Skeletal rearrangement of all-carbon spiro skeletons mediated by a Lewis acid†
Fei
Zhao
a,
Chao
Wang
a,
Lantao
Liu
ab,
Wen-Xiong
Zhang
a and
Zhenfeng
Xi
*ac
aBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China. E-mail: zfxi@pku.edu.cn; Fax: +86 10 62751708; Tel: +86 10 62759728
bInstitute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, China
First published on 11th September 2009
Abstract
Mediated by AlCl3, substituted spiro[4.5]deca-tetraenes underwent novel and selective skeletal rearrangement to generate indene derivatives in high to excellent yields.
Selective carbon–carbon bond1 and carbon–hydrogen bond2 cleavage reactions are of great importance both fundamentally and practically. Achievements in these areas have been much more realized in a wide variety of functionalized or highly active compounds than in those unfunctionalized or thermodynamically stable systems, probably due to the extreme difficulty to cut C–C or C–H bonds without any mediation or facilitation by active substituents or special structures. For example, there are many reports on breaking C–C bonds in small rings;3,4 reports also appeared on the cleavage of C–C bonds in cyclopentadienyl ligands in metallocenes5,6 or aromatic compounds,7 whereas research on the cleavage of C–C bonds in those all-carbon medium cyclic compounds has been very rare in the literature,1 although interesting transformations, surprising mechanisms, or synthetically useful protocols can be expected from these reactions. As our continuous interest in cleavage and synthetic applications of unactivated chemical bonds6,8,9, we would like to report herein our preliminary results on Lewis acid10 mediated skeletal rearrange-ment of all-carbon cyclic compounds spiro[4.5]deca-tetraene 1 or 1′ yielding indene derivatives 211via sequential cleavage of both C–C and C–H bonds (Scheme 1). Notably, different from the strained spiro compounds,12 in our results the inert sp2–sp3 C–C bond of the stable cyclopentadiene moiety was cleaved under mild conditions. To the best of our knowledge, this is the first example of such skeletal rearrangements.
 | ||
| Scheme 1 Cleavage of C–C and C–H bonds in all-carbon spiro compounds. | ||
All-carbon spiro compounds 1 and 1′could be readily prepared in high yields from 1-lithio-4-aryl-1,3-butadienes.13,14 Treatment of 1a with 1 equivalent of AlCl3 in CH2Cl2 resulted in the formation of an indene derivative 2a15 in 92% isolated yield (path A, Scheme 2), while a similar procedure using 1a′ produced the same indene derivative 2a in 71% isolated yield (path B, Scheme 2), implying the reaction of both spiro compounds 1a and 1a′ may have similar reaction pathways leading to the indene derivative mediated by AlCl3. Employing directly a mixture of 1a and 1a′ as starting materials under the above condition again afforded the same indene derivative 2a as the only product in 80% isolated yield (path C, Scheme 2).‡In situNMR experiment demonstrated that 2a was formed in the reaction mixture before quenching and work-up.
 | ||
| Scheme 2 AlCl3-Mediated formation of indene 2a from 1a and 1a′. | ||
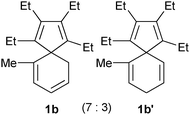
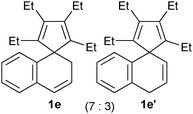
Consequently, as listed in Table 1, spiro compounds 1b–1e, and 1b′–1e′ could be transformed into the corresponding indene derivatives 2b–2e or 2c′ in good to excellent isolated yields following the procedure as described above. It is noticeable that a mixture of 1b and 1b′ afforded 2b as the sole product, whilst a mixture of 1c and 1c′ gave two isomeric products 2c and 2c′ in 73% combined isolated yield. These results are informative for the skeletal rearrangement mechanisms.
| Entry | 1 and 1′ (ratio of 1:1′) | Indene 2 |
|---|---|---|
| 1 |
|

|
| 2 |

|

|
| 3 |

|

|
| 4 |
|

|
As a fundamental reaction in organic chemistry, the reaction of Lewis acids such as AlCl3, which have an electron-deficient nature, with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond would result in the formation of reactive carbon cation species.10 Thus, for the skeletal rearrangement of spiro compounds 1 or 1′ to indene derivatives 2, a proposed mechanism including intermediacy of carbon cation species is exhibited in Scheme 3. The intermediate 3, formed through the C
C double bond would result in the formation of reactive carbon cation species.10 Thus, for the skeletal rearrangement of spiro compounds 1 or 1′ to indene derivatives 2, a proposed mechanism including intermediacy of carbon cation species is exhibited in Scheme 3. The intermediate 3, formed through the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the six-membered ring of 1 coordinating to AlCl3,16 would trigger an intramolecular proton transfervia ring-openning process and aromatization of 4, to give the diene 5. In succession, with the existance of Lewis acid, an intramolecular Friedel–Crafts reaction of 5 would herein take place and afford 2 as the final product. Indeed, diene 5 was observed during utilizing in situNMR to track the reaction. According to this mechanism, it is quite reasonable that in the case of 1c and 1c′, 2c and 2c′ were obtained as a mixture viacyclization of 5c with different regio-selectivity (Scheme 4). Although this result is helpful for testifying the possibility of the above mechanism, yet other reaction pathways still can not be ruled out (see supporting information†).
C bond in the six-membered ring of 1 coordinating to AlCl3,16 would trigger an intramolecular proton transfervia ring-openning process and aromatization of 4, to give the diene 5. In succession, with the existance of Lewis acid, an intramolecular Friedel–Crafts reaction of 5 would herein take place and afford 2 as the final product. Indeed, diene 5 was observed during utilizing in situNMR to track the reaction. According to this mechanism, it is quite reasonable that in the case of 1c and 1c′, 2c and 2c′ were obtained as a mixture viacyclization of 5c with different regio-selectivity (Scheme 4). Although this result is helpful for testifying the possibility of the above mechanism, yet other reaction pathways still can not be ruled out (see supporting information†).
 | ||
| Scheme 3 A proposed mechanism. | ||
 | ||
| Scheme 4 Two reaction pathways of cyclization of 5c. | ||
In order to get further evidence in support of the above proposed reaction mechanisms, we began to investigate the reaction between dienes 513a and AlCl3. As shown in Table 2, treated with AlCl3, dienes 5a–5d could be converted into 2a–2c, 2c′or2f in good to excellent yield. It is worth emphasizing that the ratio of 2c and 2c′ acquired from 5c is completely identical with the one from reaction of 1c and 1c′, which can be explained as the dicussion above (Scheme 4). Hence, these results not only support the above propounded mechanism, but also provide an alternative route to synthesize indene derivatives.
| Diene 5 | Ar | Indene 2 (isolated yield) |
|---|---|---|
| 5a | Ph | 2a (85%) |
| 5b | 2′-Tolyl | 2b (60%) |
| 5c | 3′-Tolyl | 2c + 2c′ (total 78%, 6:4) |
| 5d | 4′-Tolyl |

|
In summary, we have reported in this paper a facile and efficient Lewis-acid-mediated skeletal rearrangement of unstrained spiro[4.5]deca-tetraene to generate indene derivates via tandem cleavage of allylic C–H and inert C–C bonds. Further investigation on the mechanism, scope and applications are in process.
This work was supported by the National Natural Science Foundation of China and the Major State Basic Research Development Program (2006CB806105). The Cheung Kong Scholars Programme, Qiu Shi Science & Technologies Foundation, Dow Corning Corporation, Eli Lilly China and BASF are gratefully acknowledged.
Notes and references
- For recent reviews on C–C bond cleavage, see: (a) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS; (b) Y. J. Park, J.-W. Park and C.-H. Jun, Acc. Chem. Res., 2008, 41, 222 CrossRef CAS; (c) C. Nájera and J. M. Sansano, Angew. Chem., 2009, 121, 2488 CrossRef; C. Nájera and J. M. Sansano, Angew. Chem., Int. Ed., 2009, 48, 2452 CrossRef CAS.
- For recent reviews on C–H bond cleavage, see: (a) F. Kakiuchi and N. Chatani, Adv. Synth. Catal., 2003, 345, 1077 CrossRef CAS; (b) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS; (c) L. Ackermann, Top. Organomet. Chem., 2007, 24, 35 CAS; (d) K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC; (e) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef CAS; (f) J. C. Lewis, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2008, 41, 1013 CrossRef CAS; (g) B. Li, S. Yang and Z. Shi, Synlett, 2008, 7, 949; (h) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS.
- For recent examples on C–C bond cleavage in three-membered rings, see: (a) M. Shi, L.-P. Liu and J. Tang, J. Am. Chem. Soc., 2006, 128, 7430 CrossRef CAS; (b) P. A. Wender, L. O. Haustedt, J. Lim, J. A. Love, T. J. Williams and J.-Y. Yoon, J. Am. Chem. Soc., 2006, 128, 6302 CrossRef CAS; (c) V. Bagutski, N. Moszner, F. Zeuner, U. K. Fischer and A. de Meijere, Adv. Synth. Catal., 2006, 348, 2133 CrossRef CAS; (d) C. Aïssa and A. Fürstner, J. Am. Chem. Soc., 2007, 129, 14836 CrossRef CAS; (e) Z.-X. Yu, P. H.-Y. Cheong, P. Liu, C. Y. Legault, P. A. Wender and K. N. Houk, J. Am. Chem. Soc., 2008, 130, 2378 CrossRef CAS; (f) P. Mauleón, J. L. Krinsky and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 4513 CrossRef CAS; (g) L. Liu and J. Zhang, Angew. Chem., Int. Ed., 2009, 48, 6093 CrossRef CAS.
- (a) For recent examples on C–C bond cleavage in four-memdered rings, see: T. Matsuda, M. Shigeno and M. Murakami, J. Am. Chem. Soc., 2007, 129, 12086 Search PubMed; (b) T. Seiser and N. Cramer, Angew. Chem., Int. Ed., 2008, 47, 9294 CrossRef CAS; (c) C. Winter and N. Krause, Angew. Chem., Int. Ed., 2009, 48, 2460 CrossRef CAS.
- (a) A. Tillack, W. Baumann, A. Ohff, C. Lefeber, A. Spannennberg, R. Kempe and U. Rosenthal, J. Organomet. Chem., 1996, 520, 187 CrossRef; (b) T. L. Dzwiniel and J. M. Stryker, J. Am. Chem. Soc., 2004, 126, 9184 CrossRef CAS.
- (a) Z. Xi, K. Sato, Y. Gao, J. Lu and T. Takahashi, J. Am. Chem. Soc., 2003, 125, 9568 CrossRef CAS; (b) T. Takahashi, Y. Kuzuba, F. Kong, K. Nakajima and Z. Xi, J. Am. Chem. Soc., 2005, 127, 17188 CrossRef CAS; (c) T. Takahashi, Z. Song, K. Sato, Y. Kuzuba, K. Nakajima and K. Kanno, J. Am. Chem. Soc., 2007, 129, 11678 CrossRef CAS; (d) T. Takahashi, Z. Song, Y.-F. Hsieh, K. Nakajima and K. Kanno, J. Am. Chem. Soc., 2008, 130, 15236 CrossRef CAS.
- (a) A. L. Crombie, Jr., J. L. Kane, K. M. Shea and R. L. Danheiser, J. Org. Chem., 2004, 69, 8652 CrossRef; (b) P. Panne and J. M. Fox, J. Am. Chem. Soc., 2007, 129, 22 CrossRef CAS.
- (a) X. Sun, C. Wang, Z. Li, S. Zhang and Z. Xi, J. Am. Chem. Soc., 2004, 126, 7172 CrossRef CAS; (b) T. Yu, X. Sun, C. Wang, L. Deng and Z. Xi, Chem.–Eur. J., 2005, 11, 1895 CrossRef CAS; (c) C. Wang, J. Yuan, G. Li, Z. Wang, S. Zhang and Z. Xi, J. Am. Chem. Soc., 2006, 128, 4564 CrossRef CAS; (d) C. Wang, L. Deng, J. Yan, H. Wang, Q. Luo, W. Zhang and Z. Xi, Chem. Commun., 2009, 4414 RSC.
- (a) T. Takahashi, Z. Xi, Y. Obora and N. Suzuki, J. Am. Chem. Soc., 1995, 117, 2665 CrossRef CAS; (b) T. Takahashi, Z. Xi, R. Fischer, S. Huo, C. Xi and K. Nakajima, J. Am. Chem. Soc., 1997, 119, 4561 CrossRef CAS; (c) Z. Xi, R. Fischer, R. Hara, W.-H. Sun, Y. Obora, N. Suzuki, K. Nakajima and T. Takahashi, J. Am. Chem. Soc., 1997, 119, 12842 CrossRef CAS.
- C. Wang and Z. Xi, Chem. Soc. Rev., 2007, 36, 1395 RSC.
- For selected examples on synthesis of indenes, see: (a) Z. Xi, R. Guo, S. Mito, H. Yan, K. Kanno, K. Nakajima and T. Takahashi, J. Org. Chem., 2003, 68, 1252 CrossRef CAS; (b) H. Tsukamoto, T. Ueno and Y. Kondo, Org. Lett., 2007, 9, 3033 CrossRef CAS; (c) D. Zhang, Z. Liu, E. K. Yum and R. C. Larock, J. Org. Chem., 2007, 72, 251 CrossRef CAS; (d) X. Zhou, H. Zhang, X. Xie and Y. Li, J. Org. Chem., 2008, 73, 3958 CrossRef CAS; (e) Z. A. Khan and T. Wirth, Org. Lett., 2009, 11, 229 CrossRef CAS.
- (a) K. M. Joly, B. M. Kariuki, D. M. Coe and L. R. Cox, Organometallics, 2005, 24, 358 CrossRef CAS; (b) T. Matsuda, T. Tsuboi and M. Murakami, J. Am. Chem. Soc., 2007, 129, 12596 CrossRef CAS.
- (a) T. Takahashi, W.-H. Sun, C. Xi, H. Ubayama and Z. Xi, Tetrahedron, 1998, 54, 715 CrossRef CAS; (b) Z. Wang and Z. Xi, Synlett, 2006, 1275 CAS; (c) L. Liu, Z. Wang, F. Zhao and Z. Xi, J. Org. Chem., 2007, 72, 3484 CrossRef CAS.
- For reviews on reactions and applications of 1-lithio-1,3-butadiene, see: (a) W.-X. Zhang and Z. Xi, Pure Appl. Chem., 2009, 81, 235 CrossRef CAS; (b) Z. Xi, Bull. Chem. Soc. Jpn., 2007, 80, 1021 CrossRef CAS.
- X. Sun, K.-J. Izumi, C.-Q. Hu and G.-Q. Lin, Chin. J. Chem., 2006, 24, 430 CrossRef CAS.
- For examples on interaction between Lewis acid and C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, see:
(a) T. Tsuchimoto, S. Kamiyama, R. Negoro, E. Shirakawa and Y. Kawakami, Chem. Commun., 2003, 852 RSC;
(b) Y.-J. Jiang, Y.-Q. Tu, E. Zhang, S.-Y. Zhang, K. Cao and L. Shi, Adv. Synth. Catal., 2008, 350, 552 CrossRef CAS;
(c) J. Barluenga, A. Fernández, F. Rodríguez and F. J. Fañanás, J. Organomet. Chem., 2009, 694, 546 CrossRef CAS.
C double bonds, see:
(a) T. Tsuchimoto, S. Kamiyama, R. Negoro, E. Shirakawa and Y. Kawakami, Chem. Commun., 2003, 852 RSC;
(b) Y.-J. Jiang, Y.-Q. Tu, E. Zhang, S.-Y. Zhang, K. Cao and L. Shi, Adv. Synth. Catal., 2008, 350, 552 CrossRef CAS;
(c) J. Barluenga, A. Fernández, F. Rodríguez and F. J. Fañanás, J. Organomet. Chem., 2009, 694, 546 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and scanned NMR spectra of all new products. See DOI: 10.1039/b914619a |
| ‡ A typical procedure for synthesis of indene derivatives 2 from spiro compounds1. To a solution of a mixture of 1 and 1′ (1 mmol in total) in dichloromethane (5 mL) was added AlCl3 (133 mg, 1 mmol). The reaction mixture was stirred at 0 °C for 1 h. After quenching with water, the mixture was extracted with hexane. The extract was washed with brine and dried over MgSO4. The solvent was then evaporated in vacuo and the residue was purified by column chromatography (silica gel, hexane) to afford the product 2. 2a: Colorless liquid, isolated yield 80% (194 mg); 1H NMR (300 MHz, CDCl3, Me4Si): δ 0.28 (t, J = 7.5 Hz, 3H, CH3), 0.54–0.58 (m, 1H, CH2), 0.68 (t, J = 6.6 Hz, 3H, CH3), 0.72–0.78 (m, 1H, CH2), 1.13 (t, J = 7.5 Hz, 3H, CH3), 1.16 (t, J = 7.5 Hz, 3H, CH3), 1.64–1.88 (m, 4H, CH2), 2.24 (qd, J = 7.5, 1.5 Hz, 2H, CH2), 2.53 (q, J = 7.5 Hz, 2H, CH2), 7.08–7.20 (m, 4H, CH); 13C NMR (75 MHz, CDCl3, Me4Si): δ 7.97 (1 CH3), 13.92 (1 CH3), 13.99 (1 CH3), 14.46 (1 CH3), 16.83 (1 CH2), 18.21 (1 CH2), 18.68 (1 CH2), 30.25 (1 CH2), 40.00 (1 CH2), 58.67 (1 quart C), 117.89 (1 CH), 120.92 (1 CH), 123.76 (1 CH), 125.90 (1 CH), 139.56 (1 quart C), 145.89 (1 quart C), 147.28 (1 quart C), 149.63 (1 quart C); HRMS(ESI) calcd for [C18H26]H+: 243.2107; found 243.2102. |
| This journal is © The Royal Society of Chemistry 2009 |

