< 333K, 1 atm air).
C bond.1–5 An important trend in the oxidation of hydrocarbons is to use dioxygen from air as the terminal oxidant.6–12 Tetra (p-sulfonato phenyl) porphyrin rhodium complexes ((TSPP)RhIII) in water are observed to mediate the selective oxidation of alkenes to ketones by atmospheric molecular oxygen through the intermediacy of β-hydroxy alkyl complexes (< 333K, 1 atm air).
Formation of β-hydroxy alkyl rhodium porphyrin complexes in water
Porphyrin rhodium(III) bisaquo complex [(TSPP)RhIII(D2O)2]−3 (1) occurs in an equilibrium distribution with the mono and bishydroxo complexes, [(TSPP)RhIII(D2O)(OD)]−4 (2), [(TSPP)RhIII(OD)2]−5 (3) in water.13Ethene and larger terminal alkenes react regioselectively with (TSPP)RhIII in D2O solutions to form β-hydroxy alkyl complexes where rhodium is bonded to a terminal CH2 unit ((TSPP)Rh-CH2CH(OD)R(D2O)) (eqn (1)).14[(TSPP)Rh-OD(D2O)]−4 + CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR ⇌ [(TSPP)Rh-CH2CH(OD)R(D2O)]−4 CHR ⇌ [(TSPP)Rh-CH2CH(OD)R(D2O)]−4 | (1) |
The reactions to produce β-hydroxy alkyl complexes ((TSPP)Rh-CH2CH(OD)R) (4) reach completion within hours in a borate buffer (pH = 9)(Table 1)(ESI).†
|
|
||||
|---|---|---|---|---|
| Entry | Substrate | Time/h | Product | Yield |
| a The overall percentage of the formed (TSPP)Rh-CH2C(O)R in the process of the reaction. The concentration of (TSPP)RhIII is 1.9 × 10−3 M and the yield of the reaction was measured by 1H NMR spectroscopy. | ||||
| 1 |

|
3 |

|
92 (3)a |
| 2 |

|
6.7 |

|
80 (6)a |
| 3 |

|
<5 min |

|
>95 |
| 4 |

|
<5 min |

|
>95 |
| 5 |
|
82 |

|
>95 |
Formation of the β-hydroxy ethyl octaethylporphyrin rhodium complex ((OEP)Rh-CH2CH2OH) was previously reported in hydrocarbon media through hydroxide nucleophilic attack on an ethene π complex ((OEP)Rh(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2))+.15 The concentration of π complexes (TSPP)Rh(CH2
CH2))+.15 The concentration of π complexes (TSPP)Rh(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR) in water at 298K was too small for observation by 1H NMR.
CHR) in water at 298K was too small for observation by 1H NMR.
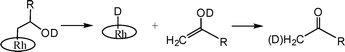
Formation of ketones through β-hydrogen elimination.
The β-hydroxy alkyl complexes in the absence of air spontaneously transform to (TSPP)RhI and ketones over a period of hours at 333K in water (eqn (2), Table 2)(ESI).†| [(TSPP)Rh-CH2CH(OD)R(D2O)]−4⇌ [(TSPP)Rh(H)(D2O)]−4 + CH2DC(O)R | (2) |
|
|
||||
|---|---|---|---|---|
| Entry | Substrate | Time/h | Solvent | Product |
| a 353K. b When the reactions were run for a longer period of time, final products turned to be CD3C(O)CD2R. The reactions were run in borate buffer with pH = 9 and the product ketones were formed quantitatively as measured by 1H NMR. | ||||
| 1 |

|
9 | H2O |
|
| D2O |
 b
b
|
|||
| 2 |

|
9 | H2O |

|
| D2O |
 b
b
|
|||
| 3 |

|
9 | D2O |
 b
b
|
| 4 |
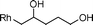
|
16.5 | H2O |

|
| 3a | D2O |

|
||
The resulting ketones were extracted by CDCl3 and determined by the 1H NMR and GC-MS compared with commercial samples of ketones(ESI).†
Thermal reaction of (TSPP)Rh-CH2CH(OD)(CH2)2CH3 in D2O results in the formation of pentanone with deuterium incorporated into the methyl group (CH2(D)C(O)CH2CH2CH3) (J1D–1H = 2.2 Hz) (Fig. 1). The position for D atom incorporation into pentanone indicates that the ketone is formed through β-C–H elimination to form an enol containing an O–D unit (Scheme 1). This aspect of the mechanism differs from the Wacker process where all of the hydrogens come from the alkene. Substantially faster β-hydrogen elimination rate of Rh-CH2CH(OD)R in water (333K, Table 2) compared to the process in benzene probably results from the efficacy of ionic pathways in water.
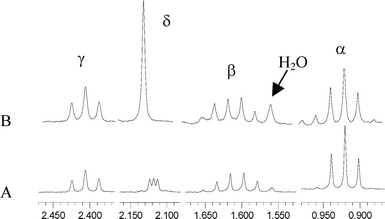 | ||
| Fig. 1 1H NMR (400 MHz; CDCl3) spectrum of pentanone produced from reaction of (TSPP)Rh-CH2CH(OH)CH2CH2CH3 in water. (A) (D2O) CH2(D)C(O)CH2CH2CH3 in CDCl3. (B) (H2O) CH3C(O)CH2CH2CH3. | ||
Aerobic oxidation of (TSPP)RhI to (TSPP)RhIII in water
The brownish aqueous solutions of [(TSPP)RhI]−5 species formed in reaction 2 undergo an immediate colour change to a clear dark red solution when exposed to air, and the distinctive pyrrole 1H NMR position for (TSPP)RhI species at 8.31 ppm is replaced by a pyrrole resonance at 9.04 ppm associated with (TSPP)RhIII species.13 The sequence of reactions for dioxygen to convert (TSPP)RhI to (TSPP)RhIII is depicted by eqn (3–7), and the overall catalytic reactions are shown in Scheme 2.| [(TSPP)RhI(D2O)]−5 + O2⇌ [(TSPP)RhIII(O2)(D2O)]−5 | (3) |
| [(TSPP)RhIII(O2)(D2O)]−5 + D2O ⇌ [(TSPP)RhIII(D2O)2]−3 + O22− | (4) |
| O22− + 2D2O ⇌ D2O2 + 2OD− | (5) |
| D2O2⇌ D2O + 1/2O2 | (6) |
| [(TSPP)RhI(D2O)]−5 + 1/2O2 + 2D2O ⇌ [(TSPP)RhIII(D2O)2]−3 +2OD− | (7) |
 | ||
| Scheme 2 Proposed mechanism for aerobic oxidations of alkenes mediated by porphyrin rhodium(III) in water. | ||
Rapid air oxidation of Rh(I) to Rh(III) completes the cycle which provides the possibility for catalytic oxidation of olefins. However, (TSPP)RhIII reacts with α-C–H groups of ketones to form β-carbonyl organometallic derivatives (eqn (8)).
| [(TSPP)RhIII(D2O)(OD)]−4 + CH3C(O)R ⇌ [(TSPP)Rh-CH2C(O)R(D2O)]−4 + HOD | (8) |
The ketone products react with the Rh(III) oxidation catalyst resulting in a product inhibited catalytic oxidation of olefins with only small numbers of turnovers.
The oxidation reactions are envisioned to proceed through a four-step cycle involving: (a) displacement of one water molecule on (TSPP)RhIII(H2O)2 by alkene to form (TSPP)RhIII(CH2=CHR) π complexes; (b) nucleophilic attack on the π complexes by hydroxide to produce β-hydroxy alkyl rhodium complexes; (c) release of ketones through β-hydrogen elimination; and (d) regeneration of (TSPP)RhIII by molecular oxygen (Scheme 2).
This work was supported by a starter grant from Peking University, NSFC (grants 20841002 and 20801002), and the U.S. Department of Energy, Division of Chemical Science, Office of Science (grants DE-FG02–86ER13615 and DE-FG02–09ER16000).
Notes and references
- (a) B. de Bruin, P. H. M. Budzelaar and A. W. Gal, Angew. Chem., Int. Ed., 2004, 43, 4142–4157 CrossRef CAS; (b) S. Thewissen, M. D. M. Reijnders, J. M. M. Smits and B. de Bruin, Organometallics, 2005, 24, 5964–5972 CrossRef CAS.
- (a) R. S. Pryadun and J. D. Atwood, Organometallics, 2007, 26, 4830–4834 CrossRef CAS; (b) R. Pryadun, D. Sukumaran, R. Bogadi and J. D. Atwood, J. Am. Chem. Soc., 2004, 126, 12414–12420 CrossRef CAS.
- (a) C. Liu, C. F. Bender, X. Han and R. A. Widenhoefer, Chem. Commun., 2007, 3607–3618 RSC; (b) A. R. Chianese, S. J. Lee and M. R. Gagne, Angew. Chem., Int. Ed., 2007, 46, 4042–4059 CrossRef CAS.
- S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400–3420 CrossRef CAS.
- P. Zhao, C. D. Incarvito and J. E. Hartwig, J. Am. Chem. Soc., 2006, 128, 9642–9643 CrossRef CAS.
- T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329–2363 CrossRef CAS.
- (a) S. S. Stahl, Science, 2005, 309, 1824 CrossRef CAS; (b) M. M. Konnick and S. S. Stahl, J. Am. Chem. Soc., 2008, 130, 5753–5762 CrossRef CAS; (c) B. A. Steinhoff and S. S. Stahl, J. Am. Chem. Soc., 2006, 128, 4348–4355 CrossRef CAS; (d) J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 2868–2869 CrossRef CAS.
- T. J. Williams, A. J. M. Caffyn, N. Hazari, P. F. Oblad, J. A. Labinger and J. E. Bercaw, J. Am. Chem. Soc., 2008, 130, 2418–2419 CrossRef CAS.
- G. Brink, I. W. C. E. Arends, G. Papadogianakis and R. A. Sheldon, Chem. Commun., 1998, 2359–2360 RSC.
- J. R. Khusnutdinova, L. L. Newman, P. Y. Zavalij, Y. Lam and Andrei N. Vedernikov, J. Am. Chem. Soc., 2008, 130, 2174–2175 CrossRef CAS.
- C. N. Cornell and M. S. Sigman, Inorg. Chem., 2007, 46, 1903–1909 CrossRef CAS.
- G. Jiang, J. Chen, H.-Y. Thu, J.-S. Huang, N. Zhu and C.-H. Che, Angew. Chem., Int. Ed., 2008, 47, 6638–6642 CrossRef CAS.
- X. Fu and B. B. Wayland, J. Am. Chem. Soc., 2004, 126, 2623–2631 CrossRef CAS.
- X. Fu, S. Li and B. B. Wayland, J. Am. Chem. Soc., 2006, 128, 8947–8954 CrossRef CAS.
- B. B. Wayland, S. L. VanVoorhees and K. J. Del Rossi, J. Am. Chem. Soc., 1987, 109, 6513–6515 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and scans of NMR spectra. See DOI: 10.1039/b904414k |
| This journal is © The Royal Society of Chemistry 2009 |