Unprecedented double migratory insertion of phenyl isocyanide into cyclopentadienyl C–H bonds†
Andrew L.
Johnson
*a,
Alexander M.
Willcocks
a,
Paul R.
Raithby
a,
Mark. R.
Warren
a,
Andrew J.
Kingsley
b and
Raj
Odedra
b
aDepartment of Chemistry, University of Bath, Bath, UK BA2 7AY. E-mail: a.l.johnson@bath.ac.uk; Fax: +44 (0) 1225 386231; Tel: +44 (0) 1225 384467
bSAFC-Hitech, Power Road, Bromborough, Wirral, UK CH62 3QF
First published on 6th January 2009
Abstract
The reaction of [(η5-C5H5)Cu(CNPh)] with phenyl-isocyanide results in an unprecedented double migratory insertion into two sp2 C–H bonds of a η5-coordinated cyclopentadienyl group, and formation of the 6-aminofulvene-2-aldimine complexes [(CNPh)Cu{κ2-N,N-C5H3-1,2-(CHNPh)2}] and [(CNPh)2Cu{κ2-N,N-C5H3-1,2-(CHNPh)2}], respectively, both of which have been structurally characterised.
Isocyanides, R–NC, have a rich organic1,2 and organometallic chemistry3–5 primarily due to the presence of a formally divalent carbon atom. Unlike carbon monoxide, with which isocyanides are formally isoelectronic, isocyanides are generally considered to be good σ-donors and weak π-acceptors6 and, pertinent to this study, contain an R group for which the steric and electronic features can be varied.7,8 This results in remarkable variability in reactivity of isocyanides. Despite the weak π-acceptor ability of isocyanides, they are susceptible to attack by nucleophiles, where the π* orbital of the isocyanide group can accept electrons from a nucleophile. As a result of this, the insertion chemistry of isocyanides into a range of polar bonds such as metal–alkyl, –aryl and –amide is well known.9–11 Less common, and understood, is the formal insertion of a single isocyanide into a C–H bond either by a metal assisted C–H activation and insertion into the resultant M–C bond,12–15 or by direct insertion into C–H bonds under the influence of Lewis acids.16,17
Here, we wish to report an unprecedented double migatory insertion of phenyl isocyanide, Ph–NC, into two sp2 C–H bonds of a η5-coordinated cyclopentadienyl group, and describe the formation and structural characterisation of the resultant 6-aminofulvene-2-aldimine (AFA) complexes [(CNPh)Cu{κ2-N,N-C5H3-1,2-(CHNPh)2}] (2) and [(CNPh)2Cu{κ2-N,N-C5H3-1,2-(CHNPh)2}] (3), respectively.
As part of a study into the organometallic chemistry of copper-isocyanide complexes, we developed a synthetic route to the mono isocyanide complex [(η5-C5H5)Cu(CNPh)] (1) by reaction of the coordination complex [CuCl(CNPh)2]2 with NaC5H5 in THF (Scheme 1).† Work-up of the reaction, after 2 h, afforded the half-sandwich complex [(η5-C5H5)Cu(CNPh)] (1) in quantitative yield. The 1H NMR spectrum of the complex shows the presence of a single resonance for the cyclopentadienyl moiety at δ = 5.95 ppm, suggesting an η5-coordinated [C5H5] group at the copper centre. This feature was confirmed by single-crystal X-ray diffraction experiments†‡, which reveal the molecular structure of 1 to be an uncommon example of a structurally characterised Cu–Cp system with the general form [(η5-C5H5)Cu–L] (L = 2 e− donor ligand) and is the first example of a structurally characterised cyclopentadienyl copper-isocyanide complex.
 | ||
| Scheme 1 | ||
Prolonged reaction times between [CuCl(CNPh)2]2 with NaC5H5 in THF, resulted in the reduced yield of 1, and the concomitant formation of a second product within the reaction mixture, indicated by the presence of three indicative resonances in the 1H NMR spectrum (i.e. a singlet at δ 8.35 ppm, a doublet at δ 7.01 ppm and a distinctive triplet at δ 6.40 ppm in a 2 : 2 : 1 intensity ratio) that where not consistent with an (η5-C5H5)Cu-based product.† A multiplet between δ 7.12–7.42 ppm with an integral corresponding to 15 hydrogen atoms was also observed. Work-up of a reaction between [CuCl(CNPh)2]2 with NaC5H5 in THF after 72 h, followed by extraction of the residue into hot hexane and recrystallisation resulted in the isolation of a crop crystals suitable for single-crystal X-ray diffraction.‡
Single-crystal X-ray diffraction experiments revealed the crystals to be the surprising N,N-diphenyl-AFA derivative (2), resulting form the serendipitous and remarkable double insertion of phenyl isocyanide into two C–H bonds of the formally η5-cyclopentadienyl group (Scheme 2) of compound 1, which is formed in situ.
 | ||
| Scheme 2 | ||
The molecular structure of 2 (Fig. 1) shows a three coordinate (the two arms of the AFA ligand and the carbon atom of a phenyl-isocyanide ligand) trigonal planar coordination geometry about the copper centre. The Cu–CNPh distance of 1.848(3) Å compares well with values found in other Cu-isocyanide complexes.18–20 The bite angle of the AFA ligand [N(2)–Cu(1)–N(3): 113.41(8)°] is considerably larger than those reported by Baily et al. for the dicyclohexyl-AFA complex [THF(CH3)Mg{κ2-N,N-C5H3-1,2-(CHNCy)2}], (Cy = cyclohexyl),21 which is presumably a reflection of both the trigonal coordination geometry in 2 and larger ionic radii of Cu(I). Complex, 2 represents a rare example of a metal complex of the AFA ligand system,21,22 despite the fact that AFA derivatives have been know since 196323–25 and are related to both the N,N′-disubstituted amino-troponimate26,27 and β-diketiminate ligand systems.28
 | ||
| Fig. 1 ORTEP (50% probability ellipsoids) diagram of (2). Selected bond lengths (Å) and angles (°): Cu(1)–C(1) 1.849(3) C(1)–N(1) 1.170(3), N(1)–C(2) 1.399(3), Cu(1)–N(2) 1.978(2), Cu(1)–N(3) 1.964(2), N(2)–C(8) 1.306(3), N(2)–C(21) 1.439(3), N(3)–C(14) 1.312(3), N(3)–C(15) 1.430(3), C(8)–C(9) 1.417(4), C(9)–C(10) 1.412(4), C(10)–C(11) 1.393(4), C(11)–C(12) 1.388(4), C(12)–C(13) 1.416(4), C(13)–C(14) 1.411(4), C(1)–Cu(1)–N(2) 117.50(10), C(1)–Cu(1)–N(3) 128.01(10), N(2)–Cu(1)–N(3) 113.41(8), N(1)–C(1)–Cu(1) 173.3(2). | ||
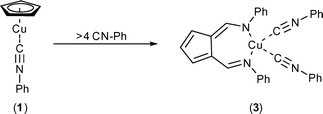
Monitoring of an NMR scale reaction of 1 with an excess of phenyl isocyanide over 72 h in THF (Scheme 3), showed the presence of peaks associated with the formation of a new AFA complex (3), with 1H NMR resonances that were shifted with respect to those observed for 2. Attempts to isolate crystalline material suitable for X-ray analysis where unsuccessful, but the reaction of [CuCl(CNPh)2]2 with NaC5H5 in the presence of an excess (> 4 equiv.) of CNPh followed by extraction into hexane, results in the isolation of a crop of yellow crystals.†1H NMR spectroscopy of the crystals, showed the product to be identical to that derived from the reaction of 1 with excess CN–Ph, with resonances at δ 6.40 (t), 7.01 (d), and 8.35 ppm (s) associated with the AFA ligand and a multiplet between δ 7.12–7.42 ppm corresponding to the 20 H of the aromatic groups.
 | ||
| Scheme 3 | ||
Single-crystal X-ray analysis confirmed the product to be the bis-isocyanide complex 3. The molecular structure of 3 (Fig. 2) shows the Cu centre is coordinated by the two nitrogen atoms of the AFA ligand and by the two divalent carbon atoms of the isocyanide ligands resulting in approximate tetrahedral coordination geometry. The cyclopentadienyl and imine portions of the AFA ligand are approximately co-planar with the Cu atom located approximately 0.57 Å above this plane. The two Cu–CNPh distances are comparable to that observed in complex 2, but unlike 2 the bite angle of the AFA ligand in 3 shows a significant decrease [N(2)–Cu(1)–N(3) 105.87(7), 3; N(2)–Cu(1)–N(3) 113.41 (8), 2] as a result of pyramidalisation at the metal centre. In both 2 and 3, the C–N and C–C bond lengths of the AFA ligand suggest multiple bond character and localisation of charge about C(10)–C(11)–C(12) and C–N bonds, which is consistent with Baily's description of the ligand as a cyclopentadienyl-dimine type system.21,22
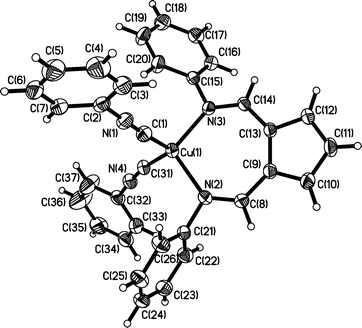 | ||
| Fig. 2 ORTEP (50% probability ellipsoids) diagram of (3). Selected bond lengths (Å): Cu(1)–C(1) 1.907(2), Cu(1)–C(31) 1.917(2), C(1)–N(1) 1.163(3), C(31)–N(4) 1.164(3), N(1)–C(2) 1.403(3), N(4)–C(32) 1.407(3), Cu(1)–N(2) 2.0367(18), Cu(1)–N(3) 2.0530(18), N(2)–C(8) 1.294(3), N(2)–C(21) 1.437(3), N(3)–C(14) 1.308(3), N(3)–C(15) 1.429(3), C(8)–C(9) 1.426(3), C(9)–C(10) 1.415(3), C(10)–C(11) 1.392(3), C(11)–C(12) 1.384 (3), C(12)–C(13) 1.421(3), C(13)–C(14) 1.416(3), C(1)–Cu(1)–C(31) 116.95(10), N(2)–Cu(1)–N(3) 105.87(7), Cu(1)–C(1)–N(1) 174.7(2), Cu(1)–C(31)–N(4) 171.24(19). | ||
To the best of our knowledge, the formation of complexes 2 and 3 represents the first examples of double migratory insertion of isocyanides into C–H bonds. Precedent for this type of migratory insertion chemistry can be found in the case of the Pd and Pt systems described by Carmona et al.,29,30 in which η1-C5H5 complexes, formed in situ, undergo a single migratory insertion of CNtBu into a the η1-M-C5H5 bond followed by tautomerisation to form metallafulvene products. The single migratory insertion of the divalent carbon species [CPh2] into one of the C–H bonds of an η5-coordinated Cp ligand has also been reported.31
While the precise mechanism for the formation of the AFA ligand in 2 and 3 is unknown, a plausible mechanism involves initial formation of a transient σ-bound [(η1-C5H5)Cu(CNPh)]32 complex, which in a process too fast to be monitored using 1H NMR inserts isocyanide to form metallafulvene intermediates which undergoes further rearrangement and a second insertion of CNPh into an adjacent σ-Cu–C bond.
In an attempt to elucidate the mechanism by which the AFA complex 2 is formed, the reaction between [CuCl(CNPh)2]2 and NaC5H5 was repeated and monitored using 1H NMR spectroscopy. Monitoring the reaction over 72 h shows the initial formation of complex 1 and free phenyl isocyanide. Over time the gradual appearance of peaks characteristic of the AFA complex are observed, which is associated with a concurrent decrease in the intensity of the resonance associated with the η5-C5H5 ligand in 1. As a result of the reaction stoichiometry (i.e. 2 : 1 ratio of [CuCl(CNPh)2]2–NaC5H5) 2 is not produced in large quantities and there is no evidence from 1H NMR of the presence of intermediate species. Significantly, attempts to react NaC5H5 with a range of isocyanide ligands in the absence of copper proved to be unsuccessful with no evidence for the production of AFA systems.
Finally, to investigate the influence of the nature of the isocyanide ligand on the reaction [(η5-C5H5)Cu(CNtBu)]33 and [(η5-C5H5)Cu(CNPh)] with an excess of the alkyl isocyanide, CNtBu (ca. 4 equiv.), was studied. 1H NMR spectroscopy failed to provide evidence of reaction. However, the analogous reaction of [(η5-C5H5)Cu(CNtBu)] with an excess (4 equiv.) of CNPh does show the slow production of an AFA complex, as evidence by the appearance of indicative resonances for the ligand system in the 1H NMR spectra, and provides an alterative route for the synthesis of complex 3. Similarly, reaction of either [(η5-C5H5)Cu(CNPh)] or [(η5-C5H5)Cu(CNtBu)] with the electron withdrawing isocyanides CN(p-C6H4F) and CN(p-C6H4NO2) show a much more rapid production of AFA complexes (< 12 h), the precise identity of which has not been fully established, indicating that the electronic nature of the isocyanide is a significant factor in the migratory insertion of isocyanides into C–H bonds of the Cu-C5H5 systems.
In conclusion, we have described the previously unprecedented 1,2-double migratory insertion of phenyl isocyanide in to the C–H bonds of a formally η5-coordinated Cp ligand, and reported the synthesis and molecular structures of two new and rare AFA complexes.
Acknowledgements
The EPSRC is thanked for the award of a Senior Research Fellowship (PRR) and studentships (AMW and MRW). Sigma-Aldrich HiTech Ltd. is also thanked for the for the funding of a case award studentship (AMW).Notes and references
- A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef.
- M. Tobisu and N. Chatani, Chem. Soc. Rev., 2008, 37, 300–307 RSC.
- R. A. Michelin, A. J. L. Pombeiro, M. Fatima and C. G. da Silva, Coord. Chem. Rev., 2001, 218, 75–112 CrossRef CAS.
- A. J. L. Pombeiro, M. da Silva and R. A. Michelin, Coord. Chem. Rev., 2001, 218, 43–74 CrossRef CAS.
- E. Singleton and H. E. Oosthuizen, Adv. Organomet. Chem., 1983, 22, 209–310 CAS.
- D. B. Beach, R. Bertoncello, G. Granozzi and W. L. Jolly, Organometallics, 1985, 4, 311–316 CrossRef.
- H. Ito, T. Kato and M. Sawamura, Chem.–Asian J., 2007, 2, 1436–1446 CrossRef CAS.
- C.-O. Ng, T.-L. Lo, S.-M. Ng and N. Zhu, Inorg. Chem., 2008, 47, 7447–7449 CrossRef CAS.
- L. D. Durfee and I. P. Rothwell, Chem. Rev., 1988, 88, 1059–1079 CrossRef CAS.
- C. K. Broder, A. E. Goeta, J. A. K. Howard, A. K. Hughes, A. L. Johnson, J. M. Malget and K. Wade, J. Chem. Soc., Dalton Trans., 2000, 3526–3533 RSC.
- F. Amor, J Sánchez-Nieves, P. Royo, H. Jacobsen, O. Blacque, H. Berke, M. Lanfranchi, M. A. Pellinghelli and A. Tiripicchio, Eur. J. Inorg. Chem., 2002, 2810–2817 CrossRef CAS.
- W. D. Jones, G. P. Foster and J. M. Putinas, J. Am. Chem. Soc., 1987, 109, 5047–5048 CrossRef.
- W. D. Jones and E. T. Hessel, Organometallics, 1990, 9, 718–727 CrossRef CAS.
- J. P. Lee, J. O. C. Jimenez-Halla, T. R. Chundari and T. B. Gunnoe, J. Organomet. Chem., 2007, 692, 2175–2186 CrossRef CAS.
- M. Tanaka, T. Sakakura, Y. Tokunaga and T. Sodeyama, Chem. Lett., 1987, 2373–2374 CrossRef CAS.
- K. Kobayashi, T. Matoba, S. Irisawa, T. Matsumoto, O. Morikawa and H. Konishi, Chem. Lett., 1998, 551–552 CrossRef CAS.
- M. Tobisu, S. Yamaguchi and N. Chatani, Org. Lett., 2007, 9, 3351–3353 CrossRef CAS.
- Y. M. Badiei and T. H. Warren, J. Organomet. Chem., 2005, 690, 5989–6000 CrossRef CAS.
- A. Toth, C. Floriani, A. Chiesi-Villa and C. Guastini, J. Chem. Soc., Dalton Trans., 1988, 1599–1605 RSC.
- B. A. Jazdzewski, P. L. Holland, M. Pink, V. G. Young Jr., D. J. Spencer and W. B. Tolman, Inorg. Chem., 2001, 40, 6097–6017 CrossRef CAS.
- P. J. Bailey, D. Loroño-González and S. Parsons, Chem. Commun., 2003, 1426–1427 RSC.
- P. J. Bailey, M. Melchionna and S. Parsons, Organometallics, 2007, 26, 128–135 CrossRef CAS.
- K. Hafner, K. H. Vopel, G. Ploss and C. Konig, Justus Liebigs Ann. Chem., 1963, 661, 52–75 CAS.
- U. Mueller-Westerhoff, J. Am. Chem. Soc., 1970, 92, 4849–4855 CrossRef.
- J. M. Lopez del Amo, U. Langer, V. Torres, G. Buntkowsky, H.-M. Vieth, M. Perez-Torralba, D. Sanz, R. M. Claramunt, J. Elguero and H.-H. Limbach, J. Am. Chem. Soc., 2008, 130, 8620–8632 CrossRef CAS.
- H. Rasika Dias, V. and W. Jin, Inorg. Chem., 1996, 35, 6546–6551 CrossRef CAS.
- H. Rasika Dias, V., W. Jin and R. E. Ratcliff, Inorg. Chem., 1995, 34, 6100–6105 CrossRef CAS.
- L. Bourget-Merle, M. F. Lappert and J. R. Severn, Chem. Rev., 2002, 102, 3031–3065 CrossRef CAS.
- F. M. Alías, T. R. Belderraín, M. Paneque, M. L. Poveda and E. Carmona, Organometallics, 1997, 16, 301–303 CrossRef CAS.
- F. M. Alías, T. R. Belderraín, M. Paneque, M. L. Poveda and E. Carmona, Organometallics, 1998, 17, 5620–5629 CrossRef CAS.
- H. Werner, Organometallics, 2005, 24, 1036–1049 CrossRef CAS.
- G. M. Whitesides and J. S. Fleming, J. Am. Chem. Soc., 1967, 89, 2855–2859 CrossRef CAS.
- T. Kruck and C. Terfloth, Chem. Ber., 1993, 126, 1101–1106 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterisation data and experimental procedures for the synthesis of 1, 2 and 3. CCDC reference numbers 706302–706304. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b822337h |
‡ Crystallography: the data were collected on a Nonius KappaCCD area detector diffractometer using MoKα radiation (λ = 0.71073 Å), and all structures were solved by direct methods and refined on all F2 data using the SHELX-97 suite of programs,34 All hydrogen atoms are in idealised positions and refined using a riding model. Crystal data: 1: C12H10Cu1N1, M = 231.75, colourless needles, crystal size 0.13 × 0.10 × 0.08 mm, monoclinic, space groupP21/n, a = 5.56900(10), b = 8.0000(2), c = 23.7374(7) Å, β = 98.6230(10)°, U = 1045.59(4) Å3, Z = 4, Dc = 1.472 g cm−3, T = 150(2) K, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429 reflections measured, 2390 unique reflections (2θ = 27.47°, Rint = 0.0609) against 128 parameters gave R1 = 0.0400 and wR2 = 0.0905 [I > 2σ(I)] (R1 = 0.0788 and wR2 = 0.1064 for all data). 2: C26H20Cu1N3, M = 437.99, yellow blocks, crystal size 0.10 × 0.10 × 0.08 mm, triclinic, space groupP 429 reflections measured, 2390 unique reflections (2θ = 27.47°, Rint = 0.0609) against 128 parameters gave R1 = 0.0400 and wR2 = 0.0905 [I > 2σ(I)] (R1 = 0.0788 and wR2 = 0.1064 for all data). 2: C26H20Cu1N3, M = 437.99, yellow blocks, crystal size 0.10 × 0.10 × 0.08 mm, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.1900(4), b = 10.1970(5), c = 10.3120(5) Å, α = 80.536(2)°, β = 80.213(2)°, γ = 83.978(3)°, U = 1038.26(8) Å3, Z = 2, Dc = 1.401 g cm−3, T = 150(2) K, 11 , a = 10.1900(4), b = 10.1970(5), c = 10.3120(5) Å, α = 80.536(2)°, β = 80.213(2)°, γ = 83.978(3)°, U = 1038.26(8) Å3, Z = 2, Dc = 1.401 g cm−3, T = 150(2) K, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479 reflections measured, 4604 unique reflections (2θ = 27.44°, Rint = 0.0734) against 272 parameters gave R1 = 0.0455 and wR2 = 0.0886 [I > 2σ(I)] (R1 = 0.0750 and wR2 = 0.0994 for all data). 3: C33H25Cu1N4, M = 541.11, yellow blocks, crystal size 0.20 × 0.13 × 0.10 mm, triclinic, space groupP 479 reflections measured, 4604 unique reflections (2θ = 27.44°, Rint = 0.0734) against 272 parameters gave R1 = 0.0455 and wR2 = 0.0886 [I > 2σ(I)] (R1 = 0.0750 and wR2 = 0.0994 for all data). 3: C33H25Cu1N4, M = 541.11, yellow blocks, crystal size 0.20 × 0.13 × 0.10 mm, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.9170(3), b = 10.8650(4), c = 14.5300(6) Å, α = 106.4740(10)°, β = 97.129(2)°, γ = 112.271(2)°, U = 1341.18(8) Å3, Z = 2, Dc = 1.340 g cm−3, T = 150(2) K, 14 , a = 9.9170(3), b = 10.8650(4), c = 14.5300(6) Å, α = 106.4740(10)°, β = 97.129(2)°, γ = 112.271(2)°, U = 1341.18(8) Å3, Z = 2, Dc = 1.340 g cm−3, T = 150(2) K, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539 reflections measured, 6046 unique reflections (2θ = 27.48°, Rint = 0.0432) against 344 parameters gave R1 = 0.0393 and wR2 = 0.0853 [I > 2σ(I)] (R1 = 0.0572 and wR2 = 0.0948 for all data). 539 reflections measured, 6046 unique reflections (2θ = 27.48°, Rint = 0.0432) against 344 parameters gave R1 = 0.0393 and wR2 = 0.0853 [I > 2σ(I)] (R1 = 0.0572 and wR2 = 0.0948 for all data). |
| This journal is © The Royal Society of Chemistry 2009 |
