Increased control over the desolvation of p-tert-butylcalix[5]arene†
Jian
Tian
a,
Scott J.
Dalgarno
ab,
Praveen K.
Thallapally‡
a and
Jerry L.
Atwood
*a
aDepartment of Chemistry, University of Missouri-Columbia, 601 S. College Avenue, Columbia, MO 65211, USA. E-mail: AtwoodJ@missouri.edu; Fax: +1 573 882 2754; Tel: +1 573 882 8374
bSchool of Engineering and Physical Sciences-Chemistry, Heriot-Watt University, Edinburgh, UK EH14 4AS
First published on 15th October 2008
Abstract
Parital de-solvation of the toluene solvate of p-tert-butylcalix[5]arene affords a frustrated organic solid that is a new type of sorbant. Increased control over this sorption is achieved by employing a high boiling solvent for incremental de-solvation, revealing the calixarene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent ratio for maximum gas sorption.
solvent ratio for maximum gas sorption.
The adsorption of gases on or in carbon nanotubes,1a activated carbons,1b zeolites,1b,c clays,1c,d metal–organic1e and covalent-organic1f frameworks is continuing to attract interest due to the fascinating nature of these various materials and the inherent potential of the ‘Hydrogen economy’.1g Our interests in the area of gas sorption lie in the use of calix[n]arenes, cyclic bowl-shaped molecules that have cavities suitable for the hosting of guest molecules.2 Although a number of our recent studies have focused on the remarkable transport of gas molecules into the cavities of a seemingly non-porous form of p-tert-butylcalix[4]arene (1, Fig. 1A),3 we have also been examining various porous phases of calix[5]arenes for gas storage properties.4,5 We found that calix[5]arene (2, Fig. 1A) sublimed to form self-included and back-to-back helices in the solid state, the former of which possess empty channels that are suitable for such purposes.6 In relation to the current contribution, we found that the relatively controllable de-solvation of the toluene solvate of p-tert-butylcalix[5]arene (3·Tol, Fig. 1A), afforded a ‘frustrated’ organic solid.4
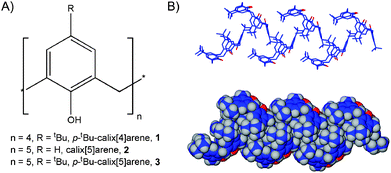 | ||
| Fig. 1 (A) Schematic of 1–3. (B) Stick and space filling representations of self-include (and non-porous) chains of 3 formed either by sublimation or by full de-solvation of 3·Tol. | ||
The term ‘frustrated’ relates not to magnetic properties, but rather to the fact that the crystal lattice becomes porous upon partial solvent removal. In this regard, the residual toluene molecules prevent the material from collapsing. Removal of these residual molecules, however, affords a non-porous material comprising self-included chains of 3 (Fig. 1B) that can also be obtained by sublimation.4 These studies focused on one of three pseudo-polymorphs of the toluene solvate of 3, and we also recently showed that the second of these materials (that can be isolated in bulk) has similar sorption properties when partially de-solvated.5
While the toluene solvates of 3 allow for relatively controllable de-solvation (by heating at 80 °C over a number of hours), we found there to be a window in which the material is at maximum sorption capability, which can only be obtained by trial and error heating. As this is the case, we sought to explore and reach the point at which maximum incremental desolvation occurs in these materials. In order to achieve this, we employed p-xylene (PXY) as a high boiling solvent on the basis that it should prove harder to remove from the crystal lattice. Although we have actually found this particular phase of TBC5·solvent to be less suitable for gas sorption purposes when considering the significantly smaller amount of residual solvent present in the most ‘frustrated form’, we show that this technique does indeed allow for more controlled desolvation of the resulting material. Maximum sorption in 3·PXY occurs when the calixarene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent ratio is approximately 1
solvent ratio is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 and shows that small differences in the the solvent may hold different preferences for preservation of the extended porous nature of these materials.
0.8 and shows that small differences in the the solvent may hold different preferences for preservation of the extended porous nature of these materials.
Crystallisation of 3·PXY was performed by heating 3 in boiling PXY, followed by cooling to room temperature and slow evaporation over a number of days, affording large colourless crystals that were suitable for single crystal X-ray diffraction studies.§Thermo-gravimetric analysis on the harvested material (which is stable toward solvent loss upon removal from the mother liquor) afforded a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY ratio of 1
PXY ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8. The crystals are in a monoclinic cell and the asymmetric unit contains one half of a molecule of 3 and one PXY residing in the calixarene cavity on a mirror plane. There is an additional PXY that is exo to the calixarene cavity, is disordered over two positions (while also residing on a mirror plane), and that was modelled at a total occupancy of 0.8 according to TGA analysis. As was the case for the three pseudo-polymorphs of 3·Tol, symmetry expansion shows the presence of a common structural feature, the base-to-base dimerisation of 3 (Fig. 2A).5 The endo-cavity PXY molecule is bound in the cavity of 3 through a total of four CH⋯π interactions, two from host to guest and vice versa (Fig. 2B), with CH⋯aromatic centroid distances in the range of 3.03–3.17 Å.
1.8. The crystals are in a monoclinic cell and the asymmetric unit contains one half of a molecule of 3 and one PXY residing in the calixarene cavity on a mirror plane. There is an additional PXY that is exo to the calixarene cavity, is disordered over two positions (while also residing on a mirror plane), and that was modelled at a total occupancy of 0.8 according to TGA analysis. As was the case for the three pseudo-polymorphs of 3·Tol, symmetry expansion shows the presence of a common structural feature, the base-to-base dimerisation of 3 (Fig. 2A).5 The endo-cavity PXY molecule is bound in the cavity of 3 through a total of four CH⋯π interactions, two from host to guest and vice versa (Fig. 2B), with CH⋯aromatic centroid distances in the range of 3.03–3.17 Å.
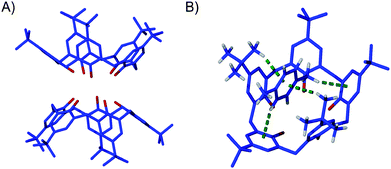 | ||
| Fig. 2 (A) The common base-to-base dimer found in both 3·PXY and 3·Tol.5 (B) The concerted CH⋯π interactions found in 3·PXY. Hydrogen atoms have been omitted completely and selectively for clarity in (A) and (B), respectively. In addition, the disordered tert-butyl group is shown in only one position for clarity. | ||
With respect to the exo-cavity PXY molecule, a cross-section of the extended structure around the disordered molecule shows it to occupy a large space generated by six neighbouring calixarenes (Fig. 3). We propose that exo-cavity PXY would be more easily removed over the endo-cavity molecules, affording a porous material with the hypothesised ‘frustration’.
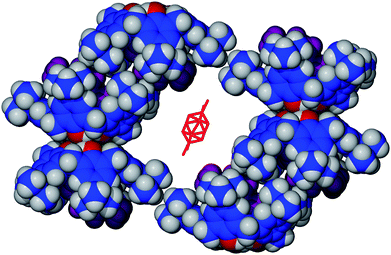 | ||
| Fig. 3 Cross-section of the extended structure of 3·PXY showing the disordered PXY molecule occupying the space generated by the packing of six neighbouring calixarenes. Hydrogen atoms are not shown on the disordered PXY, and endo-cavity PXY are shown in purple. | ||
Given the pseudo-polymorphism present in 3·Tol, the harvested crystals of 3·PXY were checked for such properties by X-ray powder diffraction (XRPD). These studies showed, when compared with a calculated powder pattern from the single crystal structure (Fig. S1),† that the bulk material contains only one form of 3·PXY.
As this was the case, we examined the bulk material for controllable solvent loss under various conditions. We found the material to be sensitive to solvent loss under vacuum at ambient temperature over extended periods of time until the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY ratio reached 1
PXY ratio reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, at which point solvent loss ceased under these conditions. Given this, de-solvation was stopped at a number of points during this timeframe and the ‘frustration’ was monitored by recording acetylene sorption and performing TGA analysis (Fig. 4A). From this observation it is clear that removing a very specific amount of PXY from the material affords a stable array that is capable of some endo-cavity guest loss prior to structural collapse (to the sublimed phase). This indicates that there are a specific number of intermolecular interactions required in order to maintain frustration within these materials.
1.1, at which point solvent loss ceased under these conditions. Given this, de-solvation was stopped at a number of points during this timeframe and the ‘frustration’ was monitored by recording acetylene sorption and performing TGA analysis (Fig. 4A). From this observation it is clear that removing a very specific amount of PXY from the material affords a stable array that is capable of some endo-cavity guest loss prior to structural collapse (to the sublimed phase). This indicates that there are a specific number of intermolecular interactions required in order to maintain frustration within these materials.
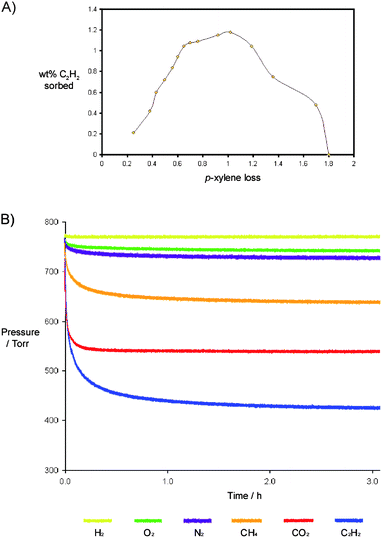 | ||
Fig. 4 (A) Graph showing the wt% acetylene sorbed by 3·PXY as a function of sequential desolvation under reduced pressure (p-xylene loss region of ∼0.2–0.7), and at reduced pressure with heating at 105 °C (p-xylene loss region of ∼0.7 – 1.8). (B) Gas sorption isotherms for the material containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 ratio of 3·PXY. 0.8 ratio of 3·PXY. | ||
Once the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 was reached using prolonged exposure to vacuum, it was necessary to heat the material under reduced pressure at > 90 °C sequentially over a number of hours to induce further solvent loss. The anticipated ‘frustration’ was similarly followed by acetylene sorption (wt%) and TGA analysis, and it was found that upon removing >1 molar equivalent of PXY from the material, i.e. with a 3·PXY ratio of 1
1.1 was reached using prolonged exposure to vacuum, it was necessary to heat the material under reduced pressure at > 90 °C sequentially over a number of hours to induce further solvent loss. The anticipated ‘frustration’ was similarly followed by acetylene sorption (wt%) and TGA analysis, and it was found that upon removing >1 molar equivalent of PXY from the material, i.e. with a 3·PXY ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <0.8, gas sorption capability (and hence porosity) is reduced by conversion to the self-included or sublimed phase. The temperature required to remove some endo-cavity PXY supports the hypothesis of ‘frustration’ (through host–guest interactions) and formation of a stable material when considering the plot in Fig. 4.
<0.8, gas sorption capability (and hence porosity) is reduced by conversion to the self-included or sublimed phase. The temperature required to remove some endo-cavity PXY supports the hypothesis of ‘frustration’ (through host–guest interactions) and formation of a stable material when considering the plot in Fig. 4.
In addition to the sorption of acetylene outlined above, the partially de-solvated material was studied for sorption of other gases at a number of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY ratios. At 1
PXY ratios. At 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, the material also displayed maximum sorption of carbon dioxide and methane. (Fig. 4B), and we observed sequential reductions in the amount of gas sorbed in the sample following each subsequent de-solvation step. All phases showed little or negligible sorption of nitrogen, oxygen and hydrogen at 1 atm pressure and room temperature.
0.8, the material also displayed maximum sorption of carbon dioxide and methane. (Fig. 4B), and we observed sequential reductions in the amount of gas sorbed in the sample following each subsequent de-solvation step. All phases showed little or negligible sorption of nitrogen, oxygen and hydrogen at 1 atm pressure and room temperature.
In order to confirm conversion towards the non-porous inactive phase after reaching a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY ratio of 1
PXY ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, combined XRPD and TGA studies were performed on various ratios (Fig. 5). Compared to our previous studies, it is clear that a phase change to the non-porous phase occurs, and is well underway by the time the 3
1, combined XRPD and TGA studies were performed on various ratios (Fig. 5). Compared to our previous studies, it is clear that a phase change to the non-porous phase occurs, and is well underway by the time the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY ratio is 1
PXY ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 (Fig. 5).
0.6 (Fig. 5).
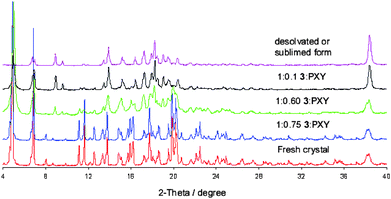 | ||
Fig. 5
X-Ray diffraction powder patterns recorded for three ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PXY between crystals of the fresh solvate (red, ratio of 1 PXY between crystals of the fresh solvate (red, ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8) and the desolvated/sublimed form (purple, ratio of 1 1.8) and the desolvated/sublimed form (purple, ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). The gradual transformation is evidenced by the development of indicative peaks (2θ values of 9 and 10°). This is consistent with previous studies.4 0). The gradual transformation is evidenced by the development of indicative peaks (2θ values of 9 and 10°). This is consistent with previous studies.4 | ||
In conclusion, we have shown that ‘frustration’ of 3 can be achieved through the use of different solvates. By employing a high boiling solvent, significantly greater control over the de-solvation of 3·solvent has been gained, thereby allowing us to probe for maximum sorption capabilities through detailed ‘frustration’ of the crystal lattice, as measured by gas sorption and TGA analysis. Furthermore, our findings suggest that our original hypothesis is likely correct with regard to structural ‘frustration’.4 Similar studies with the other xylene isomers are currently underway, along with various other solvates in order to determine the optimum extended structure for ‘frustration’ with a view to producing this type of material with maximum porosity.
References
- (a) A. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune and M. J. Heben, Nature, 1997, 386, 377 CrossRef CAS; (b) V. C. Menon and S. Komarneni, J. Porous Mater., 1998, 5, 43 CrossRef CAS; (c) D. W. Breck, Zeolites Molecular Sieves, Chemistry and Use, Wiley, New York, 1974 Search PubMed; (d) A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS; (e) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, J. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS; (f) R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238 CrossRef CAS; (g) X. Zhao, B. Xiao, A. J. Fletcher, K. M. Thomas, D. Bradshaw and M. J. Rosseinsky, Science, 2004, 306, 1012 CrossRef CAS; (h) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (i) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef CAS; (j) R. Coontz and B. Hanson, Science, 2004, 305, 957 CrossRef , Special issue on the hydrogen economy.
- C. D. Gutsche, Calixarenes, Royal Society of Chemistry, Cambridge, 1992 Search PubMed.
- J. L. Atwood, L. J. Barbour and A. Jerga, Angew. Chem., Int. Ed., 2004, 43, 2948 CrossRef CAS; J. L. Atwood, L. J. Barbour, P. K. Thallapally and T. B. Wirsig, Chem. Commun., 2005, 51 RSC; P. K. Thallapally, G. O. Lloyd, T. B. Wirsig, M. W. Bredenkamp, J. L. Atwood and L. J. Barbour, Chem. Commun., 2005, 5272 RSC; P. K. Thallapally, L. Dobrańska, T. R. Gingrich, T. B. Wirsig, L. J. Barbour and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 6506 CrossRef.
- P. K. Thallapally, S. J. Dalgarno and J. L. Atwood, J. Am. Chem. Soc., 2006, 128, 15060 CrossRef CAS.
- S. J. Dalgarno, P. K. Thallapally, J. Tian, J. L. Atwood, submitted.
- S. J. Dalgarno, J. Tian, J. E. Warren, T. Clark, M. Makha, C. L. Raston and J. L. Atwood, Chem. Commun., 2007, 4848 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic information file for 3·PXY; supplementary figures showing sample homogeneity from calculated and experimental X-ray powder diffraction patterns. CCDC reference number 690349. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b813628a |
| ‡ Current address: Energy and Environment Directorate, Pacific Northwest National Laboratory, Richland, WA 99352, USA. |
| § Crystallographic data for3·PXY: C66.2H84O5, M = 959.73, monoclinic, a = 22.334(3), b = 15.584(2), c = 17.996(2) Å, β = 99.605(2)°, U = 6175.9(15) Å3, T = 173(2) K, space groupC2/m, Z = 4, Mo Kα radiation (wavelength λ = 0.71073 Å), GOF = 1.030, agreement index R1 (I > 2σ{I}) = 0.0694, 22088 reflections measured, 7030 unique (Rint = 0.0436) which were used in all calculations. The final ωR(F2) was 0.1960 (all data). A number of restraints were applied to the disordered tBu group (of the calixarene) and p-xylene molecule. |
| This journal is © The Royal Society of Chemistry 2009 |
