An organo-silver compound that shows antimicrobial activity against Pseudomonas aeruginosa as a monomer and plasma deposited film†
Neil
Poulter
a,
Xavier
Munoz-Berbel
a,
Andrew L.
Johnson
*a,
Andrea J.
Dowling
b,
Nicholas
Waterfield
c and
A. Tobias A.
Jenkins
*a
aDepartment of Chemistry, University of Bath, Bath, UK BA2 7AY. E-mail: a.t.a.jenkins@bath.ac.uk; Fax: +44 (0)1225 386231; Tel: +44 (0)1225 386118
bCentre for Ecology and Conservation, University of Exeter in Cornwall, Penryn, UK
cDepartment of Biology & Biochemistry, University of Bath, Bath, UK BA2 7AY
First published on 2nd November 2009
Abstract
In this communication we describe the synthesis, characterisation and plasma deposition of a novel organo-silver compound for the prevention of the growth of Pseudomonas aeruginosa on both polystyrene surfaces and polypropylene non-woven fabrics.
The problem of infection and colonisation of medical devices by bacteria is gaining increasing publicity with stories of so-called hospital ‘superbugs’ now common in the media. Though receiving less publicity, Pseudomonas aeruginosa has been recognised as an emerging opportunistic nosocomial pathogen of clinical relevance.1 It can be clinically important in a range of health problems including: urinary tract infections, respiratory system infections, dermatitis, soft tissue infections, bacteremias, bone and joint infections, gastrointestinal infections and is a major factor in the mortality of sufferers of a variety of systemic infections, particularly in patients with severe burns, cystic fibrosis and in cancer and immunocompromised patients.2P. aeruginosa has become notorious for its resistance to antibiotics, in part due to permeability barrier afforded by its Gram-negative outer membrane. Also, its tendency to colonise surfaces in a biofilm form makes the cells impervious to therapeutic concentrations of antibiotics.1,2 These issues make the development of new antibiotics, or the generation of materials for which biofilm formation is inhibited, a significant challenge for both the materials chemistry and clinical medicine communities.3
The activity of silver(I) ions as an antimicrobial agent has been known for hundreds of years, but more recently has attracted significant attention with the incorporation of silver(I) systems onto a wide range of medical and associated devices used in clinical settings. Silver has been proven as an effective antibacterial agent that can avoid defence mechanisms of such bacteria and prevent biofilm formation, the cause of acute infection. Most current technologies using silver require the silver to be included into the polymer matrix. A review of methodologies currently used for this is written by Thomas et al.4 These approaches, while fairly effective, have two principal difficulties: firstly, such coatings are expensive, pushing up the cost of medical devices such as catheters as a consequence. Secondly, in some clinical situations, silver may have a retarding effect on cell growth, for example in burns.5
Plasma deposition offers a low cost, highly industrially up-scalable, solvent free method for depositing thin polymer like films on surfaces. It has the advantage of deposition taking place at near to room temperature, avoiding potential substrate melting which could take place in chemical vapour deposition (CVD), and the control of chemical group functionalities by altering the input energy.6 Moreover, it allows for the straightforward coating of complex three-dimensional objects such as tubes and fabrics.
However, recently developed iCVD and oCVD (initiated and oxidative CVD), with substrate cooling, allows for the possibility of applying polymers to low melting point substrates.7
This communication describes the plasma assisted deposition of silver containing thin films, from a silver(I) compound, on polymer substrates and the consequent control of growth of P. aeruginosa. The antimicrobial activity of both the monomer and the plasma film was measured.
The phosphine-stabilised silver maleimide complex (1) was prepared by the reaction of silver nitrate with sodium maleimide (prepared in situ), in the presence of triphenylphosphine (Fig. 1). Work up of the reaction afforded the bis-phosphine complex [(Ph3P)2Ag(Mal)] (1)(Mal = maleimide) in quantitative yield. The molecular structure of 1, determined by single crystal X-ray diffraction, †shows the presence of a central three coordinate silver atom coordinated by the phosphorous atoms of two triphenylphosphine groups and the nitrogen of the maleimide group in a trigonal arrangement (sum of angles = 359.59(6)°). The maleimide ligand is coordinated to the silver atom [Ag–N(1) = 2.239(2) Å] in a monodentate fashion through the central nitrogen atom, reminiscent of the structurally related silver(I) phthalamide bis-triphenylphosphine complex reported previously.8 The antimicrobial activity (MIC) of (1) against P. aeruginosa (PAO1) was assessed and was calculated to be 0.8 × 10−6 mol dm−3 (Fig. 2) which suggests a much greater efficacy than silver sulfadiazine, used as a topical antimicrobial for wounds, which has an MIC, when incorporated into microspheres, reported at 125 × 10−6 mol dm−3.9 Zone of inhibition measurements for compound (1) pelleted with KBr in a press and grown on agar plates confirm the high level of inhibition of P. aeruginosa growth measured in the MIC assays (Fig. 3).
 | ||
| Fig. 1 The molecular structure of the silver maleimide complex (1). Selected bond lengths/Å and angles/°: Ag(1)–P(1) 2.4286(6); Ag(1)–P(2) 2.4948(6); Ag(1)–N(1) 2.239(2); N(1)–C(101) 1.356(3); N(1)–C(104) 1.347(3); C(101)–O(1) 1.215(4); C(104)–O(2) 1.230(4); C(102)–C(103) 1.462(5); P(1)–Ag(1)–N(1) 133.68(6); P(2)–Ag(1)–N(1) 105.24(6); P(1)–Ag(1)–P(2) 120.67(2) C(101)–N(1)–C(104) 109.5(2). Hydrogen atoms and solvent of crystallisation have been omitted for clarity. | ||
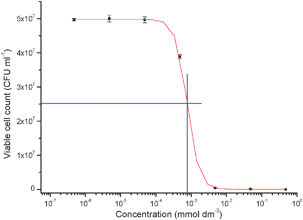 | ||
| Fig. 2 MIC measurement of 1 with P. aeruginosa. MIC measured at 0.8 μmol dm−3. | ||
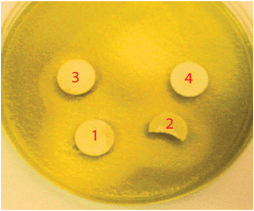 | ||
| Fig. 3 Zone of inhibition of P. aeruginosa growth around compound 1 pelleted with varying concentrations of KBr. Pellets are 10 mm in diameter. 1 = 100 wt% cpd. 1; 2 = 75% cpd. 1; 3 = 50% cpd. 1, 4 = 25% cpd. 1. | ||
Plasma deposition was used to coat complex 1 onto a range of substrates, creating a thin antimicrobial film which was effective at preventing bacterial growth on the surface. Plasma deposition took place in a home-built plasma reactor described in the ESI†. Fresh monomer ca. 150 mg was degassed by subjecting several freeze–thaw cycles in liquid nitrogen under low pressure prior to polymerisation. The monomer flow rate was determined by opening the monomer chamber to the reactor, then isolating the reactor from the vacuum. The change in pressure over 30 s allowed monomer flow rate to be calculated. All plasma polymerisation reactions were run with flow rates of 8±2 cm3stp min−1, at 50 W input power for two hours. Gold-coated glass for FTIR, glass coverslips, polystyrene Petri dishes and 3 cm × 3 cm squares of polypropylene non-woven material were used as substrates.10 Substrates were loaded to the reactor at optimised deposition points each time, with FTIR of the product taken immediately after reaction to ensure reproducibility of the resulting polymer. IR spectra of monomers and plasma deposited films were obtained using a Perkin-Elmer Spectrum 100 FT-IR spectrometer with a diamond ATR accessory. Plasma films were formed on gold-coated glass discs (Ecochemie NL) and then placed on the ATR crystal. This method significantly enhanced measurement sensitivity.
Film thickness was estimated by surface plasmon resonance (SPR) and found to be 30 nm assuming a refractive index n = 1.5. The FTIR spectrum of the deposited complex 1 shows that it is likely that the triphenylphosphine ligands were oxidised but are retained in the film, with a strong absorption at around 1050 cm−1 likely to be from phosphine oxide (formed by oxidation of the phosphine ligands in the plasma). A carbonyl stretch is observed clearly at 1600 cm−1, but a broad absorption at 3400 cm−1 is suggestive of either OH or NH stretch and opening of the maleimide ring.
P. aeruginosa PAO1 was taken from an overnight culture in LB broth. Optical density was measured at 600 nm and diluted in LB to the desired concentration for the various experiments. PSSM-coated Petri dishes were inoculated with 4 ml of P. aeruginosa at a concentration of 103 CFU ml−1. The dishes and bacteria were incubated at 37 °C for 18 h. Following growth the supernatant bacterial suspension was poured away and the attached bacteria vortexed in fresh LB (for plating) or 0.15 mol dm−3 NaCl. Optical density of the bacterial solution, fluorescence live–dead staining and plating assays were used to compare the viability of bacteria attached to the treated dishes compared with non-treated controls. A significant reduction in % live P. aeruginosa (Fig. 4) is observed following live–dead staining and fluorometric measurement of bacterial viability for the treated Petri dish compared with the control.
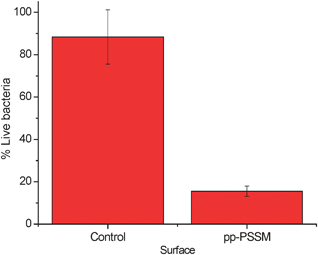 | ||
| Fig. 4 Surface killing data for P. aeruginosa on Petri dishes. | ||
The effect of plasma deposited PSSM on the viability Swiss mouse fibroblasts and human neonatal keratinocytes cultured on treated glass slides was measured using a standard XTT cell metabolic activity. No negative effect on cell viability compared with a control of clean glass was found (data included ESI†).
P. aeruginosa was grown on non-woven polypropylene fabrics 200 μl at 103 CFU ml−1 suspension for 24 h. After removal, the fabrics were stained with live–dead stain and observed using a Nikon Eclipse 90i scanning laser confocal microscope. Live bacteria fluoresce green, while dead bacteria fluoresce in the red.
The confocal microscopy images (Fig. 5), as well as showing the antimicrobial activity of the coating on the complex three-dimensional non-woven polypropylene, also show bright images on the fibres themselves. Optical microscopy showed dark particles on the fibres suggestive of metallic silver and scanning electron microscopy (SEM) of these particles showed very high silver concentrations, suggesting these are particulate silver (see ESI†). Fibre widths are approximately 10 μm.
 | ||
| Fig. 5 Scanning laser confocal images of live–dead fluorescently stained bacteria attached to non-woven polypropylene. On left is control, showing all live P. aeruginosa, on right are mainly dead. Box width/length 100 μm. | ||
Confocal microscopy of the Petri dishes and non-wovens both point to a likely ‘contact killing’ mechanism by which the coating kills bacteria, i.e. there is little leaching of the antimicrobial from the polymer into the solution. This was in part confirmed by atomic absorption spectroscopy analysis of phosphate buffered saline left in treated Petri dish for 24 h. A low concentration of 0.07 ppm silver was measured in the supernatant.
The final part of the study involved forming a thin second coating on a fluorocarbon film on the plasma film of (1) treated non-woven polypropylene fabric to render them hydrophobic and to further lessen attachment of live or dead cells which could provide growth sites for later colonisation (Fig. 6). A plasma of hexafluoroethane (Aldrich) was formed for thirty seconds on each side of the non-woven.11 This rendered the fabric intensely hydrophobic. The effect of this second layer on the plasma film of (1) greatly improved the antimicrobial performance of the non-woven fabric. The presence of the fluorinated groups was clearly observed by FTIR measurement, with a strong broad absorption centred at 1700 cm−1 being measured, attributed to fluorinated carbon groups. A modification of the Japanese Industry Standard, JIS 1902, was used to evaluate antimicrobial performance of the textile and is described in the ESI†.
 | ||
| Fig. 6 Relative growth of P. aeruginosa on compound 1–C2F6 treated non-woven polypropylene after 3 h growth. Initial bacteria concentration was 105 CFU ml−1. | ||
This communication has presented recent results which show that plasma deposition of the novel organo-silver compound (1). The effectiveness of this compound in its monomer form is far greater than many other silver based small molecules such as silver sulfadiazine and after plasma deposition is an effective way of conferring antimicrobial properties to polymeric materials, especially otherwise hard to treat textiles. The films appear not to affect the growth of sensitive mammalian cells and to be particularly effective against P. aeruginosa.
The molecules and methods detailed in this communication are currently submitted for a patent: GB0900961.4.
All authors would like to thank the European Commission’s 7th Framework programme for funding via the consortium EMBEK1-FP7 project # 211436. NP would like to acknowledge the EPSRC/University of Bath for a DTA studentship.
Notes and references
- M. Pollack, Pseudomonas aeruginosa, in Principles and Practice of Infectious Diseases, ed. G. L. Mandell, J. E. Bennett and R. Dolin, Churchill LivingstoneNew York, NY, 2000, 5th edn, , pp. 2310–2327 Search PubMed.
- A. L. Balcht and R. P. Smith, Pseudomonas aeruginosa: Infections and Treatment, Marcel Dekker Inc., New York, NY, pp. 83–84 Search PubMed.
- P. Lejeune, Trends Microbiol., 2003, 11, 179–184 CrossRef CAS.
- V. Thomas, M. Namdeo, Y. M. Mohan, S. K. Bajpai and M. Bajpai, J. Macromol. Sci., Part A: Pure Appl. Chem., 2008, 45, 107–119 Search PubMed.
- V. K. M. Poon and A. Burd, Burns, 2004, 30, 140–147 CrossRef.
- A. T. A. Jenkins, J. Hu, Y. Z. Wang, S. Schiller, R. Förch and W. Knoll, Langmuir, 2000, 16, 6381–6384 CrossRef CAS.
- S. H. Baxamusa, S. G. Im and K. K. Gleason, Phys. Chem. Chem. Phys., 2009, 11, 5227–5240 RSC.
- D. R. Whitcomb and M. Rajeswaran, J. Chem. Crystallogr., 2006, 36, 587–598 CrossRef CAS.
- N. Shanmugasundaram, J. Sundaraseelan, S. Uma, D. Selvaraj and M. Babu, J. Biomed. Mater. Res., Part B, 2006, 77b, 378–388 CrossRef CAS.
- Polypropylene non-woven cut from the topsheet of Boots™ brand disposable nappies.
- D. Liu, J. Gu, Z. Feng, D. Li and J. Niu, Thin Solid Films, 2009, 517, 3011–3019 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full details of complex synthesis, crystallographic information, protocols for MIC calculations, plasma polymerisation, bacterial counting measurements and cytotoxicity data. CCDC 742956. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b915467a |
| This journal is © The Royal Society of Chemistry 2009 |
