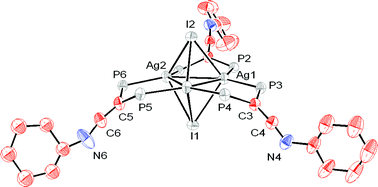
Diphosphinoketenimine ligands (PPh2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR (1a R = tBu, 1b R = Ph) were reacted with different Cu(I), Ag(I), Au(I), Pd(II), Ru(II) and Mo(0) metal complexes bearing weakly coordinating ligands yielding a number of mono-, di-, and trimetallic species with the diphosphine acting as either chelate or bridging ligand. The reactivity of some of the new complexes toward water and MeLi was investigated. The mononuclear Pd(II) complex [PdCl2{(PPh2)2C
NR (1a R = tBu, 1b R = Ph) were reacted with different Cu(I), Ag(I), Au(I), Pd(II), Ru(II) and Mo(0) metal complexes bearing weakly coordinating ligands yielding a number of mono-, di-, and trimetallic species with the diphosphine acting as either chelate or bridging ligand. The reactivity of some of the new complexes toward water and MeLi was investigated. The mononuclear Pd(II) complex [PdCl2{(PPh2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}] (6b) is transformed into the diphosphinoamide complex [PdCl2{(PPh2)2CHC(
NPh}] (6b) is transformed into the diphosphinoamide complex [PdCl2{(PPh2)2CHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(NHPh)}] (12) by nucleophilic addition of water. Similarly, treatment of 6b with MeLi yields the diphosphinoenamine complex [PdCl2{(PPh2)2C
O)(NHPh)}] (12) by nucleophilic addition of water. Similarly, treatment of 6b with MeLi yields the diphosphinoenamine complex [PdCl2{(PPh2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)(NHPh)}] (14). A different behaviour is observed in the treatment of diphosphinoketenimine with water in the presence of Cu(I) ion, which leads to hydrolysis of the ligand involving P–C bond cleavage, underscoring the influence of the coordination environment in the reactivity showed by the coordinated ligand. The liberation of the metal assisted synthesized diphosphines from the Pd(II) metal center can be achieved easily and in quantitative yields by treatment with aqueous KCN solution.
C(Me)(NHPh)}] (14). A different behaviour is observed in the treatment of diphosphinoketenimine with water in the presence of Cu(I) ion, which leads to hydrolysis of the ligand involving P–C bond cleavage, underscoring the influence of the coordination environment in the reactivity showed by the coordinated ligand. The liberation of the metal assisted synthesized diphosphines from the Pd(II) metal center can be achieved easily and in quantitative yields by treatment with aqueous KCN solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR (1a R = tBu, 1b R = Ph) were reacted with different Cu(I), Ag(I), Au(I), Pd(II), Ru(II) and Mo(0) metal complexes bearing weakly coordinating
NR (1a R = tBu, 1b R = Ph) were reacted with different Cu(I), Ag(I), Au(I), Pd(II), Ru(II) and Mo(0) metal complexes bearing weakly coordinating ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}] (6b) is transformed into the diphosphinoamide complex [PdCl2{(PPh2)2CHC(
NPh}] (6b) is transformed into the diphosphinoamide complex [PdCl2{(PPh2)2CHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(NHPh)}] (12) by nucleophilic addition of
O)(NHPh)}] (12) by nucleophilic addition of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)(NHPh)}] (14). A different behaviour is observed in the treatment of
C(Me)(NHPh)}] (14). A different behaviour is observed in the treatment of 

 Please wait while we load your content...
Please wait while we load your content...