The CO–Ne van der Waals complex: ab initio intermolecular potential energy, interaction induced electric dipole moment and polarizability surfaces, and second virial coefficients†
Abstract
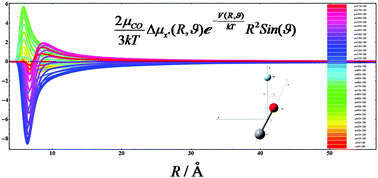
The intermolecular potential energy, interaction induced electric dipole moment and polarizability surfaces of the CO–Ne van der Waals complex are calculated using coupled cluster methods and the d-aug-cc-pVTZ basis set extended with a set of 3s3p2d1f1g midbond functions placed in the middle of the van der Waals bond. After fitting the interaction properties to appropriate analytical functions the surfaces are further used in semiclassical calculations of the pressure, the dielectric and the refractivity second virial coefficients of the system. The interaction potential energy surface has a single minimum (−49.9952 cm−1), which corresponds to R = 3.383 Å and θ = 79.4°. The computed dielectric second virial coefficient Bε≈−0.27 cm6 mol−2 around the room temperature.

 Please wait while we load your content...
Please wait while we load your content...