An enhanced fluorescence in a tunable face-to-face π⋯π stacking assembly directed by the H-bonding†
Xiao-Fei
Fu
a,
Yan-Feng
Yue
b,
Rui
Guo
a,
Le-Le
Li
b,
Wei
Sun
b,
Chen-Jie
Fang
*a,
Chun-Hu
Xu
b and
Chun-Hua
Yan
*b
aSchool of Chemical Biology and Pharmaceutical Sciences, Capital Medical University, Beijing, 100069, China. E-mail: cjfang@ccmu.edu.cn; Fax: +86 10 8391 1533; Tel: +86 10 8391 1523
bBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications & PKU-HKU Joint Laboratory on Rare Earth Materials and Bioinorganic Chemistry, Peking University, Beijing, 100871, China. E-mail: yan@pku.edu.cn; Fax: +86 10 6275 4179; Tel: +86 10 6275 4179
First published on 24th July 2009
Abstract
A simple anthryl derivative 1-((anthracen-10-yl)methylene)-4-ethylthiosemicarbazide (ANS) shows a methanol-tunable solid state fluorescence enhancement based on different molecular packing structure; the emission is stronger in crystal 2 than that in crystal 1 under the same condition. This is mainly due to the formation of face-to-face π⋯π stacking assembly in crystal 2 which is directed and enhanced by intermolecular H-bonding interactions. The observation of fluorescence decay corroborates the formation of an emissive excimer in the π⋯π stacking assembly.
As a bottom-up approach to fabricate electronic and photonic devices, the development of the tunable supramolecular structures has attracted much attention in materials science.1 For solid state materials, the property is governed by the whole collective rather than by the individual molecules, because the constituent molecules may form strong intermolecular interactions and thus assemble into packed structures.2 Moreover, the performance of the organic materials based devices is strongly dependent on the molecular assembly structure.3 So far, there is still a fundamental need to understand, and if possible to control, at the molecular level the relationships between the solid state supramolecular packing and the resulting electronic and optical properties; establishing such relationships would enable the development of a new strategy towards improvements in the performance of the organic materials.
Recently, the most extensively studied systems are π-conjugated molecules as advanced materials for applications in the field of organic electronics such as light-emitting diodes, field-effect transistors, and solar cells. Many approaches are focused on tuning fluorescent properties in solution, in contrast, only a few works on the convenient methods have been reported to control fluorescent properties in the solid state,4–6 which might be more useful for applications in devices. For example, Stalke has found that an anthracene derivative exhibits a strong solid state fluorescence depending on the inclusion of a toluene molecule.4 Ma and Yang have reported the formation of a T-shaped anthracene–anthracene dimer in the solid state upon addition of water in the anthrylpolyamine-metal hybrid system.5 Although progress has been made,7,8 it is still essential to gain an adequate characterization of the intermolecular interactions in numerous instances for the purpose of understanding and controlling the effect of molecular packing in the solid state on the optical and electronic properties.
In this communication, we present an anthracene derivative as building blocks to construct different single crystals based on H-bonding and face-to-face π⋯π interactions (Scheme 1). The methanol-mediated fluorescence enhancement effect and the relationship between the molecular packing and the optical properties of the obtained crystals are also reported. The remarkable fluorescence enhancement induced by the methanol molecule is very interesting, where the enhancement is mainly from the face-to-face π⋯π interactions between anthracene molecules directed by the stronger intermolecular H-bonds. In addition, rare solid materials exhibiting a gust-recognition ability by fluorescence change have been reported.4,5
 | ||
| Scheme 1 The ANS molecule. | ||
As large van der Waals surfaces of (supra)molecular assemblies enable the formation of an extended π-stacked structure, the π-conjugated building block anthracene prefers a herringbone packing.9 Owing to the presence of thione and amine groups which can act as H-bond acceptor and donor, ethyl thiosemicarbazide is thus introduced to offer the recognition sites for the H-bonds. This designed molecule 1-((anthracen-10-yl)methylene)-4-ethylthiosemicarbazide (ANS) was conveniently prepared in high yield from condensation of anthracene-10-carbaldehyde and ethyl thiosemicarbazide. Two kinds of the crystals suitable for single crystal X-ray diffraction analysis were grown separately by slow evaporation of the ANS solution in methanol, with 1 (ANS) made by dissolving ANS at room temperature and 2 (ANS·0.5MeOH) under heating. Crystals 1 and 2 adopted different crystallization patterns though both were from the same ANS material.‡,§Recrystallization of ANS from acetonitrile solution afforded the single crystal which was crystallized in the same space group as that of crystal 1; the structure and fluorescent property are therefore not discussed.
1 was crystallized in P21/n space group, with two molecules assembled into a dimer based on the intermolecular N–H⋯S interactions (Fig. 1). The distances of N⋯S are 3.475(2) and 3.411(2) Å, that of H⋯S are 2.70(2) and 2.66(2) Å, and the angles of N–H⋯S are 160(1) and 159(1)°.
 | ||
| Fig. 1 The ANS dimer in crystal 1. | ||
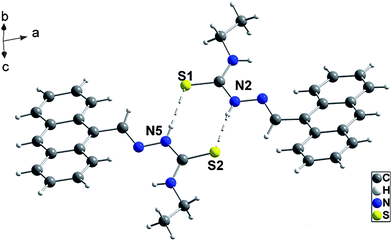
In contrast, 2 was crystallized in C2/c space group. The solvent methanol molecule which lies about a twofold axis was contained in the asymmetric unit, in which the O atom occupies three positions with different occupancy factor. The four hydrogen atoms belonging to this methanol molecule have not been included in the refinement due to the disorder of the solvent molecule. In addition, the H-bonding discussed afterwards may be affected by the disorder of the methanol molecule. The presence of methanol in crystal 2 was confirmed with microanalysis and IR spectroscopy.10 The bands at 3260 and 3220 cm−1 in the IR spectrum indicate the formation of N–H⋯O H-bonds between the ANS and methanol molecules, which held two ANS molecules together to form a dimer, with amine groups of two symmetry-related ANS molecules interacted with the same methanol molecule (Fig. 2).
 | ||
| Fig. 2 The ANS dimer in crystal 2. Selected distances (Å) and angles (°) for N–H⋯O: N2⋯O1 3.16(1), H2D⋯O1 2.37(3), N2–H2D⋯O1 153(2), N2⋯O1A 3.04(1), H2D⋯O1A 2.18(3), N2–H2D⋯O1A 173(2), N2⋯O2 2.965(5), H2D⋯O2 2.11(3), N2–H2D⋯O2 169(2) (symmetry code A: −x, y, 0.5 − z) (the dashed lines indicate the H-bonds). | ||
The packing structures assembled in the solid state are further explored. For crystal 1, Fig. 3 depicts that the considerably acute angles (31.5 and 35.2°) between the anthryl rings are somewhat reminiscent of the molecule arrangement toward a face-to-face π⋯π stacking assembly, implying that a coplanar π stacking excimer might be formed in the excited state.
 | ||
| Fig. 3 The packing diagram of ANS molecules in 1. For clarity, the H atoms are omitted. | ||
In the crystal lattice of 2, C–H⋯S and N–H⋯S H-bonding and face-to-face π⋯π interactions are observed, besides the H-bonds corresponding to the formation of the dimer as shown in Fig. 2. Therein, the distances of 2.88(3) and 3.706(3) Å were observed for S1B⋯H9 (symmetry code B: x , 1− y, −0.5 + z) and C9⋯S1B, respectively, with angle of C9–H9⋯S1B 156(3)°, meaning the formation of intermolecular H-bonds between the anthryl ring and the ethyl thiosemicarbazone of the neighbouring molecule. The molecules are thus held to form a chain along the c axis (Fig. 4). Further, the final assembly is thus formed via interchain N1–H1D⋯ S1C (symmetry code C: 0.5 − x, 0.5 + y, 0.5 − z) hydrogen-bonding interactions along the b direction, with distances of H1D⋯S1C and N1⋯S1C being 2.726(2) and 3.396(2) Å, respectively, and the angle of N1–H1D⋯S1C 137(2)°. The intermolecular face-to-face π⋯π interactions are observed between the chains, with the shortest interplanar distance of 3.50 Å and a dihedral angle of 0°. This result indicates the existence of coplanar aromatic–aromatic interactions in the new aggregated structure upon inclusion of the methanol molecule (Fig. 5). It is obvious that the face-to-face π⋯π interactions are directed and enforced by the strength and directionality of the H-bonds between the ANS molecules and those between the ANS and the solvent methanol molecules within the packing structure of the assembly.11
 | ||
| Fig. 4 The C–H⋯S and π⋯π interactions in 2. For clarity, the solvent molecules are omitted. The red dot represents the centroid of the anthracene ring. | ||
 | ||
| Fig. 5 The face-to-face π⋯π stacking structure in 2. For clarity, the H atoms are omitted. | ||
The correlation between solid state photophysical property and the arrangement of the molecules has been attracting much attention, therefore, it is interesting to compare the solid state fluorescence property of the different packing structures in the single crystals, due to the inclusion of the methanol molecule. The photoluminescence of crystals 1 and 2 originates from the π → π* electronic transition of the anthryl chromophore. Fluorescence was separately recorded on the polycrystalline samples of 1 and 2 under the same conditions. As shown in Fig. 6, crystal 1 displays a structureless emission band centred at 550 nm with a shoulder peak at around 510 nm upon excitation at 353 nm. The fluorescence decay of crystals 1 and 2 was further measured to explore the difference in the excited state. The decay was fitted to a biexponential profile. For crystal 1, the anthracene fluorescence lifetime is evaluated as 2.1 ns (66%) for the emission of monomeric chromophore and 6.7 ns (34%) for the excimer emission. While for crystal 2, the longer decay component (10.1 ns, 82%) corroborates the existence of an emissive excimer, the short decay parameter (2.6 ns, 18%) is attributed to the emission of the locally excited anthracene monomer.12
 | ||
| Fig. 6 The emission spectra of crystals 1 and 2 upon excitation at 353 nm. | ||
Different fluorescence properties between 1 and 2 are ascribed to the collective effect of intermolecular H-bonding and face-to-face π⋯π interactions between anthryl rings induced by the inclusion of the methanol molecule in the crystal lattice during crystallization. It is clear that the fluorescent properties of the crystals are strongly dependent on the molecular stacking feature.2 Therefore, it is rational to explain the emission enhancement phenomenon with the collective effect of extended H-bonds and π⋯π stacking for crystal 2 in the solid state, especially, π⋯π interactions play a dominant role in the formation of a fluorescent excimer (Fig. 7).
 | ||
| Fig. 7 Radioactive decay curves of crystals 1(upper) and 2 (bottom) monitored at emission wavelength 547 nm, λex = 353 nm. | ||
The results herein show the molecular packing features have a dramatic effect on the photoluminescent properties of the functional materials, and it is possible to tune and control the photofluorescent properties and the performance of the functional material through the regulation of the molecular structure and molecular packing features. We demonstrate an approach to control the organization of the aromatic rings of anthracene molecules in the solid state that directs and enforces the face-to-face π⋯π interactions using the strength and directionality of H-bonds. From the viewpoint of efficient charge transport, maximizing π-orbital overlap is also much desired in the organic semiconductor solid. Currently, the attempts to tune the fluorescent properties via molecular modification utilizing π⋯π stacking and H-bonding interactions, are underway in our laboratory.
The authors thank Beijing Natural Science Foundation (Grant No. 2082007), Project for Science and Technology Development, Beijing Commission of Education (Grant No. KM200810025026) and the NSFC (Grant No. 20771009) for financial support.
References
- V. Balzani, M. Venturi and A. Credi, Molecular Devices and Machines, Wiley-VCH, Weinheim, 2003 Search PubMed; J. E. Anthony, Chem. Rev., 2006, 106, 5028 Search PubMed; S. R. Forrest and M. E. Thompson, Chem. Rev., 2007, 107, 923 CrossRef CAS (Thematic issue − Organic electronics and optoelectronics).
- D. Braga, F. Grepioni and G. R. Desiraju, Chem. Rev., 1998, 98, 1375 CrossRef CAS; B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS.
- Y. Yamamoto, T. Fukushima, Y. Suna, N. Ishii, A. Saeki, S. Seki, S. Tagawa, M. Taniguchi, T. Kawai and T. Aida, Science, 2006, 314, 1761 CrossRef CAS.
- Z. Fei, N. Kocher, C. J. Mohrschladt, H. Ihmels and D. Stalke, Angew. Chem., Int. Ed., 2003, 42, 783 CrossRef CAS.
- G. Zhang, G. Yang, S. Wang, Q. Chen and J. S. Ma, Chem.–Eur. J., 2007, 13, 3630 CrossRef CAS.
- Y. Mizobe, H. Ito, I. Hisaki, M. Miyata, Y. Hasegawa and N. Tohnai, Chem. Commun., 2006, 2126 RSC.
- H. Zhang, Z. Zhang, K. Ye, J. Zhang and Y. Wang, Adv. Mater., 2006, 18, 2369 CrossRef CAS.
- Y. Mizobe, N. Tohnai, M. Miyata and Y. Hasegawa, Chem. Commun., 2005, 1839 RSC.
- G. R. Desiraju and A. Gavezzotti, J. Chem. Soc., Chem. Commun., 1989, 621 RSC; S. Tsuzuki, K. Honda and R. Azumi, J. Am. Chem. Soc., 2002, 124, 12200 CrossRef CAS; G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- The microanalysis result for crystal 1: anal. calcd. for C18H17N3S: C 70.33, H 5.57, N 13.67. Found: C 70.25, H 5.59, N 13.60%. For crystal 2: anal. calcd. for C37H38N6OS2: C 68.70, H 5.92, N 12.99. found: C 68.64, H 5.91, N 12.98%. The main IR bands for crystal 1: 3354, 3344, 3155, 2975, 2876, 1622, 1536, 1515, 1417, 1300, 1236, 1092, 882, 732, 597 cm−1; for crystal 2: 3350, 3260, 3220, 3048, 2966, 1622, 1548, 1516, 1438, 1307, 1229, 1091, 882, 731, 596 cm−1.
- A. N. Sokolov, T. Friščić and L. R. MacGillivray, J. Am. Chem. Soc., 2006, 128, 2806 CrossRef CAS.
- Y. Molard, D. M. Bassani, J.-P. Desvergne, N. Moran and J. H. R. Tucker, J. Org. Chem., 2006, 71, 8523 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The residual plots for the radioactive decay curves and crystallographic data in CIF. CCDC reference numbers 721344 and 721345. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b910263a |
| ‡ Crystal data of 1: C18H17N3S, Mr = 307.41, monoclinic, space groupP21/n, a = 20.622(4) Å, b = 7.3581(15) Å, c = 21.626(4) Å, β = 103.42(3)°, V = 3191.8(11) Å3, T = 153 (2) K, Z = 8, µ (Mo Ka) = 0.203 mm−1, 48641 reflections measured, 7322 unique (Rint = 0.0660), R1 = 0.0394 [I > 2σ(I)], wR2 = 0.1016 (all data). CCDC 721344. |
| § Crystal data of 2: C37H38N6OS2, Mr = 646.85, monoclinic, space groupC2/c, a = 18.946(4) Å, b = 8.8906(18) Å, c = 20.698(4) Å, β = 104.51(3)°, V = 3375.1(12) Å3, T = 293(2) K, Z = 4, µ (Mo Ka) = 0.197 mm−1, 9169 reflections measured, 2985 unique (Rint = 0.0276), R1 = 0.0531 [I > 2σ(I)], wR2 = 0.1371 (all data). CCDC 721345. |
| This journal is © The Royal Society of Chemistry 2009 |
