Novel enantiopure bis(pyrrolo)tetrathiafulvalene donors exhibiting chiral crystal packing arrangements†
Abstract
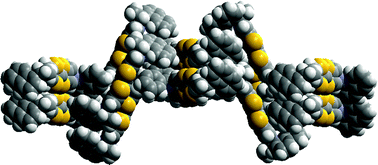
Two novel enantiopure bis(pyrrolo[3,4-d])tetrathiafulvalene derivatives, substrates for preparing chiral conducting materials, show chiral crystal packing arrangements in which successive layers are rotated in accordance with an exact or approximate 43 axis. The corresponding donors containing fused dihydropyrrole groups, and thus four more hydrogen atoms, form stacks along a crystal axis.
- This article is part of the themed collection: In memory of Peter Day

 Please wait while we load your content...
Please wait while we load your content...