A cyclic tetranuclear Ni2Gd2 complex bridged by amino acidato ligands, with an S = 9 ground state, derived from ferromagnetic spin-coupling between nickel(II) and gadolinium(III) ions†
Satoshi
Igarashi
a,
Shin-ichi
Kawaguchi
a,
Yasuhiko
Yukawa
*b,
Floriana
Tuna
*c and
Richard E. P.
Winpenny
c
aGraduate School of Science and Technology, Niigata University, 8050 Ikarashi Nino-cho, Nishi-ku, Niigata 950-2181, Japan
bDepartment of Environmental Science, Faculty of Science, Niigata University, 8050 Ikarashi Nino-cho, Nishi-ku, Niigata 950-2181, Japan. E-mail: yukawa@env.sc.niigata-u.ac.jp; Fax: +81-(0)25-262-6361; Tel: +81-(0)25-262-6361
cDepartment of Chemistry, The University of Manchester, Oxford Road, Manchester, UK M13 9PL. E-mail: Floriana.Tuna@manchester.ac.uk; Tel: +44 (0)1612 754582
First published on 24th February 2009
Abstract
Synthesis and structure of a cyclic tetranuclear Ni2Gd2 complex bridged by amino acidato ligands, with an S = 9 spin ground state, derived from ferromagnetic spin-coupling between SGd = 7/2 and SNi = 1 are reported.
The metal complexes of amino acids interest biochemists as models for the metal-binding sites on proteins. From the point of view of coordination chemistry, an amino acid, which is safe and easily obtained, can be regarded as a typical multidentate ligand. Taking advantage of the properties of the amino acid ligand, we have been carrying out the syntheses of heteronuclear complexes bridged by amino acidato ligands.1,2 Recently, such research has achieved remarkable development with contributions by many groups.3 Heteronuclear complexes have attracted special attention because of their versatile magnetic behaviour derived from large ground spin state and large magnetic anisotropy. The forthcoming target in this field is the magnetic chemistry of d–f and f–f transition metal complexes. In order to obtain information on the magnetic properties of d–f interactions, d–f heteronuclear complexes have been synthesized.4,5 In these complexes there are some single molecule magnets. The first mixed-metal 3d–4f SMM to exhibit hysteresis loops and quantum tunnelling of the magnetization, [Mn11Dy4O8(OH)6(OMe)2(O2CPh)16(NO3)5(H2O)3]·15MeCN was reported by Christou and co-workers.4b Matsumoto and co-workers have reported synthesis, structures, and magnetic properties of d–f extended cyclic tetranuclear compounds by using a ligand complex having a copper(II) ion.5 Some of these compounds exhibited the behaviour of a single molecule magnet.5c Although the ligand complex applied by Matsumoto et al. is useful to synthesize different combinations of d–f metal cyclic tetranuclear compounds, the coordination sphere of the transition metal site is square planar. Therefore, the nickel ions in the Ni2Gd2 cyclic compound are diamagnetic. Here we demonstrate a new cyclic tetranuclear Ni2Gd2 complex, [Gd2Ni2(pro)4(NO3)6(CH3CN)4] (1) (Hpro = L-proline), in which the nickel ions have an octahedral coordination geometry, by using amino acidato bridging ligands (Scheme 1).
 | ||
| Scheme 1 | ||
[Gd2Ni2(pro)4(NO3)6(CH3CN)4] 1 is formed from the reaction of hexahydrated gadolinium(III) nitrate (1 mmol) with [Ni(pro)2(H2O)2] (1 mmol) in acetonitrile (20 ml). The reaction mixture was layered with diethyl ether and kept at room temperature for several days. Although dark blue blocks were crystallized at a bottom or on the walls of a glass vessel, such crystals immediately decayed after filtration. Upon crystallization, the addition of 1,2-dihydroxybenzene resulted in large and stable crystals. It is not certain what the role of 1,2-dihydroxybenzene is during crystallization. 1,2-Dihydroxybenzene might arrange suitable conditions for crystal growth, although 1,2-dihydroxybenzene did not react.
The structure was determined by single-crystal X-ray diffraction analysis.‡Fig. 1 shows an ORTEP drawing of 1.
 | ||
| Fig. 1 ORTEP drawing of the cyclic Ni2Gd2 tetranuclear complex with selected atom labelling scheme. Thermal ellipsoids are drawn at 30% probability level. | ||
The NiII ion has a distorted octahedral coordination geometry with two acetonitrile nitrogen atoms and two amino nitrogen and two carboxylato oxygen atoms of pro ligands: N(1)–Ni(1)–N(2) = 168.2(3)°, O(1)–Ni(1)–N(11) = 172.7(3)°, O(3)–Ni(1)–N(12) = 172.0(3)°, Ni–O (2.070(5)–2.045(5) Å) and Ni–N (2.065(7)–2.099(7) Å). The carboxylato oxygen atom O(1) bridges GdIII and NiII ions: Gd(1)–O(1) = 2.495(5) Å, O(1)–Ni(1) = 2.070(5) Å, Gd(1)–O(1)–Ni(1) = 152.4(2)°, Gd(1)⋯Ni(1) = 4.434(1) Å. One carboxylato oxygen atom is coordinated to both NiII and GdIII ions and the other is coordinated to the GdIII ion, thus forming five- and four-membered chelate rings, as shown in Scheme 1. The GdIII ion has a ten-coordination geometry including the coordination of three bidentate nitrate ions (Gd–O = 2.424(8)–2.552(6) Å) and two four-membered chelate rings formed by a carboxylato group of a pro ligand (Gd–O = 2.433(6)–2.547(5) Å). The separations between metal ions in neighbouring molecules are larger than 9.58 Å, suggesting the absence of any significant intermolecular interactions.
Solid-state direct current (dc) magnetic susceptibility (χM) data for 1 were collected in the 2–300 K range, with applied magnetic fields of 0.1, 0.5 and 1 T.6 TheχMT value (Fig. 2) is 18.56 cm3K mol−1 at 300 K, which compares with the expected value of 18.17 cm3K mol−1 for two NiII and two GdIII non-interacting metal ions (SGd = 7/2, SNi = 1, gGd = 2, gNi = 2.2). This value remains almost constant down to ca. 80 K, at which the value starts to increase rapidly to reach 29.02 cm3K mol−1 at 2 K (for 0.1 T magnetic field). This is consistent with the presence of weak ferromagnetic super-exchange between NiII and GdIII ions, leading to an S = 9 ground state. This affirmation is also supported by the variable-field magnetization data depicted in the insert in Fig. 2. The χMT curves recorded at 0.5 and 1 T (not presented here) show a maximum at 3.2 (23.4 cm3K mol−1) and 5.5 K (21.4 cm3K mol−1), respectively, and a sharp decrease inχMT below these temperatures, due to zero-field splitting. We used MAGPACK7 to simultaneously simulate the susceptibility data at 0.1 T magnetic field and also the magnetization data at 2 and 4 K (inserted in Fig. 2) of 1. The best simulation was reached by full-matrix diagonalization of the following spin Hamiltonian: H = −2JGd–Ni(S1S2 + S2S3 + S3S4 + S1S4) + 2DNi[SNi,z2− (1/3)SNi(SNi + 1)], where JGd–Ni and DNi represent the isotropic exchange and single-ion ZFS parameters, respectively. The best set of parameters that match the experimental data was found to be JGd–Ni = +0.15 cm−1 and DNi = 3.0 cm−1, with gNi = 2.33 and gGd = 2.0. The solid lines in Fig. 2 correspond to the calculated curves. The J value obtained here is somewhat smaller than that reported by Costes et al. for a ferromagnetic Gd⋯Ni dimer with two phenoxo bridges of +3.6 cm−1,8 but comparable to those obtained by Yamaguchi et al. for metal complexes with Ni⋯Gd and Ni⋯Gd⋯Ni cores supported by tripodal ligands, of +0.34 cm−1 and +0.19 cm−1, respectively.9 Another phenoxo-bridged trinuclear Ni⋯Gd⋯Ni complex was also reported to be ferromagnetic (J = +0.54 cm−1).10
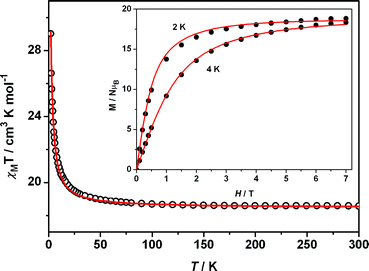 | ||
| Fig. 2 Plots of χMTvs.T at 0.1 T and M/Nβvs.H (inset) of 1. The solid curves represent the theoretical curves with the parameters of JGd–Ni = +0.15 cm−1 and DNi = 3.0 cm−1, with gNi = 2.33 and gGd = 2.0. | ||
The present study demonstrates that using amino acids as ligands it is possible to assemble cyclic 3d–4f tetranuclear compounds with a large spin ground state. Compound 1 has a remarkable S = 9 ground state that results from ferromagnetic coupling between GdIII and NiII ions. We are presently attempting to synthesize analogs containing anisotropic 4f metals such as Tb or Dy to create systems with large spin ground states and large anisotropy that might lead to potential single molecule magnets.
Notes and references
- (a) Y. Yukawa, Y. Inomata, T. Takeuchi, M. Shimoi and A. Ouchi, Bull. Chem. Soc. Jpn., 1982, 55, 3135 CAS; (b) Y. Yukawa, Y. Inomata and T. Takeuchi, Bull. Chem. Soc. Jpn., 1983, 56, 2125 CAS; (c) Y. Yukawa, N. Yasukawa, Y. Inomata and T. Takeuchi, Bull. Chem. Soc. Jpn., 1985, 58, 1591 CAS; (d) S. Itoh, Y. Yukawa, Y. Inomata and T. Takeuchi, Bull. Chem. Soc. Jpn., 1987, 60, 899 CAS; (e) Y. Yukawa, S. Nakagome, H. Yamaguchi, Y. Inomata and T. Takeuchi, Bull. Chem. Soc. Jpn., 1989, 62, 1057 CAS; (f) Y. Yukawa, J. Chem. Soc., Dalton Trans., 1992, 3217 RSC.
- (a) Y. Yukawa, S. Igarashi, A. Yamano and S. Satoh, Chem. Commun., 1997, 711 RSC; (b) S. Igarashi and Y. Yukawa, Chem. Lett., 1999, 1265 CrossRef CAS; (c) S. Igarashi, Y. Hoshino, Y. Masuda and Y. Yukawa, Inorg. Chem., 2000, 39, 2509 CrossRef CAS; (d) Y. Yukawa, S. Igarashi, Y. Masuda and M. Oguni, J. Mol. Struct., 2002, 605, 277 CrossRef CAS; (e) L.-Y. Wang, S. Igarashi, Y. Yukawa, T. Hashimoto, K. Shimizu, Y. Hoshino, A. Harrison, G. Aromí and R. E. P. Winpenny, Chem. Lett., 2003, 32, 202 CrossRef CAS; (f) L.-Y. Wang, S. Igarashi, Y. Yukawa, Y. Hoshino, O. Roubeau, G. Aromí and R. E. P. Winpenny, Dalton Trans., 2003, 2318 RSC; (g) A.-A. H. A. -Nawwas, J. Cano, P. Christian, T. Mallah, G. Rajaraman, S. J. Teat, R. E. P. Winpenny and Y. Yukawa, Chem. Commun., 2004, 314 RSC; (h) T. Komiyama, S. Igarashi, Y. Hoshino and Y. Yukawa, Chem. Lett., 2005, 34, 300 CrossRef CAS; (i) Y. Yukawa, G. Aromí, S. Igarashi, J. Ribas, S. A. Zvyagin and J. Krzystek, Angew. Chem., Int. Ed., 2005, 44, 1997 CrossRef CAS; (j) S. Maegawa, T. Sagane, T. Itou, A. Oyamada, S. Igarashi and Y. Yukawa, J. Magn. Magn. Mater., 2007, 310, 1441 CrossRef CAS; (k) T. Komiyama, S. Igarashi and Y. Yukawa, Chem. Biol., 2008, 2, 122 CAS.
- (a) S. J. W. Du, J. Dai, L. Wu, C. Cui, Z. Fu and X. Wu, J. Chem. Soc., Dalton Trans., 2001, 2963 RSC; (b) Z. Zheng, Chem. Commun., 2001, 2521 RSC; (c) J.-J. Zhang, S.-M. Hu, S.-C. Xiang, L.-S. Wang, Y.-M. Li, U.-S. Zhang and X.-T. Wu, J. Mol. Struct., 2005, 748, 129 CrossRef CAS; (d) S.-C. Xiang, S.-M. Hu, J.-J. Zhang, X.-T. Wu and J.-Q. Li, Eur. J. Inorg. Chem., 2005, 2706 CrossRef CAS; (e) S.-M. Hu, S.-C. Xiang, J.-J. Zhang, T.-L. Sheng, R.-B. Fu and X.-T. Wu, Eur. J. Inorg. Chem., 2008, 1141 CrossRef CAS.
- (a) M. Sakamoto, K. Manseki and H. Okawa, Coord. Chem. Rev., 2001, 219, 379 CrossRef; (b) A. Mishra, W. Wemsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 15648 CrossRef CAS; (c) X.-J. Kong, Y.-P. Ren, W.-X. Chen, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, Angew. Chem., Int. Ed., 2008, 47, 2398 CrossRef CAS.
- (a) T. Kido, S. Nagasato, Y. Sunatsuki and N. Matsumoto, Chem. Commun., 2000, 2113 RSC; (b) S. Osa, Y. Sunatsuki, Y. Yamamoto, M. Nakamura, T. Shimamoto, N. Matsumoto and N. Re, Inorg. Chem., 2003, 42, 5507 CrossRef CAS; (c) T. Hamamatsu, K. Yabe, M. Towatari, N. Matsumoto, N. Re, A. Pochaba and J. Mrozinski, Bull. Chem. Soc. Jpn., 2007, 80, 523 CrossRef CAS.
- Magnetic susceptibility measurements were performed on polycrystalline samples constrained in eicosane, in the temperature range 2–300 K, by using a Quantum Design MPMS-XL SQUID magnetometer equipped with a 7 T magnet. The diamagnetic corrections for the compound were estimated using Pascal's constants, and the magnetic data were corrected for diamagnetic contributions of the sample holder and of eicosane.
- J. J. Borra's-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, J. Comput. Chem., 2001, 22, 985 CrossRef CAS.
- J.-P. Costes, F. Dahan, A. Dupuis and J.-P. Laurent, Inorg. Chem., 1997, 36, 4284 CrossRef CAS.
- (a) T. Yamaguchi, Y. Sunatsuki, H. Ishida, M. Kojima, H. Akashi, N. Re, N. Matsumoto, A. Pochaba and J. Mrozinski, Inorg. Chem., 2008, 47, 5736 CrossRef CAS; (b) T. Yamaguchi, Y. Sunatsuki, H. Ishida, M. Kojima, H. Akashi, N. Re, N. Matsumoto, A. Pochaba and J. Mrozinski, Bull. Chem. Soc. Jpn., 2008, 81, 598 CrossRef CAS.
- V. Chandrasekhar, B. M. Pandian, R. Boomishankar, A. Steiner, J. J. Vittal, A. Horri and R. Clérac, Inorg. Chem., 2008, 47, 4918 CrossRef CAS.
Footnotes |
| † CCDC reference number 695407. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b823366g |
‡ Crystal data for 1: C28H52Gd2N14Ni2O30, M = 1496.69, blue prism, monoclinic, P21, a = 10.1979(3), b = 18.8871(5), c = 15.4161(5) Å, β = 99.493(1)°, V = 2928.6(2) Å3, Z = 2, T = 298(1) K, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717 reflections of which 15 717 reflections of which 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 were unique (Rint = 0.045), 672 variable parameters, R = 0.083 [based on all F2], Rw = 0.142. Four oxygen atoms of H2O were found in an asymmetric unit: two of them have occupancy 1 and the others are located on different four sites with occupancy 0.5. Hydrogen atoms of H2O could not be found. The Flack parameter supported the chirality of the structure. Data were collected over the 2θ < 60.1° on a Rigaku RAXIS-IV Imaging Plate diffractometer with graphite monochromated MoKα radiation. 892 were unique (Rint = 0.045), 672 variable parameters, R = 0.083 [based on all F2], Rw = 0.142. Four oxygen atoms of H2O were found in an asymmetric unit: two of them have occupancy 1 and the others are located on different four sites with occupancy 0.5. Hydrogen atoms of H2O could not be found. The Flack parameter supported the chirality of the structure. Data were collected over the 2θ < 60.1° on a Rigaku RAXIS-IV Imaging Plate diffractometer with graphite monochromated MoKα radiation. |
| This journal is © The Royal Society of Chemistry 2009 |
