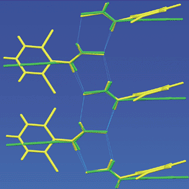
The synthesis and single crystal X-ray diffraction structures of five 2,6-disubstituted N-arylthioamides, viz.2,6-difluorophenylthioamide (1), 2,6-dichlorophenylthioamide (2), 2,6-dibromophenylthioamide (3), 2,6-dimethylphenylthioamide (4) and 2-chloro-6-methylphenylthioamide (5), are reported. The primary hydrogen-bonding motif consists of 1-D ribbons of molecules connected by N–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds and weak C–H⋯S
C hydrogen bonds and weak C–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions. All five N-arylthioamide molecules adopt the cis conformation in the solid state, defined as the conformation where the amine
C interactions. All five N-arylthioamide molecules adopt the cis conformation in the solid state, defined as the conformation where the amine